Hydroxyurea—The Good, the Bad and the Ugly
Abstract
1. Introduction
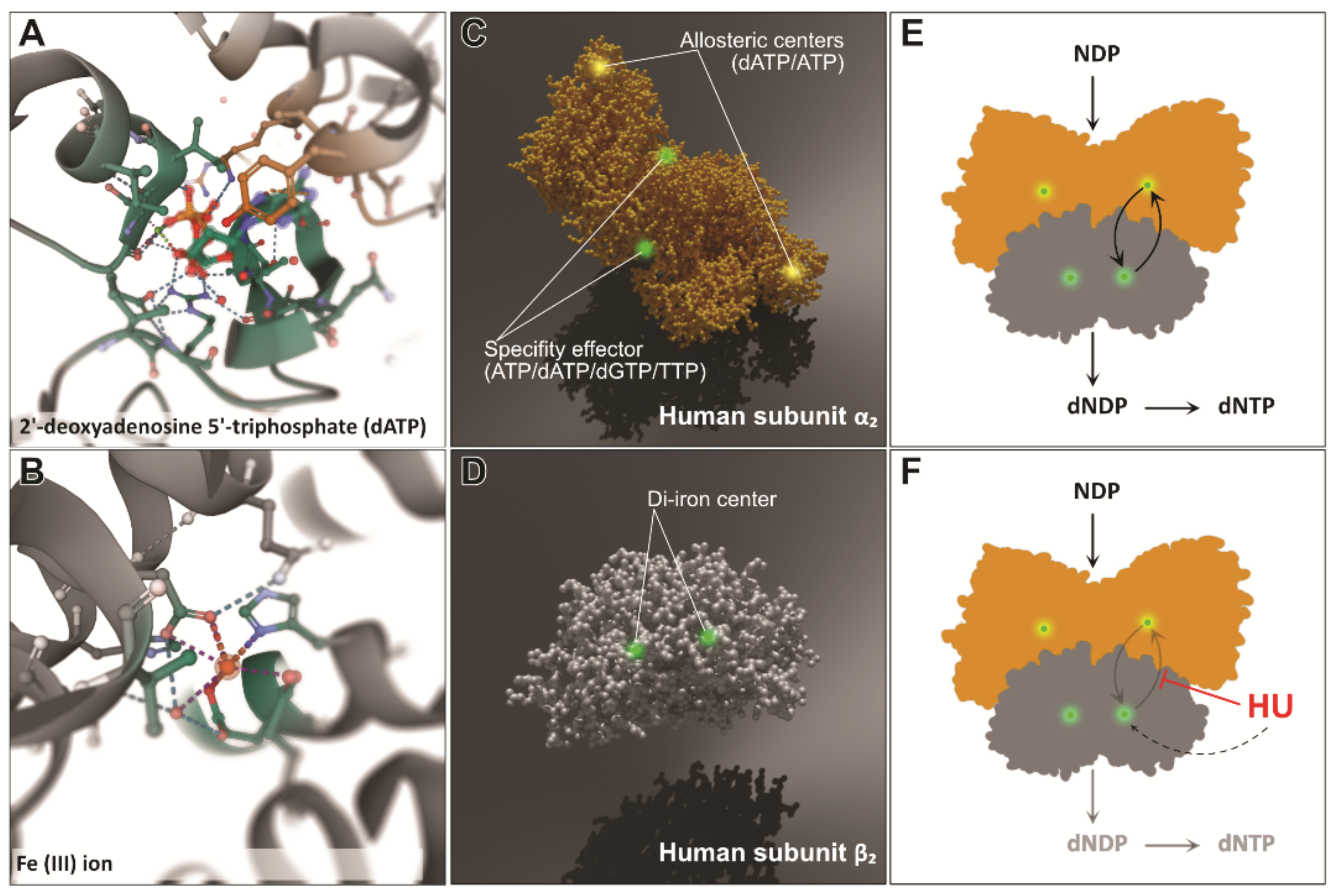
2. The Mechanism of RNR Inhibition by Hydroxyurea
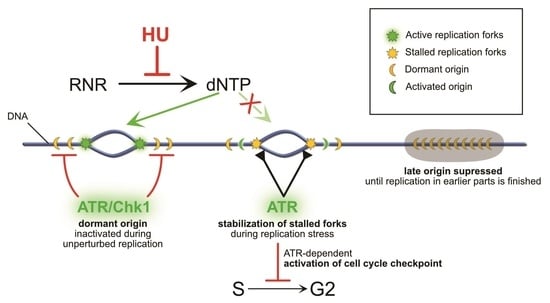
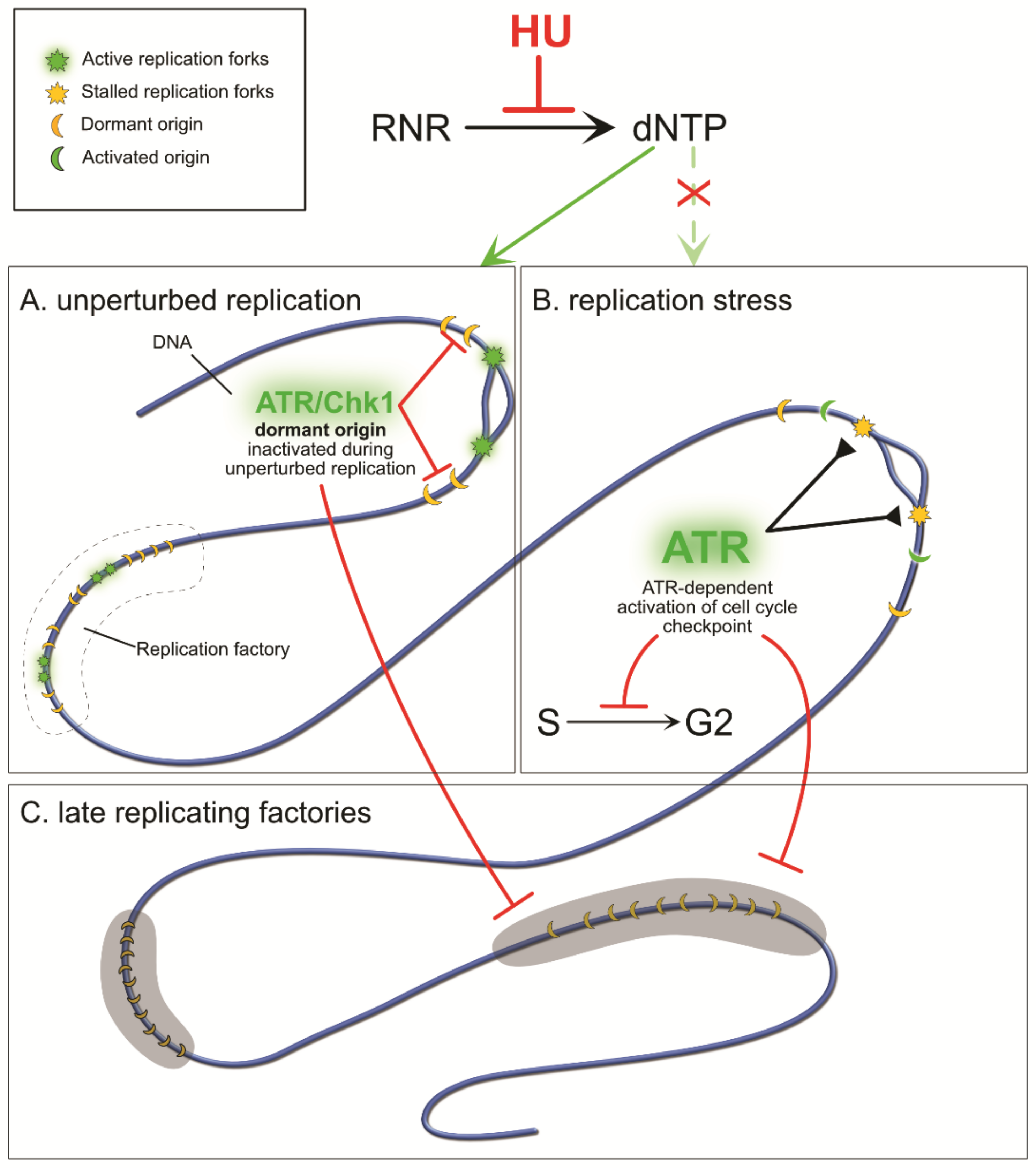
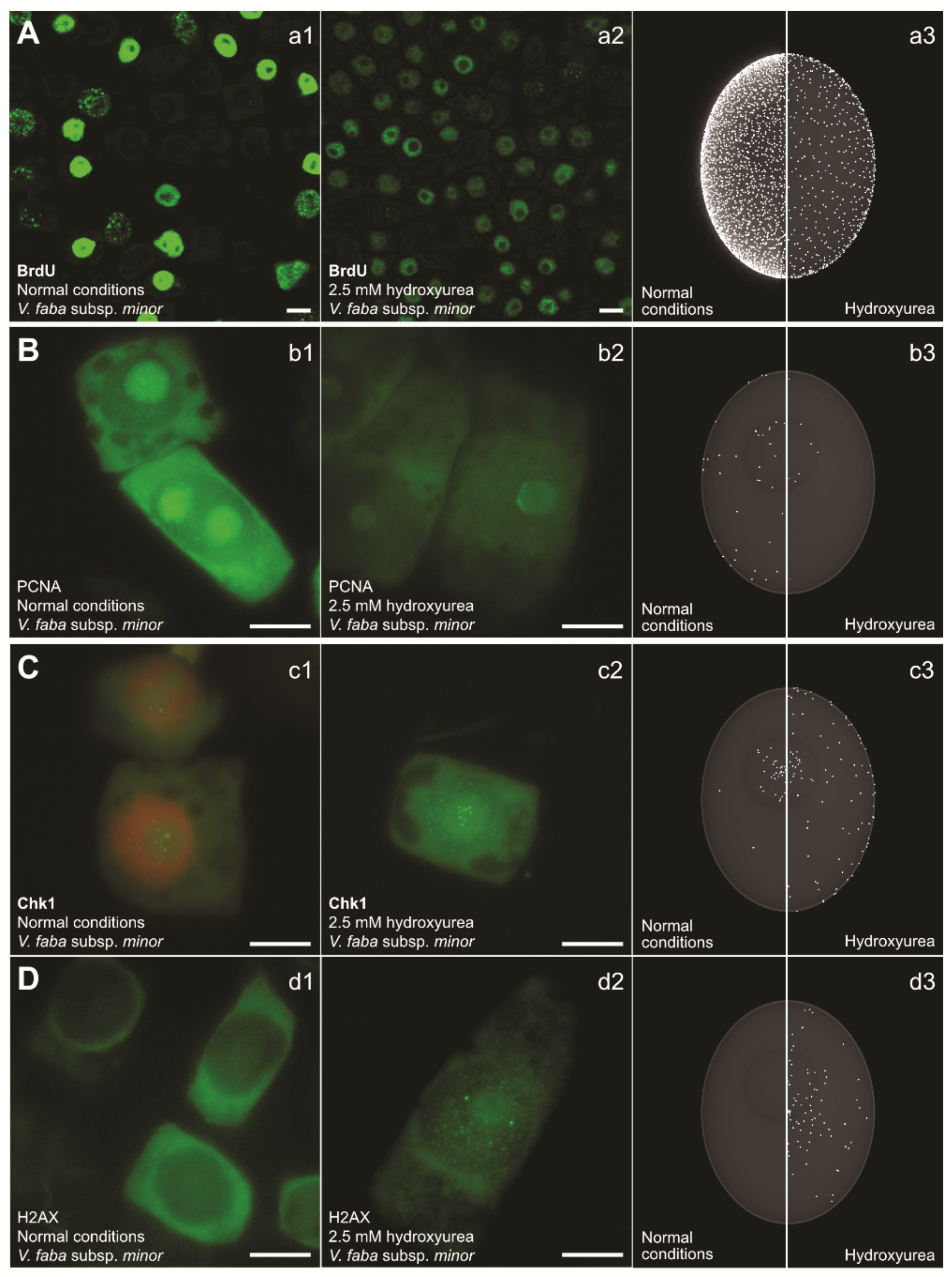
3. Stalled Fork Maintenance, Cell Cycle Checkpoint Activation, and Production of New dNTPs in Response to HU
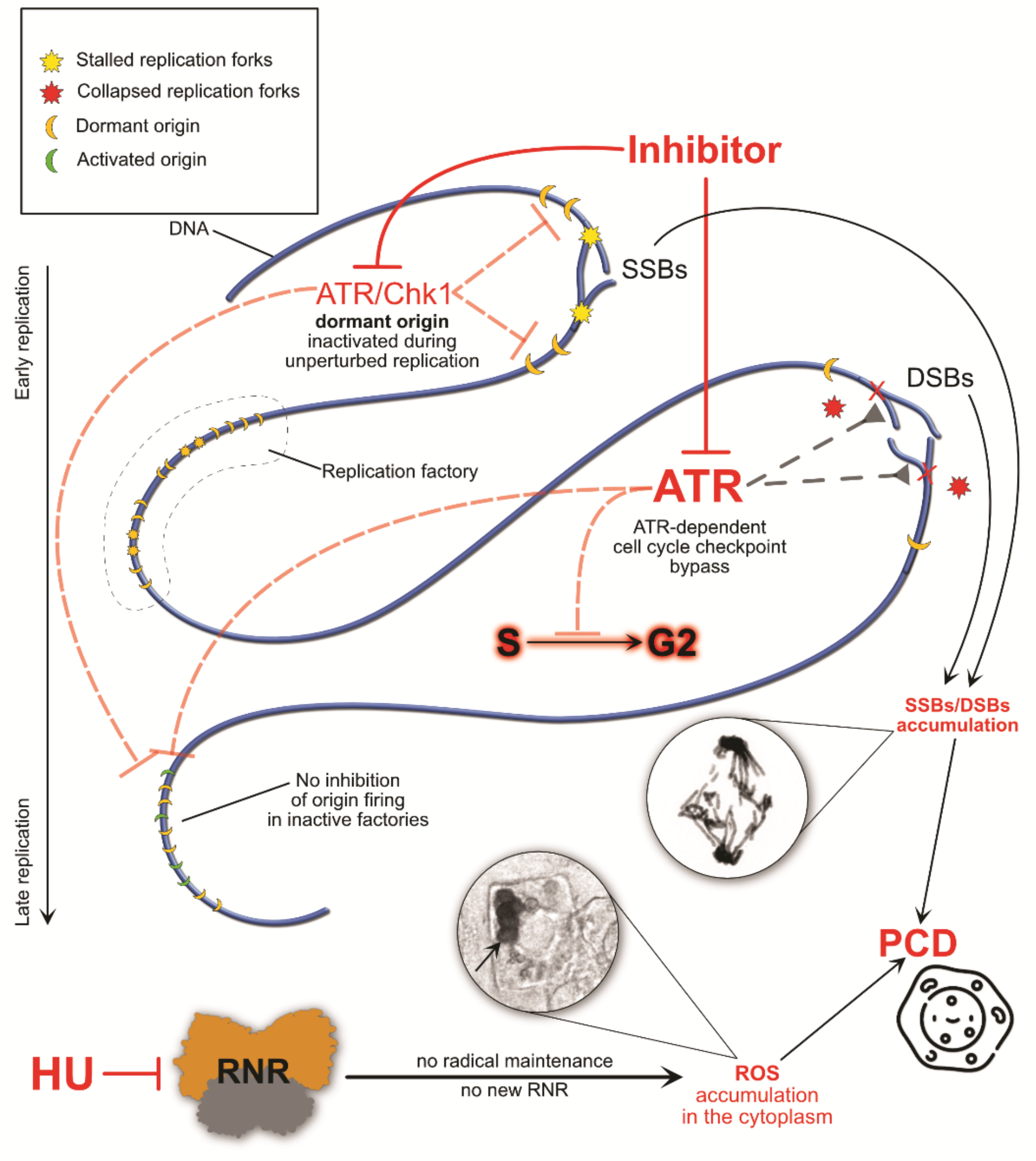
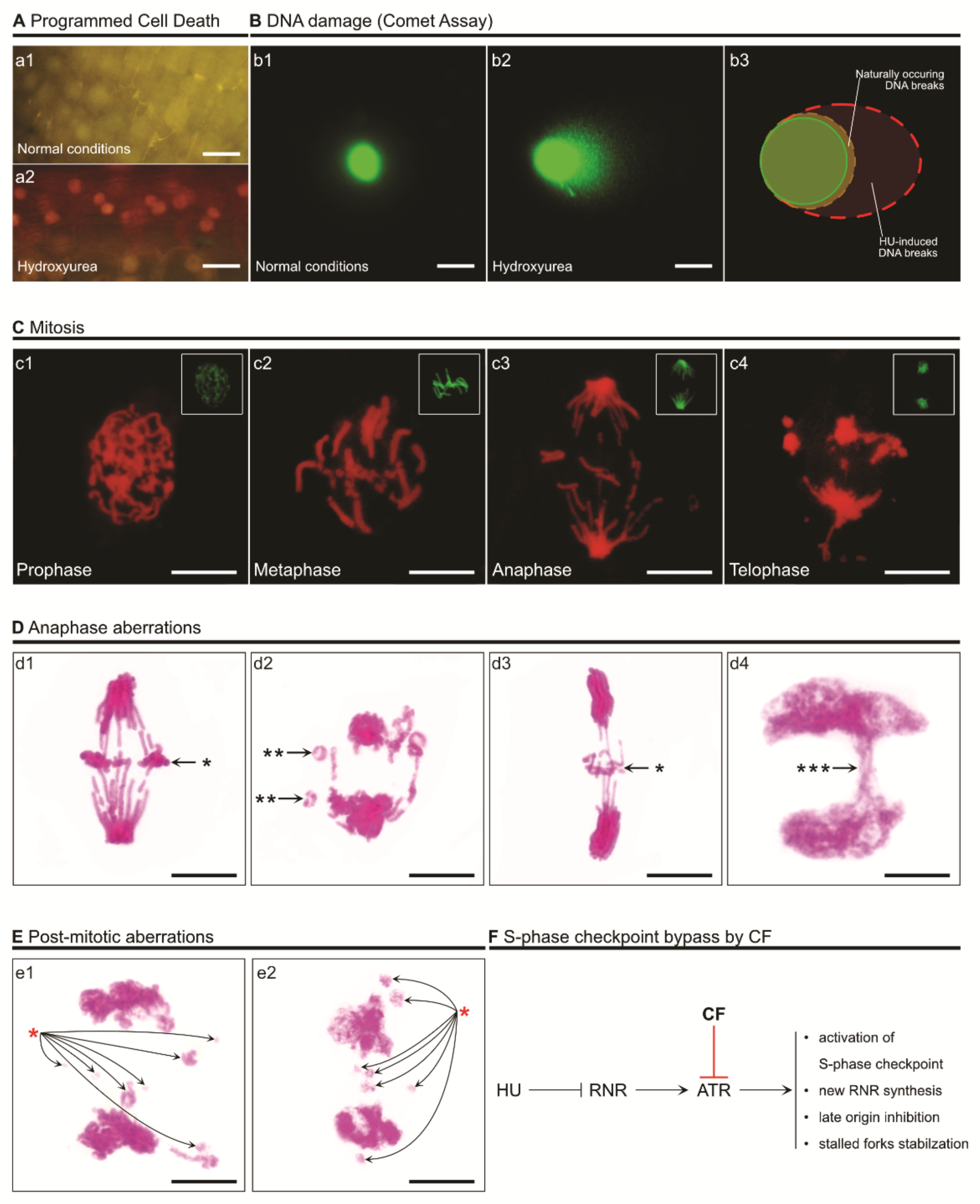
4. The Consequences of S-Phase Checkpoint Malfunction
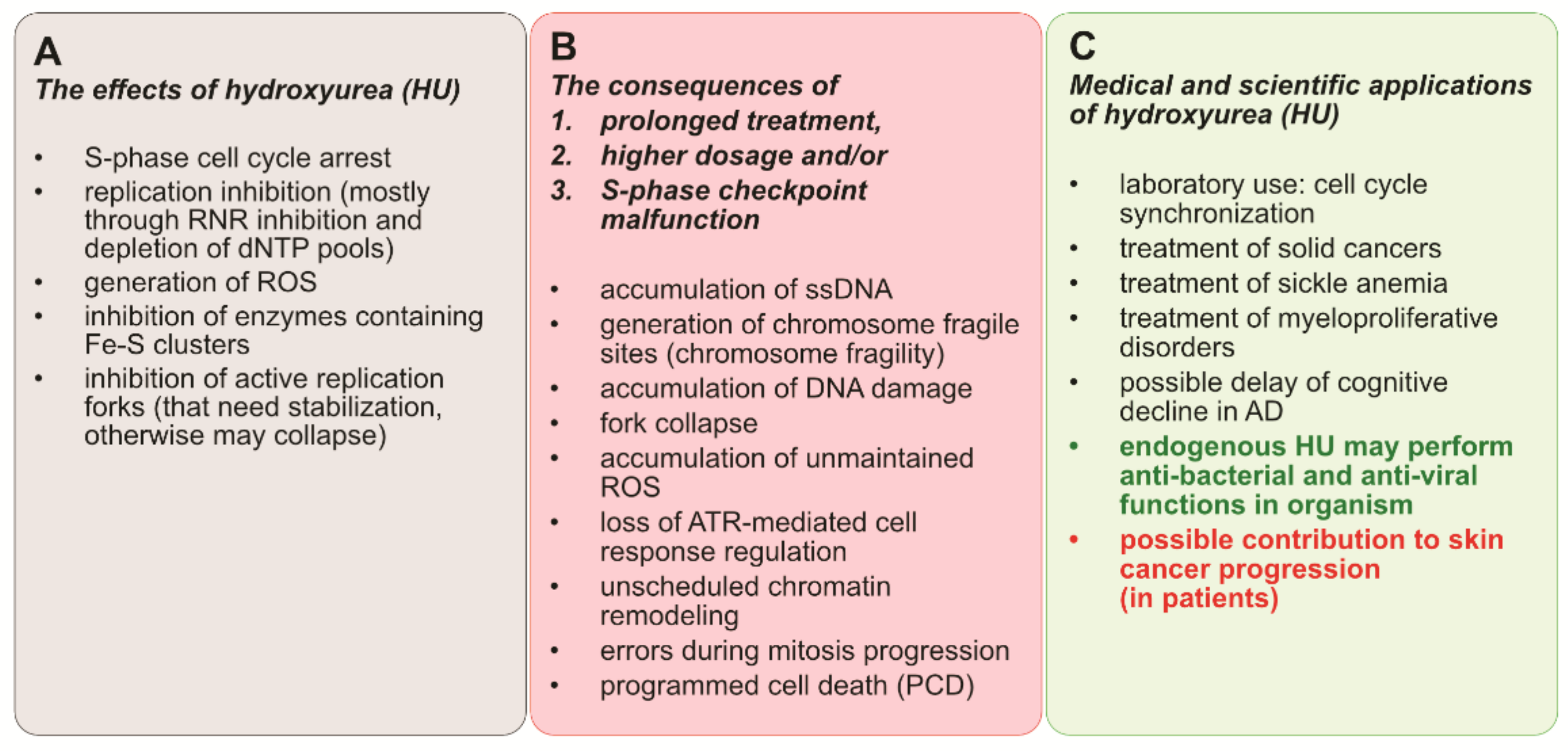
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AO | Acridine orange |
| ATR | Ataxia telangiectasia and Rad3-related |
| BLM | Bloom syndrome protein |
| CF | Caffeine |
| Chk1 | Checkpoint kinase 1 |
| dNTP | Deoxyribonucleoside triphosphate |
| DSB | Double DNA strand break |
| EB | Ethidium bromide |
| Exo1 | Exonuclease 1 |
| HU | Hydroxyurea |
| MCM | Minichromosome maintenance |
| PCET | Proton-coupled electron transfer |
| PCNA | Proliferating cell nuclear antigen |
| RIFT-IR | Reaction-induced FT-IR |
| RNR | Ribonucleotide reductase |
| ROS | Reactive oxygen species |
| RPA | Replication protein A |
| RT | Radical transfer |
References
- Rosenthal, F.; Wislicki, L.; Kollek, L. Über die Beziehungen von Schwersten Blutgiften zu Abbauprodukten des Eiweiss—Ein Beitrag zum Entstehungsmechanismus der perniziösen Anämie. Klin. Wochenschr. 1928, 7, 972–977. [Google Scholar] [CrossRef]
- Adamson, R.H. Activity of Congeners of Hydroxyurea Against Advanced Leukemia L1210. Proc. Soc. Exp. Biol. Med. 1965, 119, 456–458. [Google Scholar] [CrossRef]
- Stearns, B.; Losee, K.A.; Bernstein, J. Hydroxyurea: A New Type of Potential Antitumor Agent. J. Med. Chem. 1963, 6, 201. [Google Scholar] [CrossRef]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A key player in cancer chemotherapy. Expert Rev. Anticancer Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef]
- Spivak, J.L.; Hasselbalch, H. Hydroxycarbamide: A user’s guide for chronic myeloproliferative disorders. Expert Rev. Anticancer Ther. 2011, 11, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Yogev, O.; Anzi, S.; Inoue, K.; Shaulian, E. Induction of transcriptionally active Jun proteins regulates drug-induced senescence. J. Biol. Chem. 2006, 281, 34475–34483. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, S.J.; Jones, A.P.; Howard, J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst. Rev. 2017, 4, CD002202. [Google Scholar] [CrossRef] [PubMed]
- Tshilolo, L.; Tomlinson, G.; Williams, T.N.; Santos, B.; Olupot-Olupot, P.; Lane, A.; Aygun, B.; Stuber, S.E.; Latham, T.S.; McGann, P.T.; et al. Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa. N. Engl. J. Med. 2019, 380, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Brose, R.D.; Lehrmann, E.; Zhang, Y.; Reeves, R.H.; Smith, K.D.; Mattson, M.P. Hydroxyurea attenuates oxidative, metabolic, and excitotoxic stress in rat hippocampal neurons and improves spatial memory in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 72, 121–133. [Google Scholar] [CrossRef]
- Koç, A.; Wheeler, L.J.; Mathews, C.K.; Merrill, G.F. Hydroxyurea Arrests DNA Replication by a Mechanism that Preserves Basal dNTP Pools. J. Biol. Chem. 2004, 279, 223–230. [Google Scholar] [CrossRef]
- Berniak, K.; Rybak, P.; Bernas, T.; Zarebski, M.; Biela, E.; Zhao, H.; Darzynkiewicz, Z.; Dobrucki, J.W. Relationship between DNA damage response, initiated by camptothecin or oxidative stress, and DNA replication, analyzed by quantitative 3D image analysis. Cytom. Part. A 2013, 83, 913–924. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Busby, E.C.; Tibbetts, R.S.; Roos, P.; Taya, Y.; Karnitz, L.M.; Abraham, R.T. Inhibition of ATM and ATR Kinase Activities by the Radiosensitizing Agent, Caffeine. Cancer Res. 1999, 59, 4375–4382. [Google Scholar]
- Boddy, M.N.; Russell, P. DNA replication checkpoint. Curr. Biol. 2001, 11, R953–R956. [Google Scholar] [CrossRef]
- Rybaczek, D. Ultrastructural changes associated with the induction of premature chromosome condensation in Vicia faba root meristem cells. Plant. Cell Rep. 2014, 33, 1547–1564. [Google Scholar] [CrossRef]
- Xu, Y.J.; Singh, A.; Alter, G.M. Hydroxyurea induces cytokinesis arrest in cells expressing a mutated sterol-14α-demethylase in the ergosterol biosynthesis pathway. Genetics 2016, 204, 959–973. [Google Scholar] [CrossRef]
- Huang, M.E.; Facca, C.; Fatmi, Z.; Baïlle, D.; Bénakli, S.; Vernis, L. DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Sci. Rep. 2016, 6, 29361. [Google Scholar] [CrossRef] [PubMed]
- King, S.B. Nitric oxide production from hydroxyurea. Free Radic. Biol. Med. 2004, 37, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Trovesi, C.; Manfrini, N.; Falcettoni, M.; Longhese, M.P. Regulation of the DNA damage response by cyclin-dependent kinases. J. Mol. Biol. 2013, 425, 4756–4766. [Google Scholar] [CrossRef]
- Huh, M.S.; Ivanochko, D.; Hashem, L.E.; Curtin, M.; Delorme, M.; Goodall, E.; Yan, K.; Picketts, D.J. Stalled replication forks within heterochromatin require ATRX for protection. Cell Death Dis. 2016, 7, e2220-12. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, M.; Branzei, D. S-phase checkpoint regulations that preserve replication and chromosome integrity upon dNTP depletion. Cell. Mol. Life Sci. 2017, 74, 2361–2380. [Google Scholar] [CrossRef]
- Moiseeva, T.N.; Yin, Y.; Calderon, M.J.; Qian, C.; Schamus-Haynes, S.; Sugitani, N.; Osmanbeyoglu, H.U.; Rothenberg, E.; Watkins, S.C.; Bakkenist, C.J. An ATR and CHK1 kinase signaling mechanism that limits origin firing during unperturbed DNA replication. Proc. Natl. Acad. Sci. USA 2019, 116, 13374–13383. [Google Scholar] [CrossRef]
- Nair, J.; Huang, T.T.; Murai, J.; Haynes, B.; Steeg, P.S.; Pommier, Y.; Lee, J.M. Resistance to the CHK1 inhibitor prexasertib involves functionally distinct CHK1 activities in BRCA wild-type ovarian cancer. Oncogene 2020, 39, 5520–5535. [Google Scholar] [CrossRef] [PubMed]
- Julius, J.; Peng, J.; McCulley, A.; Caridi, C.; Arnak, R.; See, C.; Nugent, C.I.; Feng, W.; Bachant, J. Inhibition of spindle extension through the yeast S phase checkpoint is coupled to replication fork stability and the integrity of centromeric DNA. Mol. Biol. Cell 2019, 30, 2771–2789. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, B.; Chu, B.; Yen, Y. Ribonucleotide Reductase Inhibitors and Future Drug Design. Curr. Cancer Drug Targets 2006, 6, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Sethy, S.; Panda, T.; Jena, R.K. Beneficial Effect of Low Fixed Dose of Hydroxyurea in Vaso-occlusive Crisis and Transfusion Requirements in Adult HbSS Patients: A Prospective Study in a Tertiary Care Center. Indian J. Hematol. Blood Transfus. 2018, 34, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Yazinski, S.A.; Zou, L. Functions, Regulation, and Therapeutic Implications of the ATR Checkpoint Pathway. Annu. Rev. Genet. 2016, 50, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.K.; Shrivastav, M.; Nie, J.; Amerin, C.; Troksa, K.; Glanzer, J.G.; Liu, S.; Opiyo, S.O.; Dimitrova, D.D.; Le, P.; et al. DNA-PK phosphorylation of RPA32 Ser4/Ser8 regulates replication stress checkpoint activation, fork restart, homologous recombination and mitotic catastrophe. DNA Repair 2014, 21, 131–139. [Google Scholar] [CrossRef]
- Kramara, J.; Osia, B.; Malkova, A. Break Induced Replication: The where, the why, and the how. HHS Public Access. Trends Genet. 2018, 34, 518–531. [Google Scholar] [CrossRef]
- Bester, A.C.; Roniger, M.; Oren, Y.S.; Im, M.M.; Sarni, D.; Chaoat, M.; Bensimon, A.; Zamir, G.; Shewach, D.S.; Kerem, B. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 2011, 145, 435–446. [Google Scholar] [CrossRef]
- Przybyszewski, W.M.; Kasperczyk, J. Rodnikowy mechanizm ubocznej toksyczności hydroksymocznika. Postepy Hig. Med. Dosw. 2006, 60, 516–526. [Google Scholar]
- De Oliveira, E.A.M.; Boy, K.d.A.; Santos, A.P.P.; Machado, C.d.S.; Velloso-Rodrigues, C.; Gerheim, P.S.A.S.; Mendonça, L.M. Evaluation of hydroxyurea genotoxicity in patients with sickle cell disease. Einstein 2019, 17, eAO4742. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, M.; Albergante, L.; Moreno, A.; Carrington, J.T.; Blow, J.J.; Newman, T.J. Inevitability and containment of replication errors for eukaryotic genome lengths spanning megabase to gigabase. Proc. Natl. Acad. Sci. USA 2016, 113, E5765–E5774. [Google Scholar] [CrossRef]
- Elledge, S.J.; Zhou, Z.; Allen, J.B. Ribonucleotide reductase: Regulation, regulation, regulation. Trends Biochem. Sci. 1992, 17, 119–123. [Google Scholar] [CrossRef]
- Offenbacher, A.R.; Barry, B.A. A Proton Wire Mediates Proton Coupled Electron Transfer from Hydroxyurea and Other Hydroxamic Acids to Tyrosyl Radical in Class Ia Ribonucleotide Reductase. J. Phys. Chem. B 2020, 124, 345–354. [Google Scholar] [CrossRef]
- Fairman, J.W.; Wijerathna, S.R.; Ahmad, M.F.; Xu, H.; Nakano, R.; Jha, S.; Prendergast, J.; Welin, R.M.; Flodin, S.; Roos, A.; et al. Structural basis for allosteric regulation of human ribonucleotide reductase by nucleotide-induced oligomerization. Nat. Struct. Mol. Biol. 2011, 18, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Brignole, E.J.; Tsai, K.L.; Chittuluru, J.; Li, H.; Aye, Y.; Penczek, P.A.; Stubbe, J.A.; Drennan, C.L.; Asturias, F. 3.3-Å resolution cryo-EM structure of human ribonucleotide reductase with substrate and allosteric regulators bound. eLife 2018, 7, e31502. [Google Scholar] [CrossRef]
- Eriksson, M.; Uhlin, U.; Ramaswamy, S.; Ekberg, M.; Regnström, K.; Sjöberg, B.M.; Eklund, H. Binding of allosteric effectors to ribonucleotide reductase protein R1: Reduction of active-site cysteines promotes substrate binding. Structure 1997, 5, 1077–1092. [Google Scholar] [CrossRef]
- Rofougaran, R.; Vodnala, M.; Hofer, A. Enzymatically active mammalian ribonucleotide reductase exists primarily as an α6β2 octamer. J. Biol. Chem. 2006, 281, 27705–27711. [Google Scholar] [CrossRef]
- Radivoyevitch, T. Automated mass action model space generation and analysis methods for two-reactant combinatorially complex equilibriums: An analysis of ATP-induced ribonucleotide reductase R1 hexamerization data. Biol. Direct 2009, 4, 50. [Google Scholar] [CrossRef]
- Denysenkov, V.P.; Biglino, D.; Lubitz, W.; Prisner, T.F.; Bennati, M. Structure of the tyrosyl biradical in mouse R2 ribonucleotide reductase from high-field PELDOR. Angew. Chem. Int. Ed. 2008, 47, 1224–1227. [Google Scholar] [CrossRef]
- Minnihan, E.C.; Nocera, D.G.; Stubbe, J. Reversible, long-range radical transfer in E. coli class Ia ribonucleotide reductase. Acc. Chem. Res. 2013, 46, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carranza, M.; Jonna, V.R.; Lundin, D.; Sahlin, M.; Carlson, L.A.; Jemal, N.; Högbom, M.; Sjöberg, B.M.; Stenmark, P.; Hofer, A. A ribonucleotide reductase from clostridium botulinum reveals distinct evolutionary pathways to regulation via the overall activity site. J. Biol. Chem. 2020, 295, 15576–15587. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Taguchi, A.T.; Stubbe, J.A.; Drennan, C.L. Structure of a trapped radical transfer pathway within a ribonucleotide reductase holocomplex. Science 2020, 368, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, P.; Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006, 75, 681–706. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velenkar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol*Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Rofougaran, R.; Crona, M.; Vodnala, M.; Sjöberg, B.-M.; Hofer, A. Oligomerization status directs overall activity regulation of the Escherichia coli class Ia ribonucleotide reductase. J. Biol. Chem. 2008, 283, 35310–35318. [Google Scholar] [CrossRef]
- Hofer, A.; Crona, M.; Logan, D.T.; Sjöberg, B.-M. DNA building blocks: Keeping control of manufacture. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 50–63. [Google Scholar] [CrossRef]
- Håkansson, P.; Hofer, A.; Thelander, L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J. Biol. Chem. 2006, 281, 7834–7841. [Google Scholar] [CrossRef]
- Sanvisens, N.; De Llanos, R.; Puig, S. Function and regulation of yeast ribonucleotide reductase: Cell cycle, genotoxic stress, and iron bioavailability. Biomed. J. 2013, 36, 51–58. [Google Scholar]
- Guarino, E.; Salguero, I.; Kearsey, S.E. Cellular regulation of ribonucleotide reductase in eukaryotes. Semin. Cell Dev. Biol. 2014, 30, 97–103. [Google Scholar] [CrossRef]
- Lassmann, G.; Thelander, L.; Gräslund, A. EPR stopped-flow studies of the reaction of the tyrosyl radical of protein R2 from ribonucleotide reductase with hydroxyurea. Biochem. Biophys. Res. Commun. 1992, 188, 879–887. [Google Scholar] [CrossRef]
- Singh, A.; Xu, Y.J. The cell killing mechanisms of hydroxyurea. Genes 2016, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, M.; Lepoivre, M.; Elleingand, E.; Gerez, C.; Guittet, O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998, 421, 277–279. [Google Scholar] [CrossRef]
- Rawson, J.M.O.; Roth, M.E.; Xie, J.; Daly, M.B.; Clouser, C.L.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Kim, B.; Patterson, S.E.; et al. Synergistic reduction of HIV-1 infectivity by 5-azacytidine and inhibitors of ribonucleotide reductase. Bioorganic Med. Chem. 2016, 24, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Q.Q.; Lam, C.W.K.; Guo, J.R.; Zhang, W.J.; Wang, C.Y.; Wong, V.K.W.; Yao, M.C.; Zhang, W. Investigation into perturbed nucleoside metabolism and cell cycle for elucidating the cytotoxicity effect of resveratrol on human lung adenocarcinoma epithelial cells. Chin. J. Nat. Med. 2019, 17, 608–615. [Google Scholar] [CrossRef]
- Neuhard, J. Studies on the acid-soluble nucleotide pool in Escherichia coli. IV. Effects of hydroxyurea. BBA Sect. Nucleic Acids Protein Synth. 1967, 145, 1–6. [Google Scholar] [CrossRef]
- Morganroth, P.A.; Hanawalt, P.C. Role of DNA replication and repair in thymineless death in Escherichia coli. J. Bacteriol. 2006, 188, 5286–5288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuong, K.J.; Kuzminov, A. Cyanide, Peroxide and Nitric Oxide Formation in Solutions of Hydroxyurea Causes Cellular Toxicity and May Contribute to Its Therapeutic Potency. J. Mol. Biol. 2009, 390, 845–862. [Google Scholar] [CrossRef]
- Fraser, D.I.; Liu, K.T.; Reid, B.J.; Hawkins, E.; Sevier, A.; Pyle, M.; Robinson, J.W.; Ouellette, P.H.R.; Ballantyne, J.S. Widespread natural occurrence of hydroxyurea in animals. PLoS ONE 2015, 10, e0142890. [Google Scholar] [CrossRef]
- Hallmark, L.; Almeida, L.E.F.; Kamimura, S.; Smith, M.; Quezado, Z.M.N. Nitric oxide and sickle cell disease—Is there a painful connection? Exp. Biol. Med. 2021, 246, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.D.; Cook, J.G. The Temporal Regulation of S Phase Proteins during G1. Adv. Exp. Med. Biol. 2017, 1042, 335–369. [Google Scholar] [CrossRef]
- Kaykov, A.; Nurse, P. The spatial and temporal organization of origin firing during the S-phase of fission yeast. Genome Res. 2015, 25, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Walter, D.; Gillespie, P.J.; Izard, F.; Fahrenkrog, B.; Lleres, D.; Lerdrup, M.; Johansen, J.V.; Hansen, K.; Julien, E.; et al. Histone H4K20 methylation mediated chromatin compaction threshold ensures genome integrity by limiting DNA replication licensing. Nat. Commun. 2018, 9, 3704. [Google Scholar] [CrossRef] [PubMed]
- Blow, J.J. Defects in the origin licensing checkpoint stresses cells exiting G0. J. Cell Biol. 2019, 218, 2080–2081. [Google Scholar] [CrossRef]
- McIntosh, D.; Blow, J.J. Dormant origins, the licensing checkpoint, and the response to replicative stresses. Cold Spring Harb. Perspect. Biol. 2012, 4, a012955. [Google Scholar] [CrossRef]
- Musiałek, M.W.; Rybaczek, D. Behavior of replication origins in Eukaryota—Spatio-temporal dynamics of licensing and firing. Cell Cycle 2015, 14, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, J.A.; Gravells, P.; Bryant, H.E. DNA Fiber Assay for the Analysis of DNA Replication Progression in Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2020, 54, e115. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Xu, X.; Yuan, Y.; Zhang, B.; Li, Z.; Xie, Y.; Yan, R.; Zheng, Z.; Ji, J.; et al. The intra-S phase checkpoint directly regulates replication elongation to preserve the integrity of stalled replisomes. Proc. Natl. Acad. Sci USA 2021, 118, e2019183118. [Google Scholar] [CrossRef]
- Nitani, N.; Nakamura, K.; Nakagawa, C.; Masukata, H.; Nakagawa, T. Regulation of DNA replication machinery by Mrc1 in fission yeast. Genetics 2006, 174, 155–165. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, C.; Tripathi, V.; Manolika, E.M.; Heijink, A.M.; Ricci, G.; Merzouk, S.; de Boer, H.R.; Demmers, J.; van Vugt, M.A.T.M.; Ray Chaudhuri, A. RIF1 promotes replication fork protection and efficient restart to maintain genome stability. Nat. Commun. 2019, 10, 3287. [Google Scholar] [CrossRef]
- Jasencakova, Z.; Scharf, A.N.D.; Ask, K.; Corpet, A.; Imhof, A.; Almouzni, G.; Groth, A. Replication Stress Interferes with Histone Recycling and Predeposition Marking of New Histones. Mol. Cell 2010, 37, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Nazaretyan, S.A.; Savic, N.; Sadek, M.; Hackert, B.J.; Courcelle, J.; Courcelle, C.T. Replication rapidly recovers and continues in the presence of hydroxyurea in Escherichia coli. J. Bacteriol. 2018, 200, e00713-17. [Google Scholar] [CrossRef]
- Ivessa, A.S.; Lenzmeier, B.A.; Bessler, J.B.; Goudsouzian, L.K.; Schnakenberg, S.L.; Zakian, V.A. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol. Cell 2003, 12, 1525–1536. [Google Scholar] [CrossRef]
- Ercilla, A.; Feu, S.; Aranda, S.; Llopis, A.; Brynjólfsdóttir, S.H.; Sørensen, C.S.; Toledo, L.I.; Agell, N. Acute hydroxyurea-induced replication blockade results in replisome components disengagement from nascent DNA without causing fork collapse. Cell. Mol. Life Sci. 2019, 77, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Gardner, N.J.; Gillespie, P.J.; Carrington, J.T.; Shanks, E.J.; McElroy, S.P.; Haagensen, E.J.; Frearson, J.A.; Woodland, A.; Blow, J.J. The High-Affinity Interaction between ORC and DNA that Is Required for Replication Licensing Is Inhibited by 2-Arylquinolin-4-Amines. Cell Chem. Biol. 2017, 24, 981–992.e4. [Google Scholar] [CrossRef] [PubMed]
- Heidinger-Pauli, J.M.; Ünal, E.; Guacci, V.; Koshland, D. The Kleisin Subunit of Cohesin Dictates Damage-Induced Cohesion. Mol. Cell 2008, 31, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guntuku, S.; Cui, X.S.; Matsuoka, S.; Cortez, D.; Tamai, K.; Luo, G.; Carattini-Rivera, S.; DeMayo, F.; Bradley, A.; et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000, 14, 1448–1459. [Google Scholar] [CrossRef]
- Menolfi, D.; Delamarre, A.; Lengronne, A.; Pasero, P.; Branzei, D. Essential Roles of the Smc5/6 Complex in Replication through Natural Pausing Sites and Endogenous DNA Damage Tolerance. Mol. Cell 2015, 60, 835–846. [Google Scholar] [CrossRef]
- Weinert, T.A.; Kiser, G.L.; Hartwell, L.H. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994, 8, 652–665. [Google Scholar] [CrossRef]
- Zuilkoski, C.M.; Skibbens, R.V. PCNA antagonizes cohesin-dependent roles in genomic stability. PLoS ONE 2020, 15, e0235103. [Google Scholar] [CrossRef]
- Van, C.; Yan, S.; Michael, W.M.; Waga, S.; Cimprich, K.A. Continued primer synthesis at stalled replication forks contributes to checkpoint activation. J. Cell Biol. 2010, 189, 233–246. [Google Scholar] [CrossRef]
- Byun, T.S.; Pacek, M.; Yee, M.C.; Walter, J.C.; Cimprich, K.A. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005, 19, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Iyer, D.R.; Rhind, N. Replication Fork Slowing and Stalling are Distinct, Checkpoint-Independent Consequences of Replicating Damaged DNA. PLoS Genet. 2017, 13, e1006958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chabes, A.; Domkin, V.; Thelander, L.; Rothstein, R. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 2001, 20, 3544–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Muller, E.G.D.; Rothstein, R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 1998, 2, 329–340. [Google Scholar] [CrossRef]
- Zhang, Z.; Reese, J.C. Molecular Genetic Analysis of the Yeast Repressor Rfx1/Crt1 Reveals a Novel Two-Step Regulatory Mechanism. Mol. Cell. Biol. 2005, 25, 7399–7411. [Google Scholar] [CrossRef] [PubMed]
- Woolstencroft, R.N.; Bellharz, T.H.; Cook, M.A.; Preiss, T.; Durocher, D.; Tyers, M. Ccr4 contributes to tolerance of replication stress through control of CRT1 mRNA poly(A) tail length. J. Cell Sci. 2006, 119, 5178–5192. [Google Scholar] [CrossRef]
- Buisson, R.; Boisvert, J.L.; Benes, C.H.; Zou, L. Distinct but Concerted Roles of ATR, DNA-PK, and Chk1 in Countering Replication Stress during S Phase. Mol. Cell 2015, 59, 1011–1024. [Google Scholar] [CrossRef]
- Koppenhafer, S.L.; Goss, K.L.; Terry, W.W.; Gordon, D.J. Inhibition of the ATR-CHK1 pathway in ewing sarcoma cells causes DNA damage and apoptosis via the CDK2-mediated degradation of RRM2. Mol. Cancer Res. 2020, 18, 91–104. [Google Scholar] [CrossRef]
- Lopez-Contreras, A.J.; Specks, J.; Barlow, J.H.; Ambrogio, C.; Desler, C.; Vikingsson, S.; Rodrigo-Perez, S.; Green, H.; Rasmussen, L.J.; Murga, M.; et al. Increased Rrm2 gene dosage reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes Dev. 2015, 29, 690–695. [Google Scholar] [CrossRef]
- Zou, L.; Elledge, S.J. Sensing DNA Damage Through ATRIP Recognition of RPA-ssDNA Complexes. Science 2003, 300, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Frattini, C.; Villa-Hernández, S.; Pellicanò, G.; Jossen, R.; Katou, Y.; Shirahige, K.; Bermejo, R. Cohesin Ubiquitylation and Mobilization Facilitate Stalled Replication Fork Dynamics. Mol. Cell 2017, 68, 758–772.e4. [Google Scholar] [CrossRef] [PubMed]
- Litwin, I.; Pilarczyk, E.; Wysocki, R. The emerging role of cohesin in the DNA damage response. Genes 2018, 9, 581. [Google Scholar] [CrossRef]
- Rhodes, J.D.P.; Haarhuis, J.H.I.; Grimm, J.B.; Rowland, B.D.; Lavis, L.D.; Nasmyth, K.A. Cohesin Can Remain Associated with Chromosomes during DNA Replication. Cell Rep. 2017, 20, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Bot, C.; Pfeiffer, A.; Giordano, F.; Manjeera, D.E.; Dantuma, N.P.; Ström, L. Independent mechanisms recruit the cohesin loader protein NIPBL to sites of DNA damage. J. Cell Sci. 2017, 130, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.I.; Altmeyer, M.; Rask, M.B.; Lukas, C.; Larsen, D.H.; Povlsen, L.K.; Bekker-Jensen, S.; Mailand, N.; Bartek, J.; Lukas, J. XATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell 2013, 155, 1088. [Google Scholar] [CrossRef]
- Hoffman, E.A.; McCulley, A.; Haarer, B.; Arnak, R.; Feng, W. Break-seq reveals hydroxyurea-induced chromosome fragility as a result of unscheduled conflict between DNA replication and transcription. Genome Res. 2015, 25, 402–412. [Google Scholar] [CrossRef]
- Żabka, A.; Polit, J.T.; Maszewski, J. DNA replication stress induces deregulation of the cell cycle events in root meristems of Allium cepa. Ann. Bot. 2012, 110, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Rybaczek, D.; Musialek, M.W.; Balcerczyk, A. Caffeine-induced premature chromosome condensation results in the apoptosis-like programmed cell death in root meristems of Vicia faba. PLoS ONE 2015, 10, e0142307. [Google Scholar] [CrossRef] [PubMed]
- Rybaczek, D.; Musiałek, M.W.; Vrána, J.; Petrovská, B.; Pikus, E.G.; Doležel, J. Kinetics of DNA Repair in Vicia faba Meristem Regeneration Following Replication Stress. Cells 2021, 10, 88. [Google Scholar] [CrossRef]
- Palumbo, E.; Matricardi, L.; Tosoni, E.; Bensimon, A.; Russo, A. Replication dynamics at common fragile site FRA6E. Chromosoma 2010, 119, 575–587. [Google Scholar] [CrossRef]
- Hashash, N.; Johnson, A.L.; Cha, R.S. Regulation of fragile sites expression in budding yeast by MEC1, RRM3 and hydroxyurea. J. Cell Sci. 2011, 124, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Técher, H.; Koundrioukoff, S.; Carignon, S.; Wilhelm, T.; Millot, G.A.; Lopez, B.S.; Brison, O.; Debatisse, M. Signaling from Mus81-Eme2-Dependent DNA Damage Elicited by Chk1 Deficiency Modulates Replication Fork Speed and Origin Usage. Cell Rep. 2016, 14, 1114–1127. [Google Scholar] [CrossRef]
- Cortez, D. Replication-Coupled DNA Repair. Mol. Cell 2019, 74, 866–876. [Google Scholar] [CrossRef]
- Shiu, J.L.; Wu, C.K.; Chang, S.B.; Sun, Y.J.; Chen, Y.J.; Lai, C.C.; Chiu, W.T.; Chang, W.T.; Myung, K.; Su, W.P.; et al. The HLTF–PARP1 interaction in the progression and stability of damaged replication forks caused by methyl methanesulfonate. Oncogenesis 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Couch, F.B.; Cortez, D. Fork reversal, too much of a good thing. Cell Cycle 2014, 13, 1049–1050. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.H.; Rothstein, R. Rad52 recruitment is DNA replication independent and regulated by Cdc28 and the Mec1 kinase. EMBO J. 2009, 28, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Fumasoni, M.; Zwicky, K.; Vanoli, F.; Lopes, M.; Branzei, D. Error-Free DNA Damage Tolerance and Sister Chromatid Proximity during DNA Replication Rely on the Polα/Primase/Ctf4 Complex. Mol. Cell 2015, 57, 812–823. [Google Scholar] [CrossRef]
- Zellweger, R.; Dalcher, D.; Mutreja, K.; Berti, M.; Schmid, J.A.; Herrador, R.; Vindigni, A.; Lopes, M. Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J. Cell Biol. 2015, 208, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Mutreja, K.; Krietsch, J.; Hess, J.; Ursich, S.; Berti, M.; Roessler, F.K.; Zellweger, R.; Patra, M.; Gasser, G.; Lopes, M. ATR-Mediated Global Fork Slowing and Reversal Assist Fork Traverse and Prevent Chromosomal Breakage at DNA Interstrand Cross-Links. Cell Rep. 2018, 24, 2629–2642.e5. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.E.; Ajazi, A.; Carotenuto, W.; Foiani, M.; Giannattasio, M. Rad53-Mediated Regulation of Rrm3 and Pif1 DNA Helicases Contributes to Prevention of Aberrant Fork Transitions under Replication Stress. Cell Rep. 2015, 13, 80–92. [Google Scholar] [CrossRef]
- Couch, F.B.; Bansbach, C.E.; Driscoll, R.; Luzwick, J.W.; Glick, G.G.; Bétous, R.; Carroll, C.M.; Jung, S.Y.; Qin, J.; Cimprich, K.A.; et al. ATR phosphorylates SMARCAL1 to prevent replication fork collapse. Genes Dev. 2013, 27, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Ragland, R.L.; Patel, S.; Rivard, R.S.; Smith, K.; Peters, A.A.; Bielinsky, A.K.; Brown, E.J. RNF4 and PLK1 are required for replication fork collapse in ATR-deficient cells. Genes Dev. 2013, 27, 2259–2273. [Google Scholar] [CrossRef]
- Yu, C.; Gan, H.; Han, J.; Zhou, Z.X.; Jia, S.; Chabes, A.; Farrugia, G.; Ordog, T.; Zhang, Z. Strand-Specific Analysis Shows Protein Binding at Replication Forks and PCNA Unloading from Lagging Strands when Forks Stall. Mol. Cell 2014, 56, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Katou, Y.; Nakato, R.; Shirahige, K.; Donaldson, A.D. Replication-Coupled PCNA Unloading by the Elg1 Complex Occurs Genome-wide and Requires Okazaki Fragment Ligation. Cell Rep. 2015, 12, 774–787. [Google Scholar] [CrossRef]
- Ohashi, E.; Tsurimoto, T. Functions of multiple clamp and clamp-loader complexes in eukaryotic DNA replication. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1042, pp. 135–162. [Google Scholar]
- Errico, A.; Aze, A.; Costanzo, V. Mta2 promotes Tipin-dependent maintenance of replication fork integrity. Cell Cycle 2014, 13, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.; Neelsen, K.J.; Lukas, J. Molecular Cell Perspective Replication Catastrophe: When a Checkpoint Fails because of Exhaustion. Mol. Cell. 2017, 66, 735–749. [Google Scholar] [CrossRef]
- Segurado, M.; Diffley, J.F.X. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 2008, 22, 1816–1827. [Google Scholar] [CrossRef]
- Kaochar, S.; Shanks, L.; Weinert, T. Checkpoint genes and Exo1 regulate nearby inverted repeat fusions that form dicentric chromosomes in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2010, 107, 21605–21610. [Google Scholar] [CrossRef]
- Morafraile, E.C.; Bugallo, A.; Carreira, R.; Fernández, M.; Martín-Castellanos, C.; Blanco, M.G.; Segurado, M. Exo1 phosphorylation inhibits exonuclease activity and prevents fork collapse in rad53 mutants independently of the 14-3-3 proteins. Nucleic Acids Res. 2020, 48, 3053–3070. [Google Scholar] [CrossRef]
- Tomimatsu, N.; Mukherjee, B.; Harris, J.L.; Boffo, F.L.; Hardebeck, M.C.; Potts, P.R.; Khanna, K.K.; Burma, S. DNA-damage-induced degradation of EXO1 exonuclease limits DNA end resection to ensure accurate DNA repair. J. Biol. Chem. 2017, 292, 10779–10790. [Google Scholar] [CrossRef]
- Hae, Y.Y.; Shevchenko, A.; Shevchenko, A.; Dunphy, W.G. Mcm2 is a direct substrate of ATM and ATR during DNA damage and DNA replication checkpoint responses. J. Biol. Chem. 2004, 279, 53353–53364. [Google Scholar] [CrossRef]
- Shorrocks, A.M.K.; Jones, S.E.; Tsukada, K.; Morrow, C.A.; Belblidia, Z.; Shen, J.; Vendrell, I.; Fischer, R.; Kessler, B.M.; Blackford, A.N. The Bloom syndrome complex senses RPA-coated single-stranded DNA to restart stalled replication forks. Nat. Commun. 2021, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.L.; North, P.S.; Hickson, I.D. Role for BLM in replication-fork restart and suppression of origin firing after replicative stress. Nat. Struct. Mol. Biol. 2007, 14, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Franchitto, A.; Pichierri, P. Bloom’s syndrome protein is required for correct relocalization of RAD50/MRE11/NBS1 complex after replication fork arrest. J. Cell Biol. 2002, 157, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.G.; Jarosz, D.F.; Walker, F.L.; Simmons, L.A.; Walker, G.C. Y-family DNA polymerases respond to DNA damage-independent inhibition of replication fork progression. EMBO J. 2006, 25, 868–879. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. Yap1: A DNA damage responder in Saccharomyces cerevisiae. Mech. Ageing Dev. 2012, 133, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Jiang, W.; Krebs, C.; Stubbe, J. YfaE, a ferredoxin involved in diferric-tyrosyl radical maintenance in Escherichia coli ribonucleotide reductase. Biochemistry 2007, 46, 11577–11588. [Google Scholar] [CrossRef]
- Nakayashiki, T.; Mori, H. Genome-Wide screening with hydroxyurea reveals a link between nonessential ribosomal proteins and reactive oxygen species production. J. Bacteriol. 2013, 195, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Netz, D.J.A.; Stith, C.M.; Stümpfih, M.; Köpf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.J.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2011, 8, 125–132. [Google Scholar] [CrossRef]
- Rudolf, J.; Makrantoni, V.; Ingledew, W.J.; Stark, M.J.R.; White, M.F. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol. Cell 2006, 26, 801–808. [Google Scholar] [CrossRef]
- Holt, M.E.; Salay, L.E.; Chazin, W.J. A Polymerase With Potential: The Fe–S Cluster in Human DNA Primase. Methods Enzymol. 2017, 595, 361–390. [Google Scholar]
- Liu, L.; Huang, M. Essential role of the iron-sulfur cluster binding domain of the primase regulatory subunit Pri2 in DNA replication initiation. Protein Cell 2015, 6, 194–210. [Google Scholar] [CrossRef]
- Kettani, T.; Cotton, F.; Gulbis, B.; Ferster, A.; Kumps, A. Plasma hydroxyurea determined by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 446–450. [Google Scholar] [CrossRef]
- Scott, D.K.; Neville, K.; Garg, U. Determination of hydroxyurea in serum or plasma using gas chromatography-mass spectrometry (GC-MS). Methods Mol. Biol. 2010, 603, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Adragna, N.C.; Fonseca, P.; Lauf, P.K. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994, 83, 553–560. [Google Scholar] [CrossRef]
- Cokic, V.P.; Smith, R.D.; Beleslin-Cokic, B.B.; Njoroge, J.M.; Miller, J.L.; Gladwin, M.T.; Schechter, A.N. Hydroxyurea induces fetal hemoglobin by the nitric oxide–dependent activation of soluble guanylyl cyclase. J. Clin. Investig. 2003, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, C.; Kiss, N.; Naqeshbandi, A.F.; Tosti, G.; Tofani, S.; Cartoni, C.; Carmosino, I.; Cantoresi, F. Nonmelanoma skin cancer associated with Hydroxyurea treatment: Overview of the literature and our own experience. Dermatol. Ther. 2019, 32, e13043. [Google Scholar] [CrossRef] [PubMed]
- Contreras Castillo, S.; Montibus, B.; Rocha, A.; Duke, W.; von Meyenn, F.; McLornan, D.; Harrison, C.; Mullally, A.; Schulz, R.; Oakey, R.J. Hydroxycarbamide effect on DNA methylation and gene expression in myeloproliferative neoplasms. Genome Res. 2021, 9, gr-27006. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musiałek, M.W.; Rybaczek, D. Hydroxyurea—The Good, the Bad and the Ugly. Genes 2021, 12, 1096. https://doi.org/10.3390/genes12071096
Musiałek MW, Rybaczek D. Hydroxyurea—The Good, the Bad and the Ugly. Genes. 2021; 12(7):1096. https://doi.org/10.3390/genes12071096
Chicago/Turabian StyleMusiałek, Marcelina W., and Dorota Rybaczek. 2021. "Hydroxyurea—The Good, the Bad and the Ugly" Genes 12, no. 7: 1096. https://doi.org/10.3390/genes12071096
APA StyleMusiałek, M. W., & Rybaczek, D. (2021). Hydroxyurea—The Good, the Bad and the Ugly. Genes, 12(7), 1096. https://doi.org/10.3390/genes12071096