Multi-Dimensional Scaling Analysis of Key Regulatory Genes in Prostate Cancer Using the TCGA Database
Abstract
1. Introduction
2. Materials and Methods
2.1. TCGA Database
2.2. Statistical Analysis
3. Results
3.1. TCGA Database
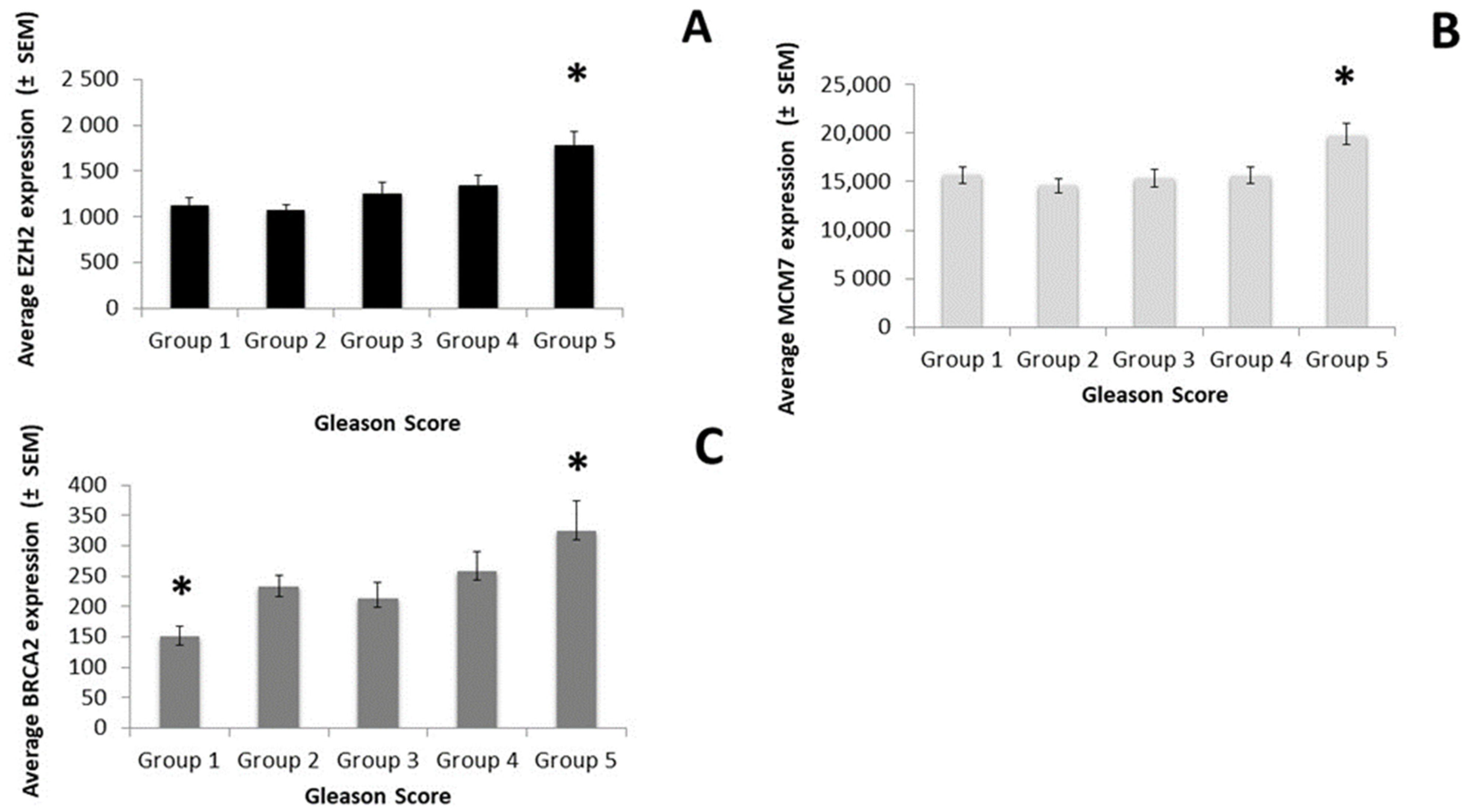
3.2. Target Genes
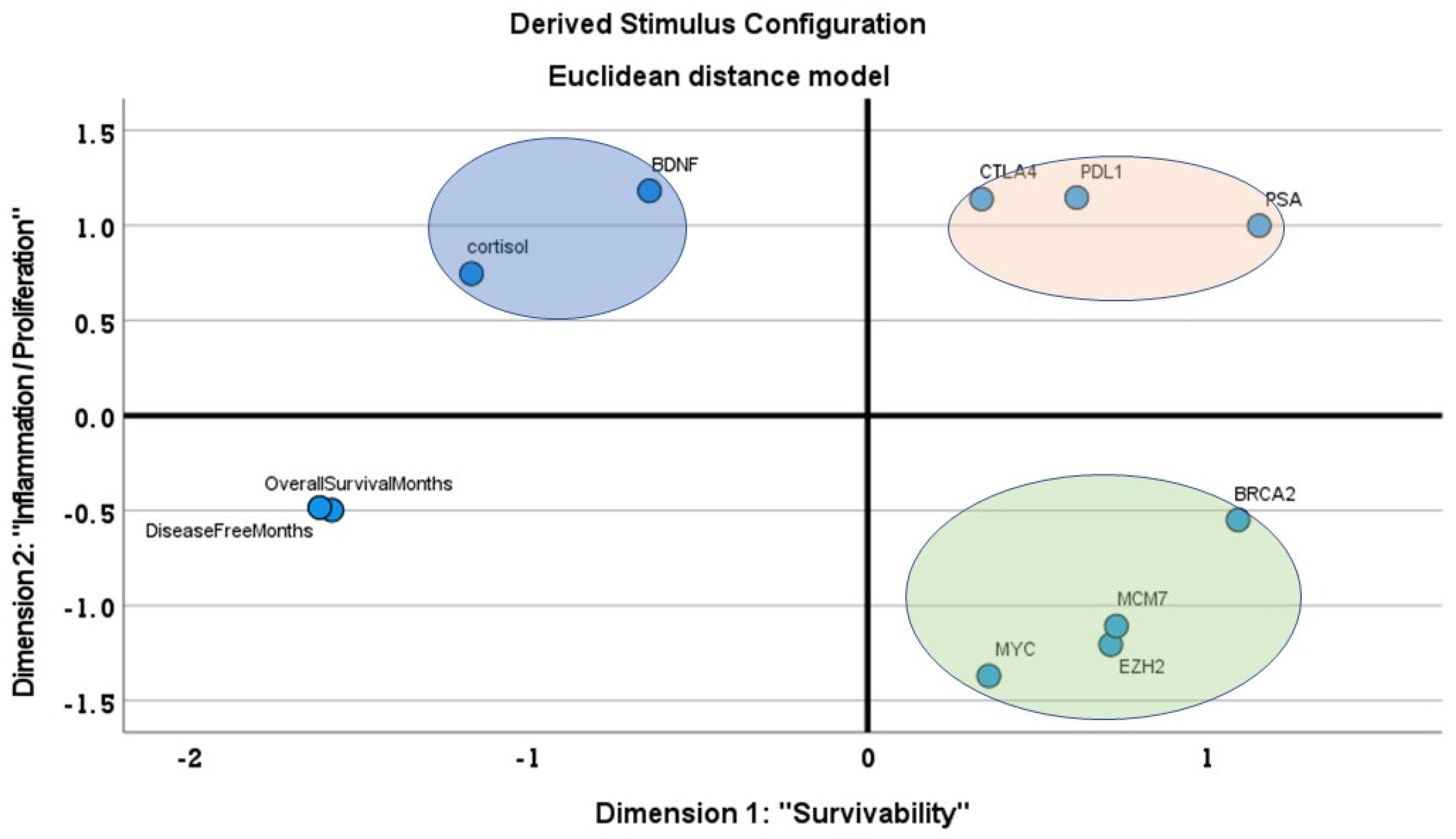
3.3. MDS Analysis
3.4. Stepwise Multiple Regression
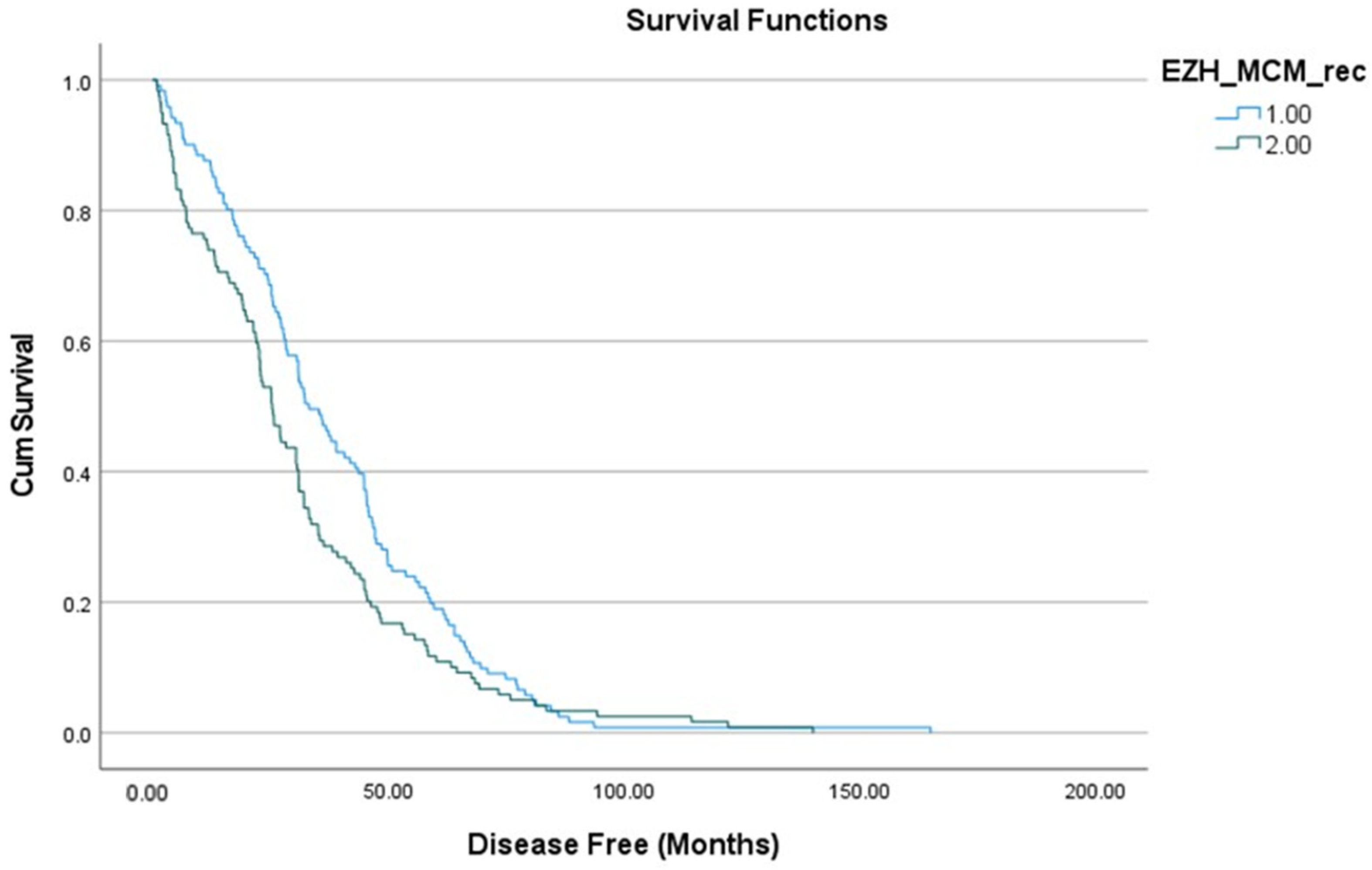
3.5. Survival Curve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Babb, P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: International comparisons. BJU 2002, 90, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2021; Available online: https://gco.iarc.fr/today (accessed on 14 January 2021).
- Draisma, G.; Etzioni, R.; Tsodikov, A.; Mariotto, A.; Wever, E.; Gulati, R.; Feuer, E.; De Koning, H. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J. Natl. Cancer Inst. 2009, 101, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. US Preventive Services Task Force. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Fondurulia, J.; Chen, M.-H.; Kaplan, I.; Beard, C.J.; Tomaszewski, J.E.; Renshaw, A.A.; Wein, A.; et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J. Clin. Oncol. 1999, 17, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Ankerst, D.; Chi, C.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Feng, Z.; Parnes, H.L.; Coltman, C.A. Assessing prostate cancer risk: Results from the prostate cancer prevention trial. J. Natl. Cancer Inst. 2006, 98, 529–534. [Google Scholar] [CrossRef]
- Egevad, L.; Granfors, T.; Karlberg, L.; Bergh, A.; Stattin, P. Prognostic value of the Gleason score in prostate cancer. BJU Int. 2002, 89, 538–542. [Google Scholar] [CrossRef]
- Rusthoven, C.G.; Carlson, J.A.; Waxweiler, T.V.; Yeh, N.; Raben, D.; Flaig, T.W.; Kavanagh, B.D. The prognostic significance of Gleason scores in metastatic prostate cancer. Urol. Oncol. 2014, 32, 707–713. [Google Scholar] [CrossRef]
- Brimo, F.; Montironi, R.; Egevad, L.; Erbersdobler, A.; Lin, D.W.; Nelson, J.B.; Rubin, M.A.; Van Der Kwast, T.; Amin, M.; Epstein, J.I.; et al. Contemporary grading for prostate cancer: Implications for patient care. Eur. Urol. 2013, 63, 892–901. [Google Scholar] [CrossRef]
- Sehn, J.K. Prostate Cancer Pathology: Recent Updates and Controversies. Mol. Med. 2018, 115, 151–155. [Google Scholar]
- McKenney, J.K.; Wei, W.; Hawley, S.; Auman, H.; Newcomb, L.F.; Boyer, H.D.; Fazli, L.; Simko, J.; Hurtado-Coll, A.; Troyer, D.A.; et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am. J. Surg. Pathol. 2016, 40, 1439–1456. [Google Scholar] [CrossRef]
- Zhou, Z.; Cheng, Y.; Jiang, Y.; Liu, S.; Zhang, M.; Liu, J.; Zhao, Q. Ten hub genes associated with progression and prognosis of pancreatic carcinoma identified by co-expression analysis. Int. J. Biol. Sci. 2018, 14, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Abate-Shen, C.; Shen, M.M. Molecular genetics of prostate cancer. Genes Dev. 2000, 14, 2410–2434. [Google Scholar] [CrossRef] [PubMed]
- Ross-Adams, H.; Lamb, A.D.; Dunning, M.J.; Halim, S.; Lindberg, J.; Massie, C.M.; Egevad, L.A.; Russell, R.; Ramos-Montoya, A.; Vowler, S.L.; et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EbioMedicine 2015, 2, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Seibert, T.M.; Fan, C.C.; Wang, Y.; Zuber, V.; Karunamuni, R.; Parsons, J.K.; Eeles, R.A.; Easton, D.F.; Kote-Jarai, Z.; Al Olama, A.A.; et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ 2018, 360, j5757. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z. A Gleason score-related outcome model for human prostate cancer: A comprehensive study based on weighted gene co-expression network analysis. Cancer Cell Int. 2020, 20, 159. [Google Scholar] [CrossRef]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Luca, B.-A.; Moulton, V.; Ellis, C.; Edwards, D.R.; Campbell, C.; Cooper, R.A.; Clark, J.; Brewer, D.S.; Cooper, C.S. A novel stratification framework for predicting outcome in patients with prostate cancer. Br. J. Cancer 2020, 122, 1467–1476. [Google Scholar] [CrossRef]
- Moghadas-Dastjerdi, H.; Sha-E-Tallat, H.R.; Sannachi, L.; Sadeghi-Naini, A.; Czarnota, G.J. A priori prediction of tumour response to neoadjuvant chemotherapy in breast cancer patients using quantitative CT and machine learning. Sci. Rep. 2020, 10, 10936. [Google Scholar] [CrossRef]
- Yang, E.V.; Glaser, R. Stress-induced immunomodulation: Implications for tumorigenesis. Brain Behav. Immun. 2003, 17, 37–40. [Google Scholar] [CrossRef]
- Bloch, S.; Love, A.; Macvean, M.; Duchesne, G.; Couper, J.; Kissane, D. Psychological adjustment of men with prostate cancer: A review of the literature. Biopsychosoc. Med. 2007, 1, 2. [Google Scholar] [CrossRef]
- Gidron, Y.; Fabre, B.; Grosman, H.; Nolazco, C.; Mesch, V.; Mazza, O.; Berg, G. Life events, cortisol and levels of prostate specific antigen: A story of synergism. Psychoneuroendocrinology 2011, 36, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B.; Grosman, H.; Gonzalez, D.; Machulsky, N.F.; Repetto, E.M.; Mesch, V.; Lopez, M.A.; Mazza, O.; Berg, G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac. J. Cancer Prev. 2016, 17, 3167–3171. [Google Scholar] [PubMed]
- Li, T.; Yu, Y.; Song, Y.; Li, X.; Lan, D.; Zhang, P.; Xiao, Y.; Xing, Y. Activation of BDNF/TrkB pathway promotes prostate cancer progression via induction of epithelial-mesenchymal transition and anoikis resistance. FASEB J. 2020, 34, 9087–9101. [Google Scholar] [CrossRef]
- Barron, D.A.; Rowley, D.R. The reactive stroma microenvironment and prostate cancer progression. Endocr. Relat. Cancer 2021, 19, R187–R204. [Google Scholar] [CrossRef] [PubMed]
- Perletti, G.; Monti, E.; Magri, V.; Cai, T.; Cleves, A.; Trinchieri, A.; Montanari, E. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch. Ital. Urol. Androl. 2017, 89, 259–265. [Google Scholar] [CrossRef]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Johansen, T.E.B.; Nesi, G. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. 2019, 20, 3833. [Google Scholar] [CrossRef] [PubMed]
- Karakiewicz, P.I.; Benayoun, S.; Begin, L.R.; Duclos, A.; Valiquette, L.; McCormack, M.; Bénard, F.; Saad, F.; Perrotte, P. Chronic inflammation is negatively associated with prostate cancer and high-grade prostatic intraepithelial neoplasia on needle biopsy. Int. J. Clin. Pract. 2007, 61, 425–430. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The tumor immune contexture of prostate cancer. Front. Immunol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Varambally, S.; Dhanasekaran, S.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P.; et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Sandra, M.G.; Otte, A.P.; Chinnaiyan, A.M.; Rubin, M.A. Multiplex biomarker approach for determining risk of prostate-specific-defined recurrence of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 661–668. [Google Scholar] [CrossRef]
- Nowinska, K.; Chmielewska, M.; Piotrowska, A.; Pula, B.; Pastuszewski, W.; Krecicki, T.; Podhorska-Okołow, M.; Zabel, M.; Dziegiel, P. Correlation between levels of expression of minichromosome maintenance proteins, Ki-67 proliferation antigen and metallothionein I/II in laryngeal squamous cell cancer. Int. J. Oncol. 2016, 48, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, X.; Guan, X.; Zhang, H.; Ma, Y.; Zhang, S.; Wang, E.; Zhang, L.; Han, Y. Overexpression of G9a and MCM7 in oesophageal squamous cell carcinoma is associated with poor prognosis. Histopathology 2015, 66, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-M.; Zhang, X.-F.; Cao, L.; Li, B.; Sui, C.-J.; Li, Y.-M.; Yin, Z. MCM7 expression predicts post-operative prognosis for hepatocellular carcinoma. Liver Int. 2012, 32, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Yu, G.; Tseng, G.C.; Cieply, K.; Gavel, T.; Nelson, J.B.; Michalopoulos, G.K.; Yu, Y.P.; Luo, J.-H. MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene 2006, 25, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, S.; Martikainen, P.M.; Tolonen, T.; Isola, J.; Tammela, T.L.; Visakorpi, T. EZH2, Ki-67 and MCM7 are prognostic markers in prostatectomy treated patients. Int. J. Cancer 2008, 122, 595–602. [Google Scholar] [CrossRef]
- Wu, Z.; Lee, S.T.; Qiao, Y.; Li, Z.; Lee, P.L.; Lee, Y.J.; Jiang, X.; Tan, J.; Aau, M.; Lim, C.Z.H.; et al. Polycomb protein EZH2 regulates cancer cell fate decision in response to DNA damage. Cell Death Differ. 2011, 18, 1771–1779. [Google Scholar] [CrossRef]
- Campbell, S.; Ismail, I.H.; Young, L.C.; Poirier, G.G.; Hendzel, M.J. Polycomb repressive complex 2 contributes to DNA double-strand break repair. Cell Cycle 2013, 12, 2675–2683. [Google Scholar] [CrossRef]
- Rondinelli, B.; Gogola, E.; Yücel, H.; Duarte, A.A.; van de Ven, M.; van der Sluijs, R.; Konstantinopoulos, P.A.; Jonkers, J.; Ceccaldi, R.; Rottenberg, S.; et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 2017, 19, 1371–1378. [Google Scholar] [CrossRef]
- Gurel, B.; Iwata, T.; Koh, C.M.; Jenkins, R.B.; Lan, F.; Dang, C.; Hicks, J.L.; Morgan, J.; Cornish, T.; Sutcliffe, S.; et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol. 2008, 21, 1156–1167. [Google Scholar] [CrossRef]
- Koh, C.M.; Bieberich, C.; Dang, C.; Nelson, W.G.; Yegnasubramanian, S.; De Marzo, A.M. MYC and prostate cancer. Genes Cancer 2012, 1, 617–628. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Favero, F.; Risch, T.; Simon, R.; Feuerbach, L.; Assenov, Y.; Heckmann, D.; Sidiropoulos, N.; Waszak, S.M.; Hübschmann, D.; et al. Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell 2018, 34, 996.e8–1011.e8. [Google Scholar] [CrossRef]
- Long, G.; Ouyang, W.; Zhang, Y.; Sun, G.; Gan, J.; Hu, Z.; Li, H. Identification of a DNA Repair Gene Signature and Establishment of a Prognostic Nomogram Predicting Biochemical-Recurrence-Free Survival of Prostate Cancer. Front. Mol. Biosci. 2021, 8, 608369. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, E. Progression-Free Survival: Patient Benefit or Lower Standard; Life Raft Group: Wayne, NJ, USA, 2007; Available online: https://liferaftgroup.org/2008/08/progression-free-survival-patient-benefit-or-lower-standard/ (accessed on 23 March 2009).
- Chakravarty, A.; Sridhara, R. Use of progression-free survival as a surrogate marker in oncology trials: Some regulatory issues. Stat. Methods Med. Res. 2008, 17, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Panageas, K.S.; Ben-Porat, L.; Dickler, M.N.; Chapman, P.B.; Schrag, D. When you look matters: The effect of assessment schedule on progression-free survival. J. Natl. Cancer Inst. 2007, 99, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, C.; Hu, Z.; Yang, C.; Zhang, R.; Ding, Y.; Wang, Z.; Tao, S.; Qin, Y. Differences in survival of prostate cancer Gleason 8–10 disease and the establishment of a new Gleason survival grading system. Cancer Med. 2021, 10, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Nelson, P.S. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Ribeiro, F.R.; Jerónimo, C.; Henrique, R.; Fonseca, D.; Oliveira, J.; Lothe, R.A.; Teixeira, M.R. 8q gain is an independent predictor of poor survival in diagnostic needle biopsies from prostate cancer suspects. Clin. Cancer Res. 2006, 12, 3961–3970. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; Macdonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell. 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Neves Filho, E.H.; Hirth, C.G.; Frederico, I.A.; Burbano, R.M.; Carneiro, T.; Rabenhorst, S.H. EZH2 expression is dependent on MYC and TP53 regulation in diffuse large B-cell lymphoma. APMIS 2020, 128, 308–315. [Google Scholar] [CrossRef]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochem. Biophys. Acta 2015, 1849, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, G.; Masuda, K.; Daigo, Y.; Cho, H.-S.; Yoshimatsu, M.; Takawa, M.; Hayami, S.; Maejima, K.; Chino, M.; I Field, H.; et al. Minichromosome Maintenance Protein 7 is a potential therapeutic target in human cancer and a novel prognostic marker of non-small cell lung cancer. Mol. Cancer 2011, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Ranza, E.; Grecchi, I.; Benericetti, E.; Ceroni, M.; Morbini, P.; Nano, R. Immunohistochemical evaluation of minichromosome maintenance protein 7 in astrocytoma grading. Anticancer Res. 2006, 26, 3513–3516. [Google Scholar] [PubMed]
- Marnerides, A.; Vassilakopoulos, T.P.; Boltetsou, E.; Levidou, G.; Angelopoulou, M.K.; Thymara, I.; Kyrtsonis, M.-C.; Pappi, V.; Tsopra, O.; Panayiotidis, P.; et al. Immunohistochemical expression and prognostic significance of CCND3, MCM2 and MCM7 in Hodgkin lymhoma. Anticancer Res. 2011, 31, 3585–3594. [Google Scholar] [PubMed]
- Shomori, K.; Nishihara, K.; Fujioka, S.; Tokuyasu, N.; Inaba, A.; Osaki, M.; Ogawa, T. Minichromosome maintenance protein 7 in colorectal cancer: Implication of prognostic significance. Int. J. Oncol. 2008, 33, 245–251. [Google Scholar] [CrossRef][Green Version]
- Ota, T.; Clayton, A.C.; Minot, D.M.; Shridhar, V.; Hartmann, L.C.; Gilks, C.B.; Chien, J.R. Minichromosome maintenance protein 7 as a potential prognostic factor for progression-free survival in high-grade serous carcinomas of the ovary. Mod. Pathol. 2011, 24, 277–287. [Google Scholar] [CrossRef]
- Qu, K.; Wang, Z.; Fan, H.; Li, J.; Liu, J.; Li, P.; Liang, Z.; An, H.; Jiang, Y.; Lin, Q.; et al. MCM7 promotes cancer progression through cyclin D1-dependent signaling and serves as a prognostic marker for patients with hepatocellular carcinoma. Cell Death Dis. 2017, 8, e2603, reprinted in Cell Death Dis. 2018, 9, 681. [Google Scholar] [CrossRef]
- Welcsh, P.L.; King, M.C. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001, 10, 705–713. [Google Scholar] [CrossRef]
- Ma, J.; Setton, J.; Lee, N.Y.; Riaz, N.; Powell, S.N. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat. Commun. 2018, 9, 3292. [Google Scholar] [CrossRef]
- Demple, B. Special problems for Base Excision Repair in coping with oxidatively-induced DNA damage. In DNA Damage, DNA Repair and Disease; The Royal Society of Chemistry: London, UK, 2020; pp. 204–219. [Google Scholar]
- Wang, Z.; Zhang, J.; Zhang, Y.; Deng, Q.; Liang, H. Expression and mutations of BRCA in breast cancer and ovarian cancer: Evidence from bioinformatics analyses. Int. J. Mol. Med. 2018, 42, 3542–3550. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Rubin, M.A. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2013, 63, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; De Bono, J.S. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Ann. Oncol. 2013, 24, 1416–1418. [Google Scholar]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Rodrigues, D.N.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef]
- Satih, S.; Savinel, H.; Rabiau, N.; Fontana, L.; Bignon, Y.J.; Bernard-Gallon, D.J. Expression analyses of nuclear receptor genes in breast cancer cell lines exposed to soy phytoestrogens after BRCA2 knockdown by TaqMan Low-Density Array (TLDA). J. Mol. Signal. 2009, 4, 3. [Google Scholar] [CrossRef]
- Blando, J.; Moore, T.; Hursting, S.; Jiang, G.; Saha, A.; Beltran, L.; Shen, J.; Repass, J.; Strom, S.; DiGiovanni, J. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev. Res. 2011, 4, 2002–2014. [Google Scholar] [CrossRef]
- Adjakly, M.; Ngollo, M.; Dagdemir, A.; Judes, G.; Pajon, A.; Karsli-Ceppioglu, S.; Penault-Llorca, F.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; et al. Prostate cancer: The main risk and protective factors-Epigenetic modifications. Ann. Endocrinol. 2015, 76, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef]
- Flores, I.E.; Sierra-Fonseca, J.A.; Davalos, O.; Saenz, L.A.; Castellanos, M.M.; Zavala, J.K.; Gosselink, K.L. Stress alters the expression of cancer-related genes in the prostate. BMC Cancer 2017, 17, 621. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Smith, M.; Lutgendorf, S.K.; Sood, A.K. Impact of stress on cancer metastasis. Future Oncol. 2010, 6, 1863–1881. [Google Scholar] [CrossRef]
- Yuan, A.; Wang, S.; Li, Z.; Huang, C. Psychological aspect of cancer: From stressor to cancer progression. Exp. Ther. Med. 2010, 1, 13–18. [Google Scholar] [CrossRef]
- Lutgendorf, S.; Sood, A. Biobehavioral factors and cancer progression: Physiological pathways and mechanisms. Psychosom. Med. 2011, 73, 724–730. [Google Scholar] [CrossRef]
- Garssen, B. Psychological factors and cancer development: Evidence after 30 years of research. Clin. Psychol. Rev. 2004, 24, 315–338. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Denaro, N.; Tomasello, L.; Russi, E.G. Cancer and stress: What’s matter? From epidemiology: The psychologist and oncologist point of view. J. Cancer Ther. Res. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Malloy, P.J.; Krishnan, A.V.; Swami, S.; Navone, N.M.; Peehl, D.M.; Feldman, D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 2000, 6, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.K. State of the science: Stress, inflammation, and cancer. Oncol. Nurs. Forum 2013, 41, 533–540. [Google Scholar] [CrossRef]
- Powell, N.D.; Tarr, A.J.; Sheridan, J.F. Psychosocial stress and inflammation in cancer. Brain Behav. Immun. 2013, 30, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Gulley, J.L. Finding an Immunologic Beachhead in the Prostate Cancer Microenvironment. J. Natl. Cancer Inst. 2019, 111, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234. [Google Scholar] [CrossRef]
- Gaudreau, P.O.; Stagg, J.; Soulieres, D.; Saad, F. The present and future of biomarkers in prostate cancer: Proteomics, genomics, and immunology advancements. Biomark. Cancer 2016, 8 (Suppl. S2), 15–33. [Google Scholar] [CrossRef]
- Intasqui, P.; Bertolla, R.P.; Sadi, M.V. Prostate cancer proteomics: Clinically useful protein biomarkers and future perspectives. Expert. Rev. Proteom. 2018, 15, 65–79. [Google Scholar] [CrossRef]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; CAP Trial Group. Effect of a low-intensity psa-based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA 2018, 319, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Pin, E.; Fredolini, C.; Petricoin, E. F, 3rd. The role of proteomics in prostate cancer research: Biomarker discovery and validation. Clin. Biochem. 2013, 46, 524–538. [Google Scholar] [CrossRef] [PubMed]
| Classification | Primary GS | Secondary GS | N. of Cases |
|---|---|---|---|
| Group 1 | 3 | 3 | 25 |
| Group 2 | 3 | 4 | 73 |
| Group 3 | 4 | 3 | 47 |
| Group 4 | 4 | 4 | 29 |
| Group 5 | 4, 5 | 4, 5 | 69 |
| Gleason Score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Total | F | p | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| OS | 34.91 | 27.63 | 38.46 | 29.14 | 37.61 | 23.86 | 41.55 | 24.46 | 36.89 | 26.36 | 37.85 | 26.53 | 0.25 | 0.91 |
| DFI | 34.91 | 27.63 | 37.85 | 29.52 | 35.24 | 22.67 | 39.85 | 24.48 | 27.68 | 22.94 | 34.47 | 25.91 | 1.79 | 0.13 |
| PSA | 0.08 | 0.20 | 0.09 | 0.40 | 0.51 | 2.37 | 0.81 | 2.33 | 8.01 | 41.93 | 2.45 | 22.27 | 1.30 | 0.27 |
| Age | 59.24 | 7.92 | 60.53 | 7.06 | 61.68 | 6.07 | 61.10 | 6.07 | 63.03 | 6.52 | 61.40 | 6.76 | 2.01 | 0.1 |
| Age | OS | DFI | MYC | EZH2 | MCM7 | BRCA2 | PDL1 | cortisol | BDNF | CTLA4 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSA | r | −0.011 | −0.115 | −0.184 ** | 0.322 ** | 0.634 ** | 0.135 * | −0.032 | 0.008 | −0.045 | −0.044 | −0.004 |
| p-value | 0.868 | 0.092 | 0.007 | 0.000 | 0.000 | 0.048 | 0.636 | 0.905 | 0.514 | 0.521 | 0.953 | |
| Age | r | −0.104 | −0.105 | 0.016 | 0.057 | 0.107 | 0.067 | 0.168 ** | −0.011 | 0.129 * | 0.096 | |
| p-value | 0.105 | 0.105 | 0.799 | 0.379 | 0.097 | 0.295 | 0.009 | 0.866 | 0.044 | 0.137 | ||
| OS | r | 0.911 ** | −0.018 | −0.096 | −0.132 * | −0.133* | −0.125 | 0.127 * | −0.077 | −0.009 | ||
| p-value | 0.000 | 0.783 | 0.134 | 0.040 | 0.039 | 0.051 | 0.048 | 0.231 | 0.885 | |||
| DFI | r | 0.008 | −0.136 * | −0.155 * | −0.127 * | −0.133 * | 0.092 | −0.078 | −0.036 | |||
| p-value | 0.897 | 0.035 | 0.017 | 0.049 | 0.039 | 0.154 | 0.229 | 0.576 |
| (a) | ||||||
| Model | Unstandardized Coefficients | Standardized Coefficients | t | p | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | 41.266 | 2.357 | 17.505 | 0.000 | |
| BRCA2 | −1.366 × 10−5 | 0.000 | −0.133 | −2.077 | 0.039 | |
| 2 | (Constant) | 37.395 | 3.001 | 12.463 | 0.000 | |
| BRCA2 | −1.407 × 10−5 | 0.000 | −0.137 | −2.152 | 0.032 | |
| cortisol | 5.340 × 10−6 | 0.000 | 0.131 | 2.063 | 0.040 | |
| 3 | (Constant) | 39.324 | 3.100 | 12.686 | 0.000 | |
| BRCA2 | −1.331 × 10−5 | 0.000 | −0.129 | −2.050 | 0.042 | |
| cortisol | 8.093 × 10−6 | 0.000 | 0.198 | 2.840 | 0.005 | |
| BDNF | −1.116 × 10−5 | 0.000 | −0.156 | −2.226 | 0.027 | |
| (b) | ||||||
| Model | Unstandardized Coefficients | Standardized Coefficients | t | p | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | 34.401 | 1.650 | 20.852 | 0.000 | |
| CLUS3 | −2.077 | 0.749 | −0.177 | −2.774 | 0.006 | |
| Dependent Variable: Disease Free (Months) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boldrini, L.; Faviana, P.; Galli, L.; Paolieri, F.; Erba, P.A.; Bardi, M. Multi-Dimensional Scaling Analysis of Key Regulatory Genes in Prostate Cancer Using the TCGA Database. Genes 2021, 12, 1350. https://doi.org/10.3390/genes12091350
Boldrini L, Faviana P, Galli L, Paolieri F, Erba PA, Bardi M. Multi-Dimensional Scaling Analysis of Key Regulatory Genes in Prostate Cancer Using the TCGA Database. Genes. 2021; 12(9):1350. https://doi.org/10.3390/genes12091350
Chicago/Turabian StyleBoldrini, Laura, Pinuccia Faviana, Luca Galli, Federico Paolieri, Paola Anna Erba, and Massimo Bardi. 2021. "Multi-Dimensional Scaling Analysis of Key Regulatory Genes in Prostate Cancer Using the TCGA Database" Genes 12, no. 9: 1350. https://doi.org/10.3390/genes12091350
APA StyleBoldrini, L., Faviana, P., Galli, L., Paolieri, F., Erba, P. A., & Bardi, M. (2021). Multi-Dimensional Scaling Analysis of Key Regulatory Genes in Prostate Cancer Using the TCGA Database. Genes, 12(9), 1350. https://doi.org/10.3390/genes12091350






