Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa
Abstract
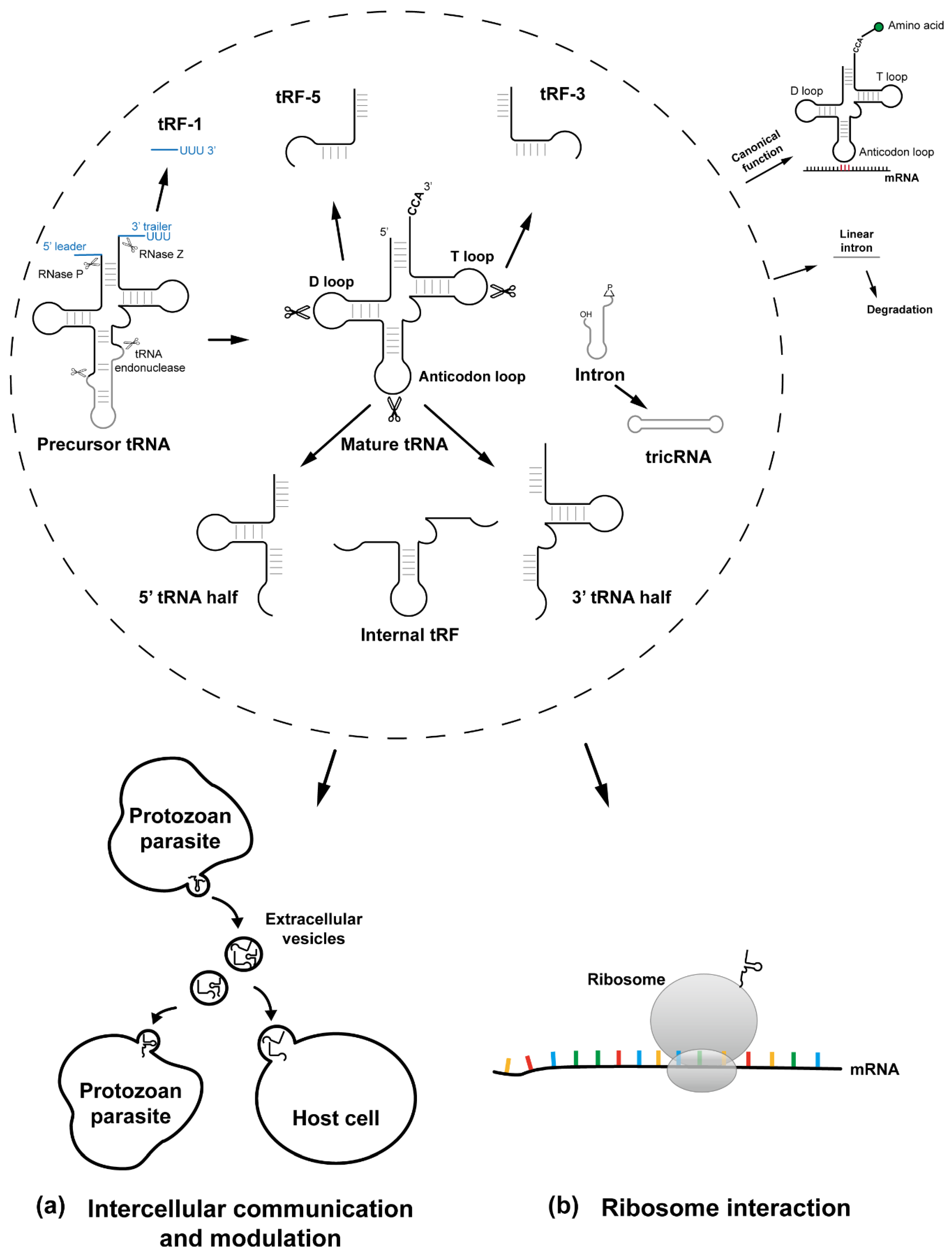
:1. Introduction
2. tRNA-Derived Small RNAs (tsRNAs) in Protozoan Parasites
2.1. Trypanosoma cruzi and Trypanosoma brucei
2.2. Leishmania donovani and Leishmania braziliensis
2.3. Plasmodium falciparum
2.4. Toxoplasma gondii
2.5. Entamoeba histolytica
2.6. Trichomonas vaginalis
2.7. Giardia lamblia
3. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, K.; Kanai, A. tRNA gene diversity in the three domains of life. Front. Genet. 2014, 5, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberbauer, V.; Schaefer, M.R. tRNA-derived small RNAs: Biogenesis, modification, function and potential impact on human disease development. Genes 2018, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, A.; Chernyakov, I.; Gu, W.; Hiley, S.L.; Hughes, T.R.; Grayhack, E.J.; Phizicky, E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell 2006, 21, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Nishikawa, K.; Nemoto, F.; Kuchino, Y.; Nishimura, S.; Miyazawa, T.; Yokoyama, S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 1988, 336, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Putz, J.; Florentz, C.; Benseler, F.; Giege, R. A single methyl group prevents the mischarging of a tRNA. Nat. Struct. Biol. 1994, 1, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Leidel, S.; Pedrioli, P.G.; Bucher, T.; Brost, R.; Costanzo, M.; Schmidt, A.; Aebersold, R.; Boone, C.; Hofmann, K.; Peter, M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 2009, 458, 228–232. [Google Scholar] [CrossRef]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and function of transfer RNA-related fragments (tRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Cole, C.; Sobala, A.; Lu, C.; Thatcher, S.R.; Bowman, A.; Brown, J.W.; Green, P.J.; Barton, G.J.; Hutvagner, G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA 2009, 15, 2147–2160. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.Y.; Ma, L.M.; Guo, Y.H.; Zhang, Y.C.; Zhou, H.; Shao, P.; Chen, Y.Q.; Qu, L.H. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS ONE 2010, 5, e10563. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, G. Anticodon nucleases. Trends Biochem. Sci. 2000, 25, 70–74. [Google Scholar] [CrossRef]
- Lin, J.J.; Newton, D.L.; Mikulski, S.M.; Kung, H.F.; Youle, R.J.; Rybak, S.M. Characterization of the mechanism of cellular and cell free protein synthesis inhibition by an anti-tumor ribonuclease. Biochem. Biophys. Res. Commun. 1994, 204, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Rybak, S.M.; Davey, R.T., Jr.; Youle, R.J.; Ackerman, E.J. Angiogenin is a cytotoxic, tRNA-specific ribonuclease in the RNase A superfamily. J. Biol. Chem. 1992, 267, 21982–21986. [Google Scholar] [CrossRef]
- St Clair, D.K.; Rybak, S.M.; Riordan, J.F.; Vallee, B.L. Angiogenin abolishes cell-free protein synthesis by specific ribonucleolytic inactivation of ribosomes. Proc. Natl. Acad. Sci. USA 1987, 84, 8330–8334. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Stanton, B.A. Transfer RNA-Derived Fragments, the Underappreciated regulatory small RNAs in microbial pathogenesis. Front. Microbiol 2021, 12, 687632. [Google Scholar] [CrossRef]
- Su, Z.; Wilson, B.; Kumar, P.; Dutta, A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu Rev. Genet. 2020, 54, 47–69. [Google Scholar] [CrossRef]
- Fu, H.; Feng, J.; Liu, Q.; Sun, F.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009, 583, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yao, L.; Yu, X.; Ruan, Y.; Li, Z.; Guo, J. Action mechanisms and research methods of tRNA-derived small RNAs. Signal. Transduct Target. Ther. 2020, 5, 109. [Google Scholar] [CrossRef]
- Lu, Z.; Filonov, G.S.; Noto, J.J.; Schmidt, C.A.; Hatkevich, T.L.; Wen, Y.; Jaffrey, S.R.; Matera, A.G. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA 2015, 21, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.; Ibba, M. Bacterial transfer RNAs. FEMS Microbiol. Rev. 2015, 39, 280–300. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, M.; Kirino, Y. tRNA-derived short non-coding RNA as interacting partners of argonaute proteins. Gene Regul. Syst. Biol. 2015, 9, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Langenberger, D.; Cakir, M.V.; Hoffmann, S.; Stadler, P.F. Dicer-processed small RNAs: Rules and exceptions. J. Exp. Zool. Part B Mol. Dev. Evol. 2013, 320, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ender, C.; Meister, G.; Moore, P.S.; Chang, Y.; John, B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucl. Acids Res. 2012, 40, 6787–6799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maute, R.L.; Schneider, C.; Sumazin, P.; Holmes, A.; Califano, A.; Basso, K.; Dalla-Favera, R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1404–1409. [Google Scholar] [CrossRef] [Green Version]
- Tao, E.W.; Cheng, W.Y.; Li, W.L.; Yu, J.; Gao, Q.Y. tiRNAs: A novel class of small noncoding RNAs that helps cells respond to stressors and plays roles in cancer progression. J. Cell. Physiol. 2020, 235, 683–690. [Google Scholar] [CrossRef]
- Thompson, D.M.; Parker, R. Stressing out over tRNA cleavage. Cell 2009, 138, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Yeri, A.; Cheah, P.S.; Chung, A.; Danielson, K.; De Hoff, P.; Filant, J.; Laurent, C.D.; Laurent, L.D.; Magee, R.; et al. Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell 2019, 177, 446–462.e16. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [Green Version]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large differences in small RNA composition between human biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Frost, E.L.; Lammert, C.R.; Przanowska, R.K.; Lukens, J.R.; Dutta, A. tRNA-derived fragments and microRNAs in the maternal-fetal interface of a mouse maternal-immune-activation autism model. RNA Biol. 2020, 17, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Dhahbi, J.M. 5′ tRNA Halves: The next generation of immune signaling molecules. Front. Immunol. 2015, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, M.M.; Nitz, N.; Araujo, P.F.; Sousa, A.O.; Rosa Ade, C.; Gomes, D.A.; Leonardecz, E.; Teixeira, A.R. Inheritance of DNA transferred from American trypanosomes to human hosts. PLoS ONE 2010, 5, e9181. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Frugier, M.; Tosar, J.P.; Correa-Dominguez, A.; Ronalte-Alves, L.; Parodi-Talice, A.; Rovira, C.; Robello, C.; Goldenberg, S.; Cayota, A. A population of tRNA-derived small RNAs is actively produced in Trypanosoma cruzi and recruited to specific cytoplasmic granules. Mol. Biochem. Parasitol. 2010, 171, 64–73. [Google Scholar] [CrossRef]
- Trocoli Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; NC, E.S.; de Almeida Abrahamsohn, I.; Colli, W.; Manso Alves, M.J. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Cabrera-Cabrera, F.; das Neves, R.F.; Souto-Padron, T.; de Souza, W.; Cayota, A. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: Relevance of tRNA-derived halves. Biomed. Res. Int. 2014, 2014, 305239. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Silva, M.R.; das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Lima, F.M.; Ruiz, J.C.; Almeida, I.C.; da Silveira, J.F. Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol. Biochem. Parasitol. 2014, 193, 71–74. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Asady, B.; Docampo, R. Acidocalcisome-mitochondrion membrane contact sites in Trypanosoma brucei. Pathogens 2018, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles in Trypanosomatids: Host cell communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef] [PubMed]
- Parreira de Aquino, G.; Mendes Gomes, M.A.; Kopke Salinas, R.; Laranjeira-Silva, M.F. Lipid and fatty acid metabolism in trypanosomatids. Microb. Cell. 2021, 8, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.; Brogli, R.; Luidalepp, H.; Wyss, L.; Fasnacht, M.; Joss, O.; Zywicki, M.; Helm, M.; Schneider, A.; Cristodero, M.; et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat. Commun. 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambertz, U.; Oviedo Ovando, M.E.; Vasconcelos, E.J.; Unrau, P.J.; Myler, P.J.; Reiner, N.E. Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved exosomal RNA Packaging. BMC Genom. 2015, 16, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wei, C.; Hao, X.; Deng, W.; Zhang, L.; Wang, Z.; Wang, H. Genome-wide identification and characterization of transfer RNA-derived small RNAs in Plasmodium falciparum. Parasit. Vectors 2019, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, K.A.; Mbagwu, S.; Hernandez-Castaneda, M.A.; Adapa, S.R.; Walch, M.; Filgueira, L.; Falquet, L.; Jiang, R.H.Y.; Ghiran, I.; Mantel, P.Y. Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci. Rep. 2018, 8, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galizi, R.; Spano, F.; Giubilei, M.A.; Capuccini, B.; Magini, A.; Urbanelli, L.; Ogawa, T.; Dubey, J.P.; Spaccapelo, R.; Emiliani, C.; et al. Evidence of tRNA cleavage in apicomplexan parasites: Half-tRNAs as new potential regulatory molecules of Toxoplasma gondii and Plasmodium berghei. Mol. Biochem. Parasitol. 2013, 188, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.Z.; Zhang, H.; Ehrenkaufer, G.; Singh, U. Stress response in Entamoeba histolytica is associated with robust processing of tRNA to tRNA-halves. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhou, H.C.; Wei, C.Y.; Wang, Z.H.; Hao, X.; Zhang, L.H.; Li, J.Z.; Wang, Z.L.; Wang, H. Global survey of miRNAs and tRNA-derived small RNAs from the human parasitic protist Trichomonas vaginalis. Parasit. Vectors 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Artuyants, A.; Campos, T.L.; Rai, A.K.; Johnson, P.J.; Dauros-Singorenko, P.; Phillips, A.; Simoes-Barbosa, A. Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA-derived small RNAs. Int. J. Parasitol. 2020, 50, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, J.; Zhou, H.; Liao, J.Y.; Ma, L.M.; Chen, Y.Q.; Qu, L.H. Stress-induced tRNA-derived RNAs: A novel class of small RNAs in the primitive eukaryote Giardia Lamblia. Nucleic Acids Res. 2008, 36, 6048–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.Y.; Guo, Y.H.; Zheng, L.L.; Li, Y.; Xu, W.L.; Zhang, Y.C.; Zhou, H.; Lun, Z.R.; Ayala, F.J.; Qu, L.H. Both endo-siRNAs and tRNA-derived small RNAs are involved in the differentiation of primitive eukaryote Giardia lamblia. Proc. Natl. Acad. Sci. USA 2014, 111, 14159–14164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and other extracellular vesicles: The new communicators in parasite infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tan, M.S.Y.; Blackman, M.J. Malaria parasite egress at a glance. J. Cell Sci. 2021, 134, jcs237345. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.S.; Fidock, D.A. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.J.; Nozaki, T. Interorganellar communication and membrane contact sites in protozoan parasites. Parasitol. Int. 2021, 83, 102372. [Google Scholar] [CrossRef]
- Mantel, P.Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Zhang, Q.; Huang, Y.; Feng, L.; Pan, W. No miRNA were found in Plasmodium and the ones identified in erythrocytes could not be correlated with infection. Malar. J. 2008, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Wassmer, S.C. Harnessing the potential of miRNAs in malaria diagnostic and prevention. Front. Cell Infect. Microbiol. 2021, 11, 793954. [Google Scholar] [CrossRef]
- Gupta, H.; Rubio, M.; Sitoe, A.; Varo, R.; Cistero, P.; Madrid, L.; Cuamba, I.; Jimenez, A.; Martianez-Vendrell, X.; Barrios, D.; et al. Plasma microRNA profiling of Plasmodium falciparum biomass and association with severity of malaria disease. Emerg. Infect. Dis. 2021, 27, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Braun, L.; Cannella, D.; Ortet, P.; Barakat, M.; Sautel, C.F.; Kieffer, S.; Garin, J.; Bastien, O.; Voinnet, O.; Hakimi, M.A. A complex small RNA repertoire is generated by a plant/fungal-like machinery and effected by a metazoan-like Argonaute in the single-cell human parasite Toxoplasma gondii. PLoS Pathog. 2010, 6, e1000920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, H.; Ogawa, T. The modes of action of colicins E5 and D, and related cytotoxic tRNases. Biochimie 2002, 84, 433–438. [Google Scholar] [CrossRef]
- Peng, R.; Yoshinari, S.; Kawano-Sugaya, T.; Jeelani, G.; Nozaki, T. Identification and functional characterization of divergent 3′-phosphate tRNA ligase from Entamoeba histolytica. Front. Cell Infect. Microbiol. 2021, 11, 746261. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.J.; Imai, K.; Makiuchi, T.; Tomii, K.; Horton, P.; Nozawa, A.; Okada, K.; Tozawa, Y.; Nozaki, T. Novel lineage-specific transmembrane beta-barrel proteins in the endoplasmic reticulum of Entamoeba histolytica. FEBS J. 2019, 286, 3416–3432. [Google Scholar] [CrossRef]
- Santos, H.J.; Hanadate, Y.; Imai, K.; Watanabe, H.; Nozaki, T. Entamoeba histolytica EHD1 is involved in mitosome-endosome contact. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sharma, M.; Morgado, P.; Zhang, H.; Ehrenkaufer, G.; Manna, D.; Singh, U. Characterization of extracellular vesicles from Entamoeba histolytica identifies roles in intercellular communication that regulates parasite growth and development. Infect. Immun. 2020, 88, e00349-20. [Google Scholar] [CrossRef]
- Jochl, C.; Rederstorff, M.; Hertel, J.; Stadler, P.F.; Hofacker, I.L.; Schrettl, M.; Haas, H.; Huttenhofer, A. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic. Acids Res. 2008, 36, 2677–2689. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Collins, K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J. Biol. Chem. 2005, 280, 42744–42749. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.M.; Lu, C.; Green, P.J.; Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 2008, 14, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Sun, L.; Kragler, F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant. Physiol. 2009, 150, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraja, S.; Cai, M.W.; Sun, J.; Varet, H.; Sarid, L.; Trebicz-Geffen, M.; Shaulov, Y.; Mazumdar, M.; Legendre, R.; Coppee, J.Y.; et al. Queuine is a nutritional regulator of Entamoeba histolytica response to oxidative stress and a virulence attenuator. mBio 2021, 12, e03549-20. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.; Adamski, A. Trichomoniasis and HIV interactions: A review. Sex. Transm. Infect. 2013, 89, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beri, D.; Yadav, P.; Devi, H.R.N.; Narayana, C.; Gadara, D.; Tatu, U. Demonstration and characterization of cyst-like structures in the life cycle of Trichomonas vaginalis. Front. Cell. Infect. Microbiol. 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Ortiz, L.M.; Barajas-Mendiola, M.A.; Barrios-Rodiles, M.; Castellano, L.E.; Arias-Negrete, S.; Avila, E.E.; Cuellar-Mata, P. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. 2017, 39, e12426. [Google Scholar] [CrossRef]
- Ankarklev, J.; Jerlstrom-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svard, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef]
- Levitz, R.; Chapman, D.; Amitsur, M.; Green, R.; Snyder, L.; Kaufmann, G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 1990, 9, 1383–1389. [Google Scholar] [CrossRef]
- Zheng, L.L.; Xu, W.L.; Liu, S.; Sun, W.J.; Li, J.H.; Wu, J.; Yang, J.H.; Qu, L.H. tRF2Cancer: A web server to detect tRNA-derived small RNA fragments (tRFs) and their expression in multiple cancers. Nucleic Acids Res. 2016, 44, W185–W193. [Google Scholar] [CrossRef]
- Pliatsika, V.; Loher, P.; Magee, R.; Telonis, A.G.; Londin, E.; Shigematsu, M.; Kirino, Y.; Rigoutsos, I. MINTbase v2.0: A comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic Acids Res. 2018, 46, D152–D159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar] [CrossRef]
- Huang, B.; Yang, H.; Cheng, X.; Wang, D.; Fu, S.; Shen, W.; Zhang, Q.; Zhang, L.; Xue, Z.; Li, Y.; et al. tRF/miR-1280 Suppresses stem cell-like cells and metastasis in colorectal cancer. Cancer Res. 2017, 77, 3194–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell 2015, 161, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Mo, D.; Jiang, P.; Yang, Y.; Mao, X.; Tan, X.; Tang, X.; Wei, D.; Li, B.; Wang, X.; Tang, L.; et al. A tRNA fragment, 5′-tiRNA(Val), suppresses the Wnt/beta-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019, 457, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Loher, P.; Shigematsu, M.; Palazzo, J.P.; Suzuki, R.; Imoto, I.; Rigoutsos, I.; Kirino, Y. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl. Acad. Sci. USA 2015, 112, E3816–E3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steidinger, T.U.; Standaert, D.G.; Yacoubian, T.A. A neuroprotective role for angiogenin in models of Parkinson′s disease. J. Neurochem. 2011, 116, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, M.; Jobava, R.; Parisien, M.; Putnam, A.; Krokowski, D.; Gao, X.H.; Guan, B.J.; Yuan, Y.; Jankowsky, E.; Feng, Z.; et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol. Cell. Biol. 2014, 34, 2450–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, P.; O’Day, E.; Emara, M.M.; Wagner, G.; Lieberman, J.; Anderson, P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA 2014, 111, 18201–18206. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, X.; Shi, J.; Tuorto, F.; Li, X.; Liu, Y.; Liebers, R.; Zhang, L.; Qu, Y.; Qian, J.; et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 2018, 20, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Sarker, G.; Sun, W.; Rosenkranz, D.; Pelczar, P.; Opitz, L.; Efthymiou, V.; Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 10547–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, C.; Toivonen, S.; Diaz Villamil, E.; Atta, M.; Ravanat, J.L.; Demine, S.; Schiavo, A.A.; Pachera, N.; Deglasse, J.P.; Jonas, J.C.; et al. Pancreatic beta-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res. 2018, 46, 10302–10318. [Google Scholar] [CrossRef]
- Guzzi, N.; Ciesla, M.; Ngoc, P.C.T.; Lang, S.; Arora, S.; Dimitriou, M.; Pimkova, K.; Sommarin, M.N.E.; Munita, R.; Lubas, M.; et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell 2018, 173, 1204–1216.e26. [Google Scholar] [CrossRef] [Green Version]
- Sobala, A.; Hutvagner, G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013, 10, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Lyons, S.M.; Achorn, C.; Kedersha, N.L.; Anderson, P.J.; Ivanov, P. YB-1 regulates tiRNA-induced Stress Granule formation but not translational repression. Nucleic Acids Res. 2016, 44, 6949–6960. [Google Scholar] [CrossRef]
- Kuscu, C.; Kumar, P.; Kiran, M.; Su, Z.; Malik, A.; Dutta, A. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 2018, 24, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.; Yim, D.G.; Lakshmanan, V.; Tirumalai, V.; Koh, J.L.; Park, J.E.; Cheong, J.K.; Low, J.L.; Lim, M.J.; Sze, S.K.; et al. Dynamic expression of tRNA-derived small RNAs define cellular states. EMBO Rep. 2019, 20, e47789. [Google Scholar] [CrossRef]
- Durdevic, Z.; Mobin, M.B.; Hanna, K.; Lyko, F.; Schaefer, M. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 2013, 4, 931–937. [Google Scholar] [CrossRef] [Green Version]
- Couvillion, M.T.; Sachidanandam, R.; Collins, K. A growth-essential Tetrahymena Piwi protein carries tRNA fragment cargo. Genes Dev. 2010, 24, 2742–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskovic, A.; Bing, X.Y.; Kaymak, E.; Rando, O.J. Control of noncoding RNA production and histone levels by a 5′ tRNA fragment. Genes Dev. 2020, 34, 118–131. [Google Scholar] [CrossRef] [PubMed]
| Protozoan Species | Category of Identified tsRNAs | Validated or Proposed Functions and Important Findings | Predominant tRNAs That tsRNAs Were Identified for | References |
|---|---|---|---|---|
| Trypanosoma cruzi | tRNA halves | Induced by nutritional stress | tRNAAsp(GUC), tRNAGlu(CUC), and tRNAAla(CGC) | [35] |
| tRNA halves | Enriched in extracellular vesicles and transferred to host cells. Secreted tRNAThr halves directly regulate gene expression in HeLa cells. | tRNALeu, tRNAThr, tRNAGlu, tRNA Gly, and tRNAArg | [37,38] | |
| Trypanosoma brucei | tRNA halves | 3′ tRNAThr halves stimulate translation for stress recovery during starvation. | tRNAThr, tRNAAla, and tRNAAsp | [43] |
| Leishmania donovani and Leishmania braziliensis | Mostly tRNA halves | Contained in exosomes. Exosomal RNA cargoes can be delivered to macrophages. | tRNAAsp, tRNAGln, tRNAGlu, and tRNALeu | [44] |
| Plasmodium falciparum | Mostly tRF-5 | Unknown | tRNAPro, tRNAPhe, tRNAAsn, tRNAGly, tRNACys, tRNAGln, tRNAHis, and tRNAAla | [45] |
| Mosly tRNA halves | Exosomal RNA cargoes can be delivered to human endothelial cells. | tRNAGlu, tRNASer, tRNAMet, tRNAPro, tRNAArg, tRNATrp, tRNAArg, and tRNACys | [46] | |
| Toxoplasma gondii | tRNA halves | Production of tRNA halves is higher in avirulent strains and in metabolically quiescent stages | tRNAAla(UGC), tRNAGln(CUG), tRNAGly(GCC), tRNAPro(UGG), tRNAMet(CAU), and tRNAGly(UCC) | [47] |
| Entamoeba histolytica | tRNA halves and tRFs | Assumed to mediate intercellular communication. | tRNAAla(AGC), tRNAAla(UGC), tRNAArg(UCU), tRNAAsp(GUC) | [48] |
| Trichomonas vaginalis | tRNA halves and tRFs | Unknown | tRNAGlu, tRNAGly, tRNAPhe, tRNALys, tRNAVal, tRNAArg, tRNAAsn, and tRNATyr | [49,50] |
| Giardia lamblia | tRNA halves and tRFs | Low metabolic conditions can induce formation of tsRNAs. | tRNAGlu(CUC), tRNAHis(GUG), and tRNACys(GCA) | [51,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Santos, H.J.; Nozaki, T. Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa. Genes 2022, 13, 286. https://doi.org/10.3390/genes13020286
Peng R, Santos HJ, Nozaki T. Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa. Genes. 2022; 13(2):286. https://doi.org/10.3390/genes13020286
Chicago/Turabian StylePeng, Ruofan, Herbert J. Santos, and Tomoyoshi Nozaki. 2022. "Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa" Genes 13, no. 2: 286. https://doi.org/10.3390/genes13020286
APA StylePeng, R., Santos, H. J., & Nozaki, T. (2022). Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa. Genes, 13(2), 286. https://doi.org/10.3390/genes13020286






