The PI3K/AKT Pathway and PTEN Gene Are Involved in “Tree-Top Disease” of Lymantria dispar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect and Virus
2.2. Sample Preparation
2.3. Quantitative Real-Time RT-PCR Analysis of Core Genes in the PI3K/AKT Pathway
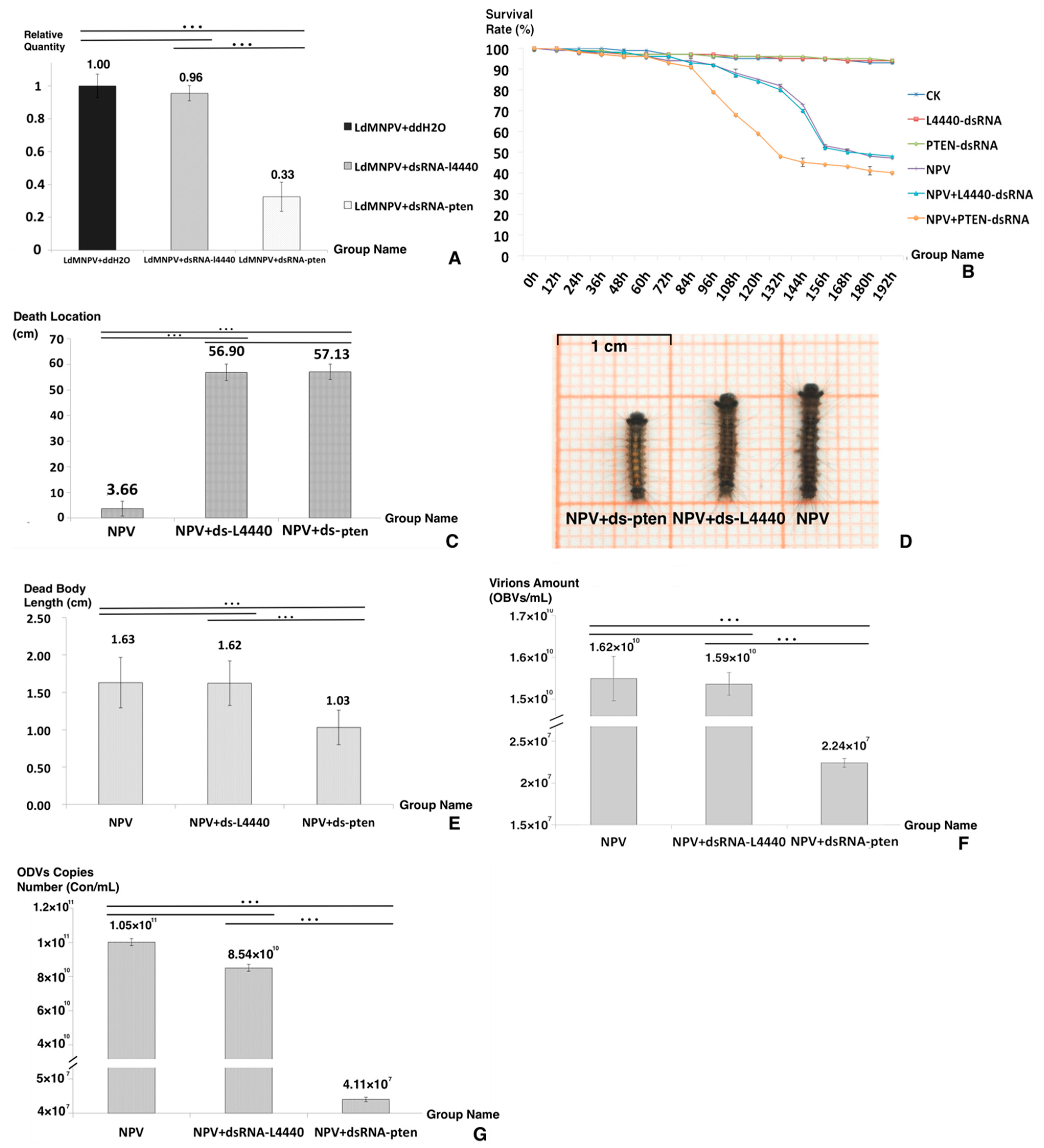
2.4. Synthesis and Validation of dsRNA-Expressing Bacteria
2.5. Delivery of dsRNA via Feeding, Mortality and Behavioral Assays
3. Results
3.1. Preliminary Screening of Genes in PI3K/AKT Signaling Pathway
3.2. Quantitative Real-Time PCR Analysis of Core Genes in the PI3K/AKT Pathway
3.3. Synthesis and Validation of DsRNA-Expressing Bacteria
3.4. Delivery of dsRNA via Feeding, Mortality Assays and Behavioral Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biron, D.G.; Ponton, F.; Marche, L.; Galeotti, N.; Renault, L.; Demey-Thomas, E.; Poncet, J.; Brown, S.P.; Jouin, P.; Thomas, F. ‘Suicide’ of crickets harbouring hairworms: A proteomics investigation. Insect Mol. Biol. 2006, 15, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Otalora-Luna, F.; Lefevre, T.; Guerin, P.M.; Lebarbenchon, C.; Duneau, D.; Biron, D.G.; Thomas, F. Water-seeking behavior in worm-infected crickets and reversibility of parasitic manipulation. Behav. Ecol. 2011, 22, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, R.; Maure, F. Host manipulation by parasites: A look back before moving forward. Trends Parasitol. 2015, 31, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.D.; Cory, J.S.; Wilson, K.R.; Sait, S.M.; Hails, R.S. Modified behavior in baculovirus-infected lepidopteran larvae and its impact on the spatial distribution of inoculum. Biol. Control 1996, 7, 299–306. [Google Scholar] [CrossRef]
- Hofmann, O. Die Schlaffsucht (Flacherie) der Nonne (Liparis monacha) nebst einem Anhang. Insektentötende Pilze mit Besonderer Berücksichtigung der Nonne. 1891. Entomol. Sci. 2004, 7, 219–223. [Google Scholar]
- Rohrmann, G.F. Baculovirus Molecular Biology [Internet], 4th ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019. [Google Scholar]
- Goulson, D. Wipfelkrankheit: Modification of host behaviour during baculoviral infection. Oecologia 1997, 109, 219–228. [Google Scholar] [CrossRef]
- Kamita, S.G.; Nagasaka, K.; Chua, J.W.; Shimada, T.; Mita, K.; Kobayashi, M.; Maeda, S.; Hammock, B.D. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc. Natl. Acad. Sci. USA 2005, 102, 2584–2589. [Google Scholar] [CrossRef] [Green Version]
- Herniou, E.A.; Olszewski, J.A.; Cory, J.S.; O’Reilly, D.R. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 2003, 48, 211–234. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Keesey, I.W.; Koerte, S.; Khallaf, M.A.; Retzke, T.; Guillou, A.; Grosse-Wilde, E.; Buchon, N.; Knaden, M.; Hansson, B.S. Pathogenic bacteria enhance dispersal through alteration of Drosophila social communication. Nat. Commun. 2017, 8, 625. [Google Scholar] [CrossRef] [Green Version]
- van Houte, S.; Ros, V.I.D.; Mastenbroek, T.G.; Vendrig, N.J.; Hoover, K.; Spitzen, J.; van Oers, M.M. Protein tyrosine phosphatase-induced hyperactivity is a conserved strategy of a subset of baculoviruses to manipulate lepidopteran host behavior. PLoS ONE 2012, 7, e46933. [Google Scholar] [CrossRef] [PubMed]
- Hoover, K.; Grove, M.; Gardner, M.; Hughes, D.P.; McNeil, J.; Slavicek, J. A gene for an extended phenotype. Science 2011, 333, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; van Houte, S.; Drees, G.F.; van Oers, M.M.; Ros, V.I.D. Parasitic manipulation of host behaviour: Baculovirus SeMNPV EGT facilitates tree-top disease in Spodoptera exigua larvae by extending the time to death. Insects 2015, 6, 716–731. [Google Scholar] [CrossRef] [Green Version]
- Biernat, M.A.; Eker, A.P.M.; van Oers, M.M.; Vlak, J.M.; van der Horst, G.T.J.; Chaves, I. A baculovirus photolyase with DNA repair activity and circadian clock regulatory function. J. Biol. Rhythms 2012, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.B.; Zhang, J.J.; Shen, Y.W.; Zheng, Q.; Feng, M.; Xiang, X.W.; Wu, X.F. Transcriptome analysis of the brain of the silkworm Bombyx mori infected with Bombyx mori nucleopolyhedrovirus: A new insight into the molecular mechanism of enhanced locomotor activity induced by viral infection. J. Invertebr. Pathol. 2015, 128, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, U.R.; Li, F.J.; Bhattarai, M.K.; Masoudi, A.; Wang, D. Phototransduction and circadian entrainment are the key pathways in the signaling mechanism for the baculovirus induced tree-top disease in the lepidopteran larvae. Sci. Rep. 2018, 8, 17528. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C.; Passarelli, A.L. Viral fibroblast growth factor, matrix metalloproteases, and caspases are associated with enhancing systemic infection by baculoviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 9825–9830. [Google Scholar] [CrossRef] [Green Version]
- Clem, R.J.; Fechheimer, M.; Miller, L.K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science 1991, 254, 1388–1390. [Google Scholar] [CrossRef]
- Yamada, H.; Kitaguchi, K.; Hamajima, R.; Kobayashi, M.; Ikeda, M. Novel apoptosis suppressor apsup from the baculovirus Lymantria dispar multiple nucleopolyhedrovirus precludes apoptosis by preventing proteolytic processing of initiator caspase dronc. J. Virol. 2013, 87, 12925–12934. [Google Scholar] [CrossRef] [Green Version]
- Byers, N.M.; Vandergaast, R.L.; Friesen, P.D. Baculovirus inhibitor-of-apoptosis Op-IAP3 blocks apoptosis by interaction with and stabilization of a host insect cellular IAP. J. Virol. 2016, 90, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Q.Y.; Fu, K.Y.; Guo, W.C.; Li, G.Q. RNA interference against the putative insulin receptor substrate gene chico affects metamorphosis in Leptinotarsa decemlineata. Insect Biochem. Mol. Biol. 2018, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Pareek, A.; Newar, K.; Dixit, N.M. Mutational pathway maps and founder effects define the within-host spectrum of hepatitis C virus mutants resistant to drugs. PLoS Pathog. 2019, 15, e1007701. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Castro, C.; Thaa, B.; Liu, L.F.; Mutso, M.; Liu, X.; Mahalingam, S.; Griffin, J.L.; Marsh, M.; McInerney, G.M. Alphavirus-induced hyperactivation of PI3K/AKT directs pro-viral metabolic changes. PLoS Pathog. 2018, 14, e1006835. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Timmons, L.; Fire, A. Specific interference by ingested dsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef]
- Tenllado, F.; Barajas, D.; Vargas, M.; Atencio, F.A.; Gonzalez-Jara, P.; Diaz-Ruiz, J.R. Transient expression of homologous hairpin RNA causes interference with plant virus infection and is overcome by a virus encoded suppressor of gene silencing. Mol. Plant-Microbe Interact. 2003, 16, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Liu, L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef]
- Tokuhira, N.; Kitagishi, Y.; Suzuki, M.; Minami, A.; Nakanishi, A.; Ono, Y.; Kobayashi, K.; Matsuda, S.; Ogura, Y. PI3K/AKT/PTEN pathway as a target for Crohn’s disease therapy. Int. J. Mol. Med. 2015, 35, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Waite, K.A.; Eng, C. Protean PTEN: Form and function. Am. J. Hum. Genet. 2002, 70, 829–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 127–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Cruise, E.S.; Dowling, R.J.O.; Stambolic, V. PTEN nuclear functions. Cold Spring Harb. Perspect. Med. 2020, 10, a036079. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Fine, B.; Steinbach, N.; Dendy, M.; Rapp, Z.; Shaw, J.; Pappas, K.; Yu, J.S.; Hodakoski, C.; Mense, S.; et al. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 2013, 341, 399–402. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, C.M.; Perl, A.; Leonard, D.; Sangodkar, J.; Narla, G. Therapeutic targeting of PP2A. Int. J. Biochem. Cell Biol. 2018, 96, 182–193. [Google Scholar] [CrossRef]
- Leslie, N.R.; Batty, I.H.; Maccario, H.; Davidson, L.; Downes, C.P. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene 2008, 27, 5464–5476. [Google Scholar] [CrossRef] [Green Version]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [Green Version]
- Katsuma, S.; Horie, S.; Daimon, T.; Iwanaga, M.; Shimada, T. In vivo and in vitro analyses of a Bombyx mori nucleopolyhedrovirus mutant lacking functional vfgf. Virology 2006, 355, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Detvisitsakun, C.; Cain, E.L.; Passarelli, A.L. The Autographa californica M nucleopolyhedrovirus fibroblast growth factor accelerates host mortality. Virology 2007, 365, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Courtney, R.J.; Steiner, S.M.; Benyesh-Melnick, M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology 1973, 52, 447–455. [Google Scholar] [CrossRef]
- Landini, M.P. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 1984, 65, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006, 2, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greseth, M.D.; Traktman, P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014, 10, e1004021. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, K.A.; Camarda, R.; Lagunoff, M. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014, 88, 4366–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogev, O.; Lagos, D.; Enver, T.; Boshoff, C. Kaposi’s sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathog. 2014, 10, e1004400. [Google Scholar] [CrossRef] [Green Version]
- Marat, A.L.; Haucke, V. Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 2016, 35, 561–579. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Smith, W.A.; Lamattina, A.; Collins, M. Insulin signaling pathways in lepidopteran ecdysone secretion. Front. Physiol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xia, X.; Cui, A.; Xiong, Z.; Yan, Y.; Luo, J.; Chen, G.; Zeng, Y.; Cai, D.; Hou, L.; et al. The precursor of PI(3,4,5)P3 alleviates aging by activating daf-18(Pten) and independent of daf-16. Nat. Commun. 2020, 11, 4496. [Google Scholar] [CrossRef] [PubMed]

| Group Name | First Feed | Second Feed |
|---|---|---|
| CK | Isopyknic dd-H2O | Buffer (dsRNA) 1 |
| dsRNA-L4440 | Isopyknic dd-H2O | dsRNA-L4440 |
| dsRNA-PTEN | Isopyknic dd-H2O | dsRNA-PTEN |
| NPV | 106 OBs/larva of LdMNPV | Buffer (dsRNA) |
| NPV + dsRNA-L4440 | 106 OBs/larva of LdMNPV | dsRNA-L4440 |
| NPV+ dsRNA-PTEN | 106 OBs/larva of LdMNPV | dsRNA-PTEN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, L.; Yu, X.; Rensing, C.; Wang, D. The PI3K/AKT Pathway and PTEN Gene Are Involved in “Tree-Top Disease” of Lymantria dispar. Genes 2022, 13, 247. https://doi.org/10.3390/genes13020247
Li F, Liu L, Yu X, Rensing C, Wang D. The PI3K/AKT Pathway and PTEN Gene Are Involved in “Tree-Top Disease” of Lymantria dispar. Genes. 2022; 13(2):247. https://doi.org/10.3390/genes13020247
Chicago/Turabian StyleLi, Fengjiao, Long Liu, Xiao Yu, Christopher Rensing, and Dun Wang. 2022. "The PI3K/AKT Pathway and PTEN Gene Are Involved in “Tree-Top Disease” of Lymantria dispar" Genes 13, no. 2: 247. https://doi.org/10.3390/genes13020247
APA StyleLi, F., Liu, L., Yu, X., Rensing, C., & Wang, D. (2022). The PI3K/AKT Pathway and PTEN Gene Are Involved in “Tree-Top Disease” of Lymantria dispar. Genes, 13(2), 247. https://doi.org/10.3390/genes13020247








