Circular RNA circYPEL2: A Novel Biomarker in Cervical Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Treatment and Cell Culture
2.2. Processing of Sequencing Data
2.3. Total RNA and Genomic DNA Isolation
2.4. RT-PCR and qPCR
2.5. RNase R and Actinomycin D Assay
2.6. Fractionation of Nuclear and Cytoplasma
2.7. Plasmid Construction and Cell Transfection
2.8. Colony Formation Assay
2.9. CCK-8 Assay
2.10. Cell Migration and Invasion Assay
2.11. Statistical Analysis
3. Results
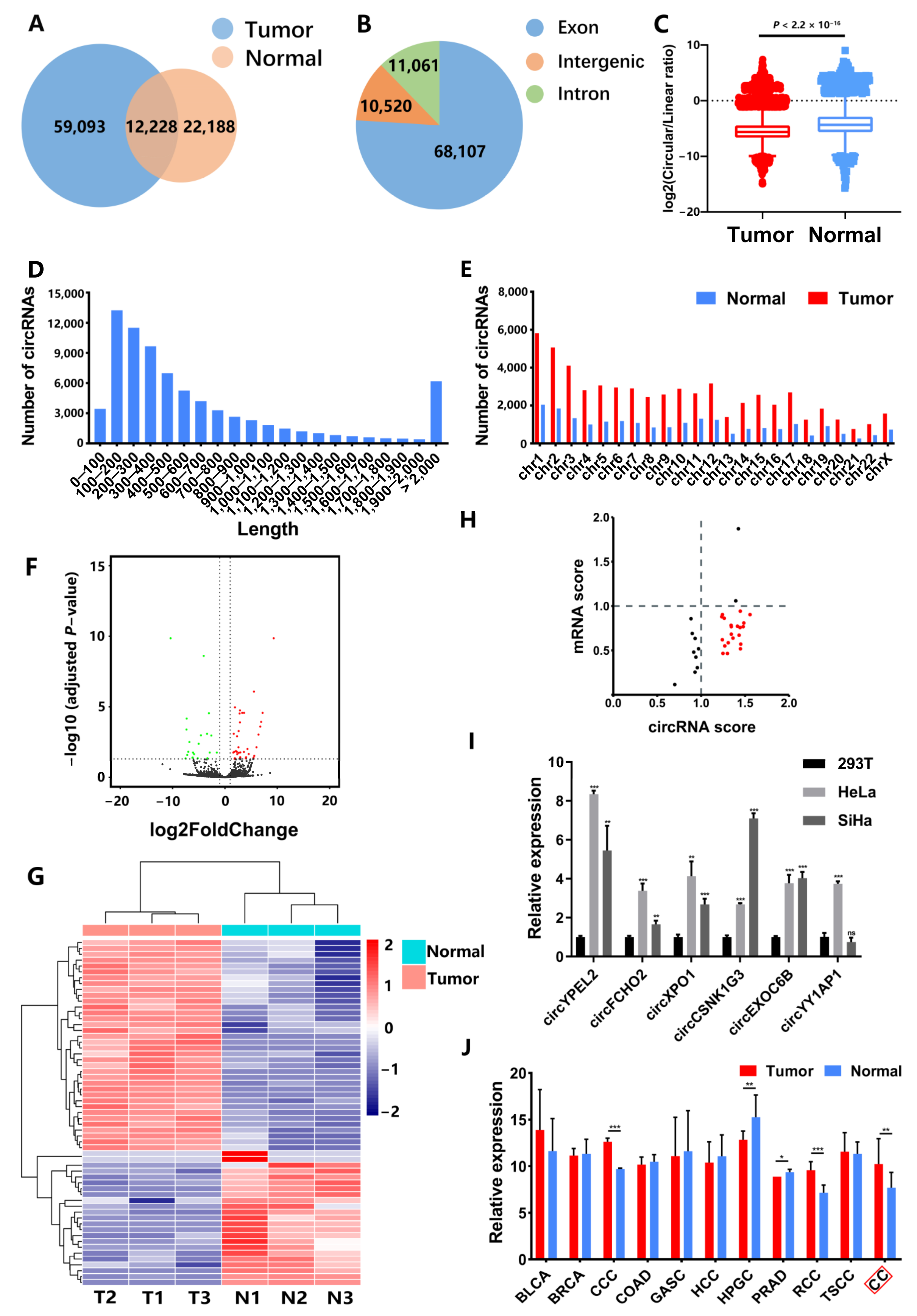
3.1. Identification of CC-Associated circRNAs
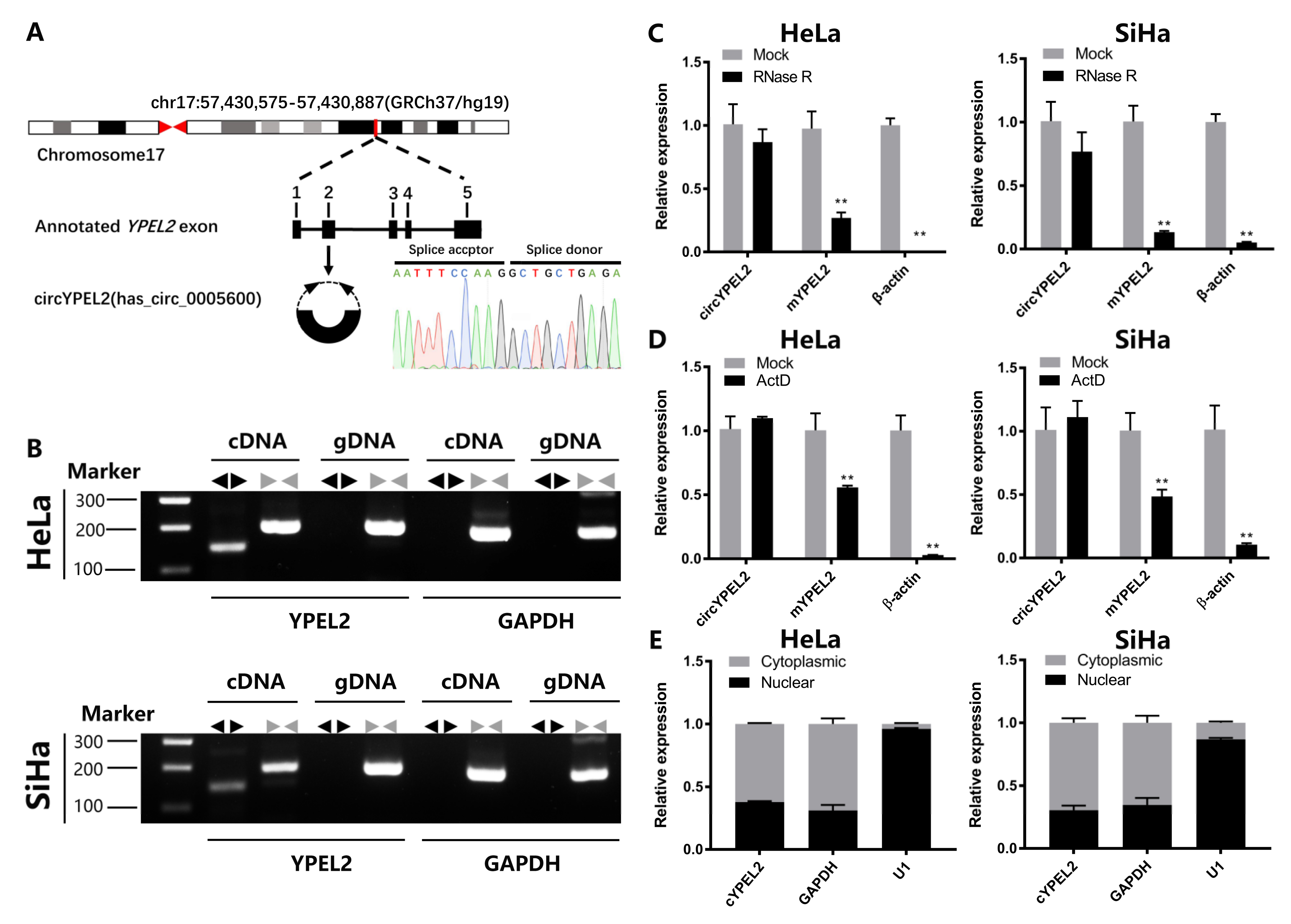
3.2. CircRNA Exerts Strong Stability in CC Cell Lines
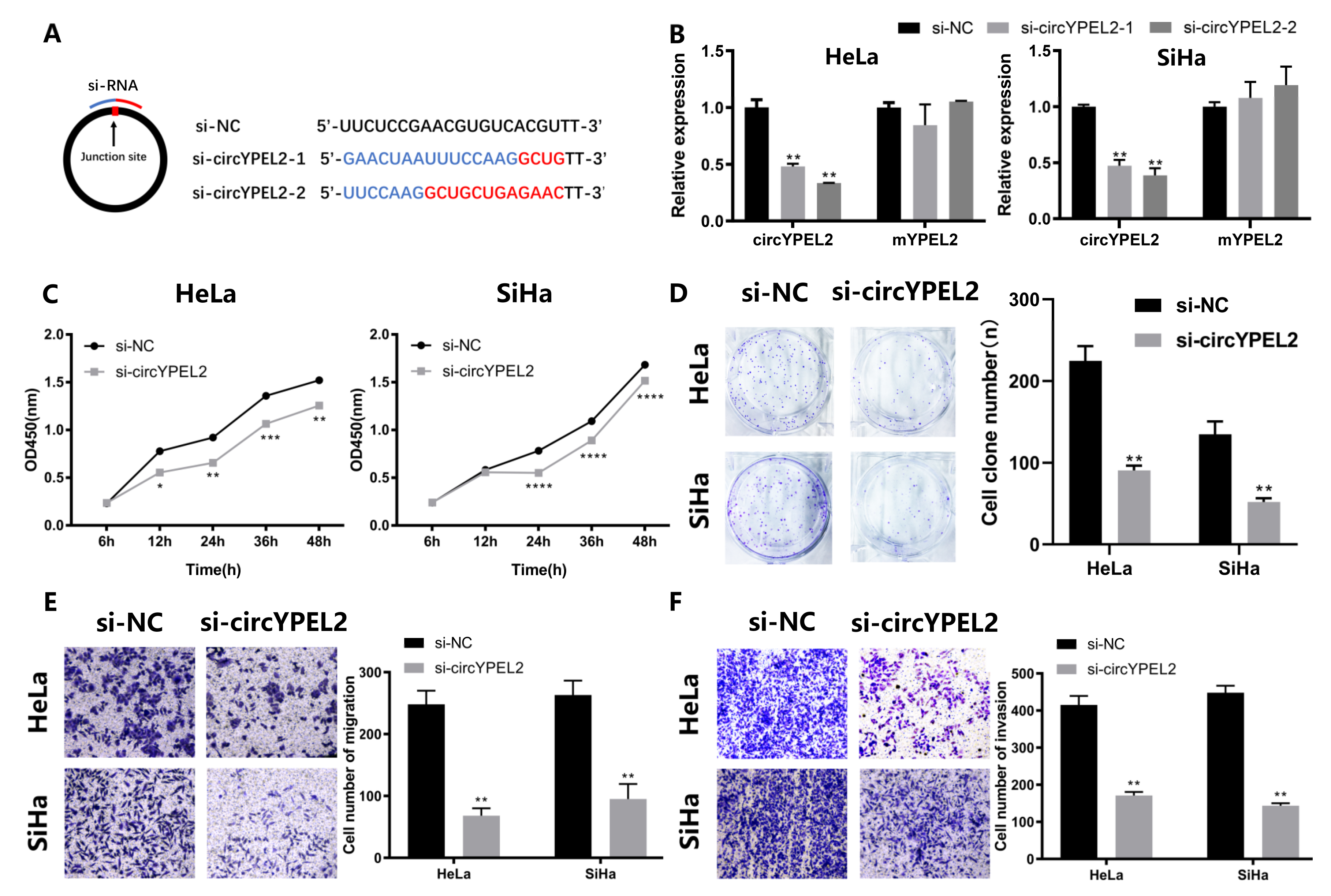
3.3. Knockdown of circYPEL2 Attenuates the Proliferation, Migration and Invasion of CC
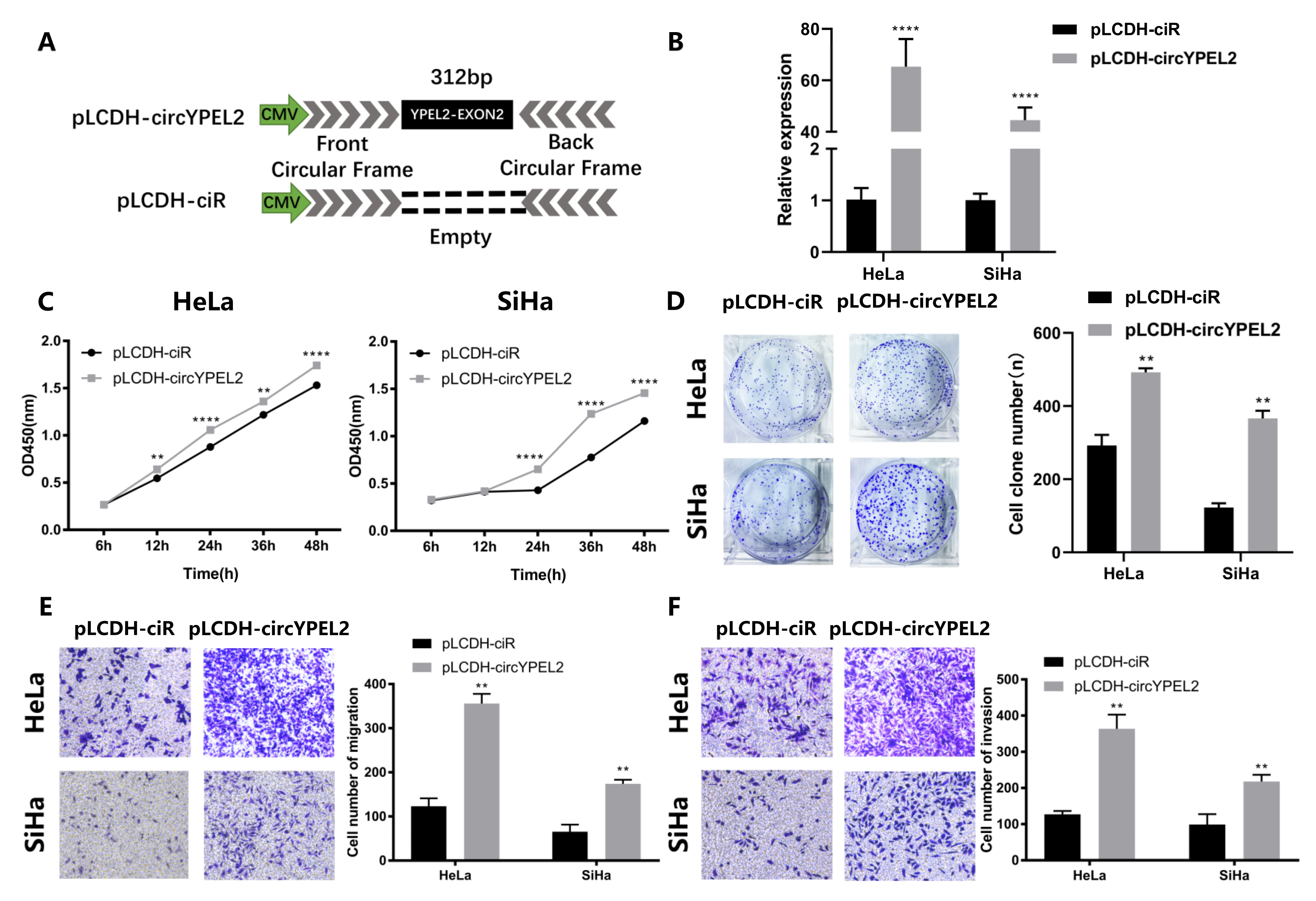
3.4. Overexpression of circYPEL2 Promotes the Proliferation, Migration and Invasion of CC
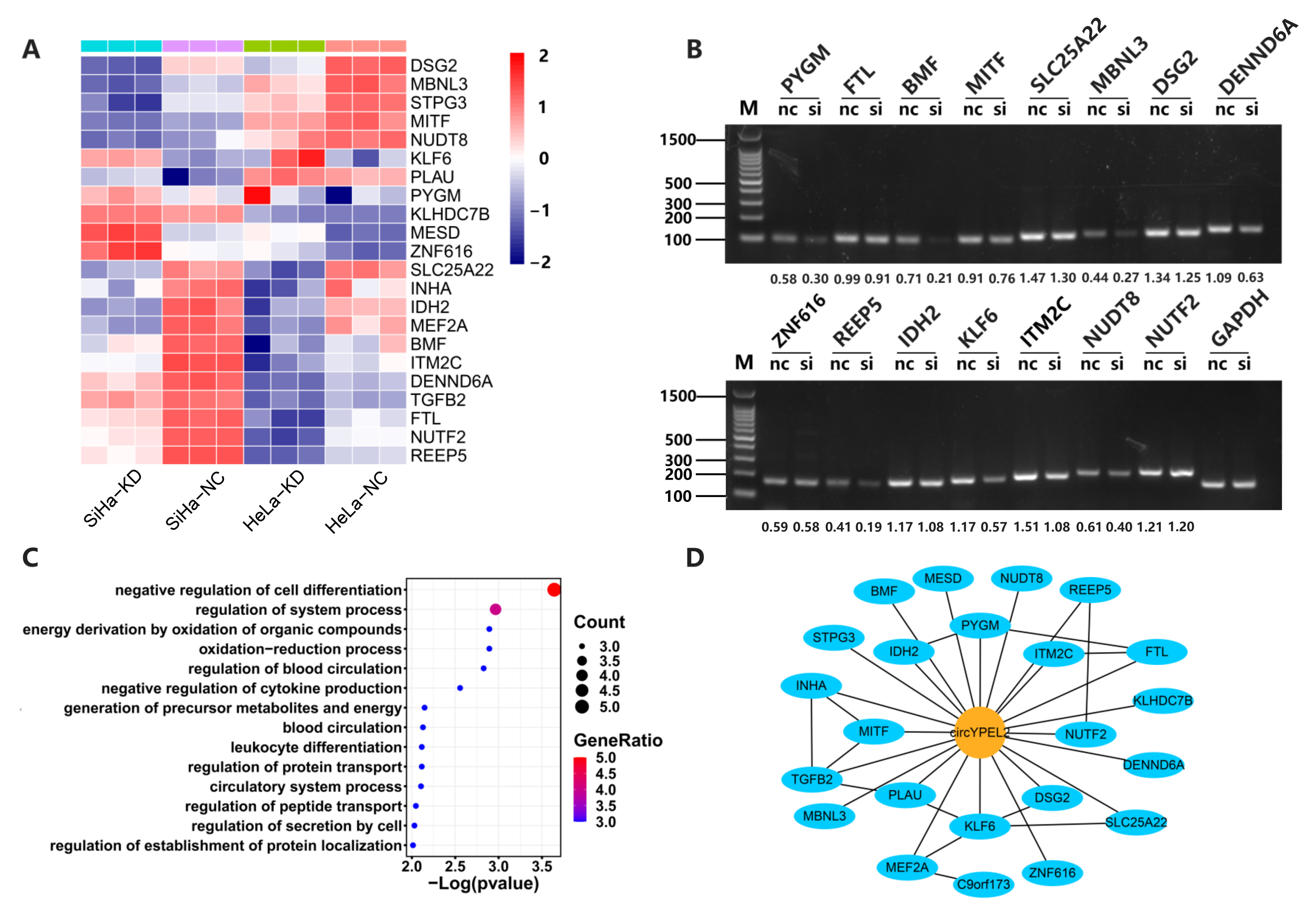
3.5. CircYPEL2 May Regulate CC via Downstream Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Munoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Rossi, P.G.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical cancer: Screening, diagnosis and staging. J. Buon 2016, 21, 320–325. [Google Scholar]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef] [Green Version]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.-M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.M.; Wang, W.-T.; Zeng, Z.-C.; Chen, T.-Q.; Han, C.; Pan, Q.; Huang, W.; Fang, K.; Sun, L.-Y.; Zhou, Y.-F.; et al. circMYBL2, a circRNA from MYBL2, regulates FLT3 translation by recruiting PTBP1 to promote FLT3-ITD AML progression. Blood 2019, 134, 1533–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, J.; Tian, Y.; Gao, Y.; Dong, X.; Chen, W.; Yuan, X.; Yin, W.; Xu, J.; Chen, K.; et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 2020, 19, 128. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e9. [Google Scholar] [CrossRef] [Green Version]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017, 24, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, J.; Wang, H.; Su, X.; Hou, J.; Gu, Y.; Qian, C.; Lin, Y.; Liu, X.; Huang, M.; et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 2017, 66, 1151–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.F.; Huang, C.; Zou, Y.; Ye, J.; Yu, J.; Gui, Y. CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol. Cancer 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, T.; Yang, R.; Zhao, X.; Guo, H. Circular RNA Circslc8a1 Acts as a Sponge of Mir-130b/Mir-494 in Suppressing Bladder Cancer Progression Via Regulating Pten. J. Urol. 2020, 203, E3. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.K.; Wang, M.-R.; Liu, C.-X.; Dong, R.; Carmichael, G.G.; Chen, L.-L.; Yang, L. CIRCexplorer3: A CLEAR Pipeline for Direct Comparison of Circular and Linear RNA Expression. Genom. Proteom. Bioinform. 2019, 17, 511–521. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.C.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics-a J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol. Rep. 2010, 23, 605–614. [Google Scholar]
- Chen, H.F.; Gu, B.; Zhao, X.; Zhao, Y.; Huo, S.; Liu, X.; Lu, H. Circular RNA hsa_circ_0007364 increases cervical cancer progression through activating methionine adenosyltransferase II α (MAT2A) expression by restraining microRNA-101-5p. Bioengineered 2020, 11, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, X.; Zhou, J.; Sheng, M.; Yin, X.; Ko, E.-A.; Zhou, T.; Gu, W. Quantifying circular RNA expression from RNA-seq data using model-based framework. Bioinformatics 2017, 33, 2131–2139. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wu, Y.; Ge, F.; Chen, Y.; Xiong, Q. The circular RNA CDR1as regulate cell proliferation via TMED2 and TMED10. BMC Cancer 2020, 20, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanaja, G.R.; Ramulu, H.G.; Kalle, A.M. Overexpressed HDAC8 in cervical cancer cells shows functional redundancy of tubulin deacetylation with HDAC6. Cell Commun. Signal 2018, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Jinawath, N.; Shiao, M.-S.; Chanpanitkitchote, P.; Svasti, J.; Furukawa, Y.; Nakamura, Y. Enhancement of Migration and Invasion of Gastric Cancer Cells by IQGAP3. Biomolecules 2020, 10, 1194. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. TET2 inhibits tumorigenesis of breast cancer cells by regulating caspase-4. Sci. Rep. 2018, 8, 16167. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Feng, J.; Chen, K.; Ma, Y.; Gong, J.; Cai, F.; Jin, Y.; Gao, Y.; Xia, L.; Chang, H.; et al. CSCD: A database for cancer-specific circular RNAs. Nucleic Acids Res. 2018, 46, D925–D929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [Green Version]
- Rodic, S.; Vincent, M.D. Reactive oxygen species (ROS) are a key determinant of cancer’s metabolic phenotype. Int. J. Cancer 2018, 142, 440–448. [Google Scholar]
- Cocquerelle, C.; Mascrez, B.; Hétuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Wu, J.; Qi, X.; Liu, L.; Hu, X.; Liu, J.; Yang, J.; Yang, J.; Lu, L.; Zhang, Z.; Ma, S.; et al. Emerging Epigenetic Regulation of Circular RNAs in Human Cancer. Mol. Ther. Nucleic Acids 2019, 16, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Sui, W.G.; Gan, Q.; Chang, Y.; Ou, M.; Chen, J.; Lin, H.; Xue, W.; Wu, Y.; He, H.; Tang, D.; et al. Differential expression profile study and gene function analysis of maternal foetal-derived circRNA for screening for Down’s syndrome. Exp. Ther. Med. 2020, 19, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Huang, Y.; Chen, L.; Wang, J. miR-221 regulates proliferation and apoptosis of ovarian cancer cells by targeting BMF. Oncol. Lett. 2018, 16, 6697–6704. [Google Scholar]
- Zhao, Y.; Qin, X.-P.; Lang, Y.-P.; Kou, D.; Shao, Z.-W. Circular RNA circ-SMAD7 promoted ovarian cancer cell proliferation and metastasis by suppressing KLF6. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7563. [Google Scholar] [PubMed]
- Han, X.T.; Jiang, J.-Q.; Li, M.-Z.; Cong, Q.-M. Circular RNA circ-ABCB10 promotes the proliferation and invasion of thyroid cancer by targeting KLF6. Eur Rev. Med. Pharmacol. Sci. 2020, 24, 1271–1277. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, S.; Chen, W.; Dong, X.; Zhang, R.; Ye, H.; Mei, X.; Liu, H.; Fang, Y.; He, C. Circular RNA circYPEL2: A Novel Biomarker in Cervical Cancer. Genes 2022, 13, 38. https://doi.org/10.3390/genes13010038
Zhang X, Yang S, Chen W, Dong X, Zhang R, Ye H, Mei X, Liu H, Fang Y, He C. Circular RNA circYPEL2: A Novel Biomarker in Cervical Cancer. Genes. 2022; 13(1):38. https://doi.org/10.3390/genes13010038
Chicago/Turabian StyleZhang, Xinyang, Siqi Yang, Wenbo Chen, Xin Dong, Rongyu Zhang, Haidong Ye, Xiangfei Mei, Huan Liu, Yu Fang, and Chunjiang He. 2022. "Circular RNA circYPEL2: A Novel Biomarker in Cervical Cancer" Genes 13, no. 1: 38. https://doi.org/10.3390/genes13010038
APA StyleZhang, X., Yang, S., Chen, W., Dong, X., Zhang, R., Ye, H., Mei, X., Liu, H., Fang, Y., & He, C. (2022). Circular RNA circYPEL2: A Novel Biomarker in Cervical Cancer. Genes, 13(1), 38. https://doi.org/10.3390/genes13010038





