Analyses of Insecticide Resistance Genes in Aedes aegypti and Aedes albopictus Mosquito Populations from Cameroon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Collection of Mosquito Larvae, Rearing and Processing
2.3. Insecticide Susceptibility Tests
2.4. Total Nucleic Acids (NAs) Extraction from Mosquito Pools and gDNA Extraction from Individual Mosquitoes
2.5. Genotyping of Mosquito Sample and Multiplex RT-qPCR for Gene Expression Analysis
2.6. Statistical Analysis
3. Results
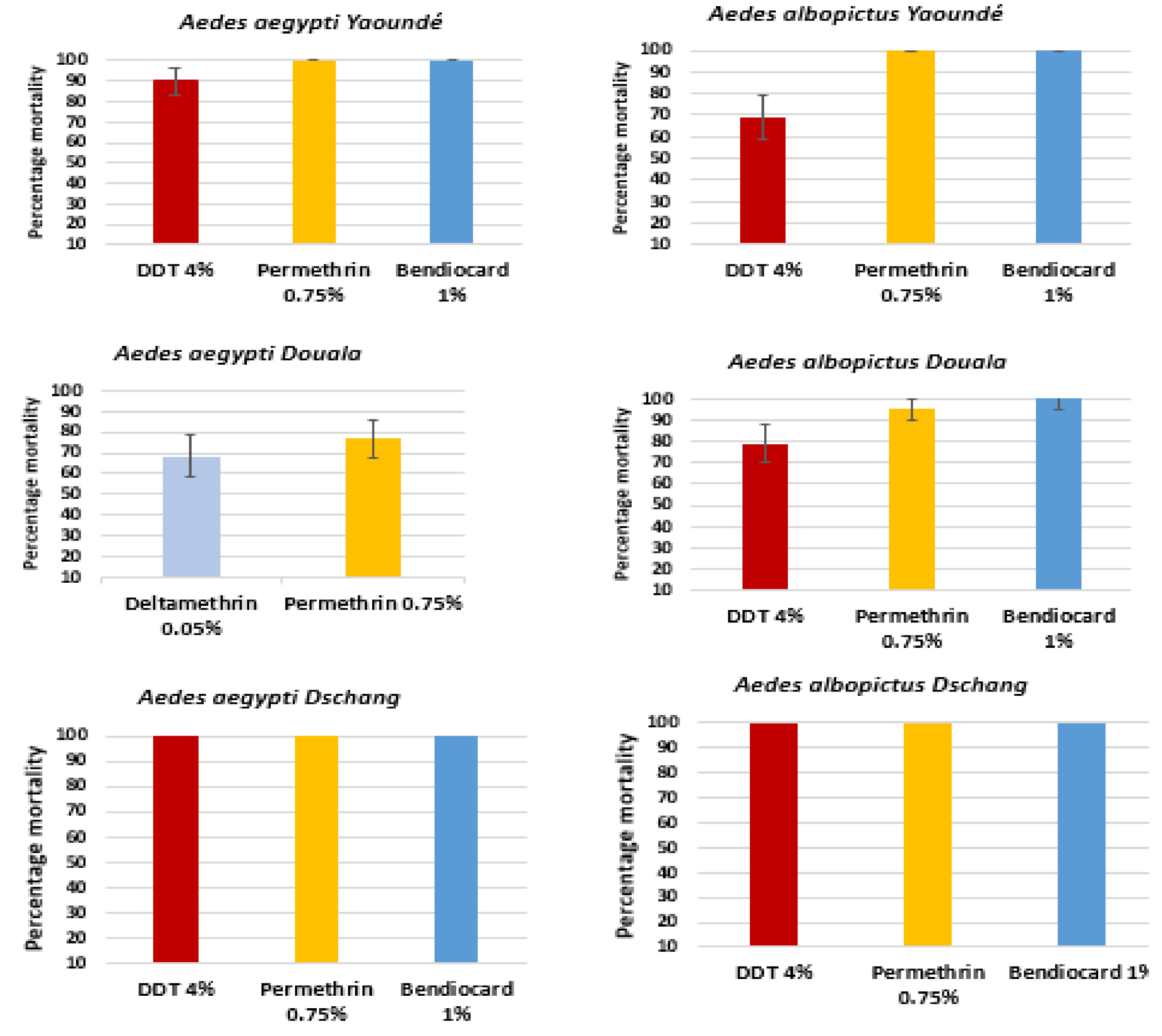
3.1. Insecticide Bioassays Results
3.2. Species Identification
3.3. Screening of Target Site Mutations (kdr F1534C, V1016G, V1016I and S989P)
3.4. Analysis of Detoxification Genes Expression Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuervo-Parra, J.A.; Cortés, T.R.; Ramirez-Lepe, M. Mosquito-Borne diseases, pesticides used for mosquito control, and development of resistance to insecticides. Insectic. Resist. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Aedes albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Toty, C.; Boyer, S.; Bouetard, A.; Bastien, F.; Fontenille, D. Evidence of habitat structuring Aedes albopictus populations in Réunion Island. PLoS Negl. Trop. Dis. 2013, 7, e2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayi, M.P.A.; Bamou, R.; Djiappi-Tchamen, B.; Fontaine, A.; Jeffries, C.L.; Walker, T.; Antonio-Nkondjio, C.; Cornel, A.J.; Tchuinkam, T. Habitat and seasonality affect mosquito community composition in the west region of Cameroon. Insects 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Barrera, R.; Makio, A.; Mutisya, J.; Koka, H.; Owaka, S.; Koskei, E.; Nyunja, A.; Eyase, F.; Coldren, R.; et al. Dengue outbreak in Mombasa City, Kenya, 2013–2014: Entomologic investigations. PLoS Negl. Trop. Dis. 2016, 10, e0004981. [Google Scholar] [CrossRef]
- Ayolabi, C.I.; Olusola, B.A.; Ibemgbo, S.A.; Okonkwo, G.O. Detection of dengue viruses among febrile patients in Lagos, Nigeria and phylogenetics of circulating dengue serotypes in Africa. Infect. Genet. Evol. 2019, 75, 103947. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Degroote, S.; Barro, S.A.; Some, A.; Bonnet, E.; Ridde, V. Épidémies recurrentes de la dengue au burkina faso: Préférences communautaires pour une intervention de prevention de la maladie. Rev. d’Epidémiologie St. Publique 2019, 67, 375–382. [Google Scholar] [CrossRef]
- Otu, A.; Udoh, U.; Ita, O.; Hicks, J.; Ukpeh, I.; Walley, J. Prevalence of Zika and Malaria in patients with fever in secondary healthcare facilities in South-Eastern Nigeria. Trop. Dr. 2020, 50, 22–30. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Rousset, D.; Pastorino, B.A.; Pouillot, R.; Bessaud, M.; Tock, F.; Mansaray, H.; Merle, O.L.; Pascual, A.M.; Paupy, C. Chikungunya virus, cameroon, 2006. Emerg. Infect. Dis. 2007, 13, 768–771. [Google Scholar] [CrossRef]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, pathogenesis, clinical features, management, and prevention. Infect. Dis. Clin. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Nkoghe, D.; Kassa, R.F.; Caron, M.; Grard, G.; Mombo, I.; Bikié, B.; Paupy, C.; Becquart, P.; Bisvigou, U.; Leroy, E.M. Clinical forms of chikungunya in Gabon, 2010. PLoS Negl. Trop. Dis. 2012, 6, e1517. [Google Scholar] [CrossRef]
- Proesmans, S.; Katshongo, F.; Milambu, J.; Fungula, B.; Mavoko, H.M.; Ahuka-Mundeke, S.; da Luz, R.I.; Esbroeck, M.V.; Ariën, K.K.; Cnops, L.; et al. Dengue and chikungunya among outpatients with acute undifferentiated fever in Kinshasa, Democratic Republic of Congo: A cross-sectional study. PLoS Negl. Trop. Dis. 2019, 13, e0007047. [Google Scholar] [CrossRef] [Green Version]
- Im, J.; Balasubramanian, R.; Ouedraogo, M.; Wandji Nana, L.R.; Mogeni, O.D.; Jeon, H.J.; van Pomeren, T.; Haselbeck, A.; Lim, J.K.; Prifti, K.; et al. The epidemiology of dengue outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef] [Green Version]
- Namountougou, M.; Soma, D.; Balboné, M.; Kaboré, D.; Kientega, M.; Hien, A.; Coulibaly, A.; Ouattara, P.; Meda, B.; Drabo, S.; et al. Monitoring insecticide susceptibility in Aedes aegypti populations from the two biggest cities, Ouagadougou and Bobo-Dioulasso, in Burkina Faso: Implication of metabolic resistance. Trop. Med. Infect. Dis. 2020, 5, 84. [Google Scholar] [CrossRef]
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization is a main driver for the larval ecology of Aedes Mosquitoes in arbovirus-endemic settings in South-Eastern Côte d’Ivoire. PLoS Negl. Trop. Dis. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Fagbohun, I.K.; Idowu, E.T.; Olakiigbe, A.K.; Oyeniyi, A.T.; Otubanjo, O.A.; Awolola, T.S. Metabolic resistance mechanism in Aedes aegypti from Lagos State, Nigeria. J. Basic Appl. Zool. 2020, 81, 59. [Google Scholar] [CrossRef]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl. Trop. Dis. 2019, 13, e0007137. [Google Scholar] [CrossRef] [Green Version]
- Fontenille, D.; Toto, J.C. Aedes (Stegomyia) albopictus (Skuse), vecteur potentiel du virus de la dengue, a envahi les villes du sud du Cameroun. Bull. Société Pathol. Exot. 2003, 96, 1. [Google Scholar]
- Demanou, M.; Pouillot, R.; Grandadam, M.; Boisier, P.; Kamgang, B.; Hervé, J.P.; Rogier, C.; Rousset, D.; Paupy, C. Evidence of dengue virus transmission and factors associated with the presence of anti-dengue virus antibodies in humans in three major towns in Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2950. [Google Scholar] [CrossRef]
- Yousseu, F.B.S.; Nemg, F.B.S.; Ngouanet, S.A.; Mekanda, F.M.O.; Demanou, M. Detection and serotyping of dengue viruses in febrile patients consulting at the New-Bell District Hospital in Douala, Cameroon. PLoS ONE 2018, 13, e0204143. [Google Scholar] [CrossRef]
- Tchuandom, S.B.; Tchadji, J.C.; Tchouangueu, T.F.; Biloa, M.Z.; Atabonkeng, E.P.; Fumba, M.I.M.; Massom, E.S.; Nchinda, G.; Kuiate, J.-R. A Cross-sectional study of acute dengue infection in paediatric clinics in Cameroon. BMC Public Health 2019, 19, 958. [Google Scholar] [CrossRef] [Green Version]
- Tchuandom, S.B.; Tchouangueu, T.F.; Antonio-Nkondjio, C.; Lissom, A.; Djang, J.O.N.; Atabonkeng, E.P.; Kechia, A.; Nchinda, G.; Kuiate, J.-R. Seroprevalence of dengue virus among children presenting with febrile illness in some public health facilities in Cameroon. Pan Afr. Med. J. 2018, 31. [Google Scholar] [CrossRef]
- Simo, F.B.N.; Yousseu, F.B.S.; Mbarga, A.E.; Bigna, J.J.; Melong, A.; Ntoude, A.; Kamgang, B.; Bouyne, R.; Fewou, P.M.; Demanou, M. Investigation of an outbreak of dengue virus serotype 1 in a rural area of Kribi, South Cameroon: A cross-sectional study. Intervirology 2018, 61, 265–271. [Google Scholar] [CrossRef]
- Gake, B.; Vernet, M.A.; Leparc-Goffart, I.; Drexler, J.F.; Gould, E.A.; Gallian, P.; de Lamballerie, X.; Gake, B.; Vernet, M.A.; Leparc-Goffart, I.; et al. Low seroprevalence of zika virus in cameroonian blood donors. Braz. J. Infect. Dis. 2017, 21, 481–483. [Google Scholar] [CrossRef]
- Abramides, G.C.; Roiz, D.; Guitart, R.; Quintana, S.; Guerrero, I.; Giménez, N. Effectiveness of a multiple intervention strategy for the control of the tiger mosquito (Aedes albopictus) in Spain. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, D.M.; Unlu, I.; Crepeau, T.; Farajollahi, A.; Healy, S.P.; Bartlett-Healy, K.; Strickman, D.; Gaugler, R.; Hamilton, G.; Kline, D.; et al. Area-wide management of Aedes albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest. Manag. Sci. 2013, 69, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2009 Dengue Guidelines for Diagnosis, Traitement, Prevention and Control., TDR for Research on Diseases and Poverty, new ed.; WHO: Geneva, Switzerland, 2009; p. 160. [Google Scholar]
- Buhler, C.; Winkler, V.; Runge-Ranzinger, S.; Boyce, R.; Horstick, O. Environmental methods for dengue vector control—A systematic review and meta-analysis. PLoS Negl. Trop. Dis 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-Borne diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.; Mutalip, H.; Lodz, N.A.; Muhammad, E.; Yoep, N.; Hasim, H.; Paiwai, F.; Rajarethinam, J.; Aik, J.; Muhammad, N. Environmental management for dengue control: A systematic review protocol. BMJ Open 2019, 9, e026101. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Ndo, C.; Njiokou, F.; Bigoga, J.D.; Awono-Ambene, P.; Etang, J.; Ekobo, A.S.; Wondji, C.S. Review of malaria situation in Cameroon: Technical viewpoint on challenges and prospects for disease elimination. Parasites Vectors 2019, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines for Malaria Vector Control; WHO: Geneva, Switzerland, 2019; p. 171. [Google Scholar]
- Nchoutpouen, E.; Abdou, T.; Djiappi-Tchamen, B.; Djamouko-Djonkam, L.; Kopya, E.; Ngadjeu, C.; Doumbe, B.; Awono-Ambene, P.; Kekeunou, S.; Wondji, C.; et al. Culex species diversity, susceptibility to insecticides and role as potential vector of lymphatic filariasis in the city of Yaoundé, Cameroon. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasites Vectors 2017, 10, 469. [Google Scholar] [CrossRef]
- Ngo, O.; Akono, P.; Ngo, J.; Nko’o, E.; Tonga, C.; Foko, G.; Kekeunou, S. Adaptation compétitive d’Aedes albopictus Skuse, 1894 en présence d’Aedes aegypti Linné, 1862 dans quelques gîtes larvaires temporaires de la ville de Douala (Cameroun) dans un contexte de résistance aux pyréthrinoïdes. Bull. Soc. Pathol. Exot. 2020, 9. [Google Scholar]
- Seixas, G.; Grigoraki, L.; Weetman, D.; Vicente, J.L.; Silva, A.C.; Pinto, J.; Vontas, J.; Sousa, C.A. Insecticide resistance is mediated by multiple mechanisms in recently introduced Aedes aegypti from Madeira Island (Portugal). PLoS Negl. Trop. Dis. 2017, 11, e0005799. [Google Scholar] [CrossRef] [Green Version]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors 2013, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Sathantriphop, S.; White, S.A.; Achee, N.L.; Sanguanpong, U. Chareonviriyaphap, eeraphap behavioral responses of Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles minimus against various synthetic and natural repellent compounds. J. Vector Ecol. 2014, 39. [Google Scholar] [CrossRef]
- Wood, O.; Hanrahan, S.; Coetzee, M.; Koekemoer, L.; Brooke, B. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasites Vectors 2010, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Yahouédo, G.A.; Chandre, F.; Rossignol, M.; Ginibre, C.; Balabanidou, V.; Mendez, N.G.A.; Pigeon, O.; Vontas, J.; Cornelie, S. Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci. Rep. 2017, 7, 11091. [Google Scholar] [CrossRef] [Green Version]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Simma, E.A.; Dermauw, W.; Balabanidou, V.; Snoeck, S.; Bryon, A.; Clark, R.M.; Yewhalaw, D.; Vontas, J.; Duchateau, L.; Leeuwen, T.V. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with pyrethroid resistance in Anopheles arabiensis populations from Ethiopia. Pest. Manag. Sci. 2018. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in Mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Hemingway, J.; Field, L.; Vontas, J. An overview of insecticide resistance. Science 2002, 298, 96–97. [Google Scholar] [CrossRef]
- Ranson, H.; N’guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African Anopheline Mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef]
- Brogdon, W.; Mcallister, J. Insecticide resistance and vector control. Emerg. Infect. Dis. 2004, 4, 605–613. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione s-transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, S.; Komagata, O.; Itokawa, K.; Shono, T.; Ng, L.C.; Kobayashi, M.; Tomita, T. Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: Target site insensitivity, penetration, and metabolism. PLoS Negl. Trop. Dis. 2014, 8, e2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Sears, C.; Sun, H.; Mertz, R.W.; Kasai, S.; Scott, J.G. CYP-mediated resistance and cross-resistance to pyrethroids and organophosphates in Aedes aegypti in the presence and absence of Kdr. Pestic. Biochem. Physiol. 2019, 160, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes Mosquito Vectors: Muddying the waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef]
- Ishak, I.H.; Riveron, J.M.; Ibrahim, S.S.; Stott, R.; Longbottom, J.; Irving, H.; Wondji, C.S. the cytochrome P450 gene CYP6P12 confers pyrethroid resistance in Kdr-free Malaysian populations of the Dengue Vector Aedes albopictus. Sci. Rep. 2016, 6, 24707. [Google Scholar] [CrossRef] [Green Version]
- Yougang, A.P.; Kamgang, B.; Bahun, T.A.W.; Tedjou, A.N.; Nguiffo-Nguete, D.; Njiokou, F.; Wondji, C.S. First detection of f1534c knockdown resistance mutation in Aedes aegypti (Diptera: Culicidae) from Cameroon. Infect. Dis. Poverty 2020, 9, 152. [Google Scholar] [CrossRef]
- Yougang, P.; Kamgang, B.; Armel, T.; Wilson-Bahun, T.; Njiokou, F.; Wondji, C. Nationwide profiling of insecticide resistance in Aedes albopictus (Diptera: Culicidae) in Cameroon. PLoS ONE 2020, 15, e0234572. [Google Scholar] [CrossRef]
- Jupp, P. Mosquitoes of Southern Africa; Ekogilde Publishers: Johannesburg, South Africa, 1996; p. 156. [Google Scholar]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors Mosquitoes, 2nd ed; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Mavridis, K.; Wipf, N.; Medves, S.; Erquiaga, I.; Müller, P.; Vontas, J. Rapid multiplex gene expression assays for monitoring metabolic resistance in the Major Malaria Vector Anopheles gambiae. Parasites Vectors 2019, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Kothera, L.; Byrd, B.; Savage, H.M. Duplex real-time PCR assay distinguishes Aedes aegypti from Aedes albopictus (Diptera: Culicidae) using DNA from Sonicated first-instar larvae. J. Med. Entomol. 2017, 54, 1567–1572. [Google Scholar] [CrossRef]
- Sene, N.; Mavridis, K.; Ndiaye, E.; Diagne, C.; Gaye, A.; Ngom, E.; Ba, Y.; Diallo, D.; Vontas, J.; Dia, I.; et al. Insecticide resistance status and mechanisms in Aedes aegypti populations from SenegaL. PLoS Negl. Trop. Dis. Accept. 2021. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic. Acids. Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 9, 36. [Google Scholar] [CrossRef]
- Kamgang, B.; Vazeille, M.; Yougang, A.P.; Tedjou, A.N.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Potential of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to transmit yellow fever virus in urban areas in central Africa. Emerg. Microbes Infect. 2019, 8, 1636–1641. [Google Scholar] [CrossRef] [Green Version]
- Kamgang, B.; Vazeille, M.; Tedjou, A.; Yougang, A.P.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Different Populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from central Africa are susceptible to zika virus infection. PLoS Negl. Trop. Dis. 2020, 14, e0008163. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Medina, J.; Chase, C.; Cardenas, G.; Mutebi, J.-P.; Petrie, W.D.; Beier, J.C. Proliferation of Aedes aegypti in urban environments mediated by the availability of key aquatic habitats. Sci. Rep. 2020, 10, 12925. [Google Scholar] [CrossRef]
- Dzib-Florez, S.; Ponce-García, G.; Che-Mendoza, A.; Medina-Barreiro, A.; Gray, L.; González-Olvera, G.; Delfin-Gonzalez, H.; Chan-Espinoza, D.; Vadillo-Sánchez, J.; del Castillo-Centeno, L.; et al. Bio-efficacy of commercially available residual insecticides for the control of Aedes aegypti in Mexico. J. Am. Mosq. Control Assoc. 2020, 36, 16–21. [Google Scholar] [CrossRef]
- Gray, L.; Florez, S.D.; Barreiro, A.M.; Vadillo-Sánchez, J.; González-Olvera, G.; Lenhart, A.; Manrique-Saide, P.; Vazquez-Prokopec, G.M. Experimental evaluation of the impact of household aerosolized insecticides on pyrethroid resistant Aedes aegypti. Sci. Rep. 2018, 8, 12535. [Google Scholar] [CrossRef] [PubMed]
- Ponlawat, A.; Scott, J.G.; Harrington, L.C. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J. Med. Entomol. 2005, 42, 821–825. [Google Scholar] [CrossRef]
- Ouattara, L.P.E.; Sangaré, I.; Namountougou, M.; Hien, A.; Ouari, A.; Soma, D.D.; Kassié, D.; Diabaté, A.; Gnankiné, O.; Bonnet, E.; et al. Surveys of arboviruses vectors in four cities stretching along a railway transect of burkina faso: Risk transmission and insecticide susceptibility status of potential vectors. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.-P.; Corbel, V.; Sutherland, I.W.; Hertz, J.C.; et al. Distribution of Insecticide Resistance and Mechanisms Involved in the Arbovirus vector Aedes aegypti in laos and implication for vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.; Chee Dhang, C.; Azirun, M.; Van Lun, L.L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasites Vectors 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- WHO. Monitoring and Managing Insecticide Resistance in Aedes Mosquito Populations Interim Guidance for Entomologists; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Badolo, A.; Sombié, A.; Pignatelli, P.M.; Sanon, A.; Yaméogo, F.; Wangrawa, D.W.; Sanon, A.; Kanuka, H.; McCall, P.J.; Weetman, D. Insecticide resistance levels and mechanisms in Aedes aegypti populations in and around Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007439. [Google Scholar] [CrossRef] [PubMed]
- Sombié, A.; Saiki, E.; Yaméogo, F.; Sakurai, T.; Shirozu, T.; Fukumoto, S.; Sanon, A.; Weetman, D.; McCall, P.J.; Kanuka, H.; et al. High frequencies of F1534C and V1016I Kdr mutations and association with pyrethroid resistance in Aedes aegypti from Somgandé (Ouagadougou), Burkina Faso. Trop. Med. Health 2019, 47, 2. [Google Scholar] [CrossRef] [PubMed]
- Kudom, A.A. Entomological surveillance to assess potential outbreak of aedes-borne arboviruses and insecticide resistance status of Aedes aegypti from Cape Coast, Ghana. Acta. Tropica. 2020, 202, 105257. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Higa, Y.; Futami, K.; Muranami, Y.; Kawashima, E.; Osei, J.H.N.; Sakyi, K.Y.; Dadzie, S.; de Souza, D.K.; Appawu, M.; et al. Discovery of point mutations in the voltage-gated sodium channel from African Aedes aegypti populations: Potential phylogenetic reasons for gene introgression. PLoS Negl. Trop. Dis. 2016, 10, e0004780. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, R.B.S.; Dykes, C.L.; Kapoor, N.; Adak, T.; Singh, O.P. Pyrethroid-resistance and presence of two knockdown resistance (Kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e3332. [Google Scholar] [CrossRef] [Green Version]
- Hirata, K.; Komagata, O.; Itokawa, K.; Yamamoto, A.; Tomita, T.; Kasai, S. A single crossing-over event in voltage-sensitive Na+ channel genes may cause critical failure of dengue mosquito control by insecticides. PLoS Negl. Trop. Dis. 2014, 8, e3085. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-X.; Kaufman, P.E.; Xue, R.-D.; Zhao, M.-H.; Wang, G.; Yan, T.; Guo, X.-X.; Zhang, Y.-M.; Dong, Y.-D.; Xing, D.; et al. Relationship between insecticide resistance and Kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasites Vectors 2015, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Kamgang, B.; Wilson-Bahun, T.A.; Yougang, A.P.; Lenga, A.; Wondji, C.S. Contrasting resistance patterns to Type I and II pyrethroids in two major arbovirus vectors Aedes aegypti and Aedes albopictus in the Republic of the Congo, Central Africa. Infect. Dis. Poverty 2020, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Ngoagouni, C.; Kamgang, B.; Brengues, C.; Yahouedo, G.; Paupy, C.; Nakouné, E.; Kazanji, M.; Chandre, F. Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic. Parasites Vectors 2016, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, K.; Wang, X.; Yang, X.; Lin, Y.; Cai, F.; Zhong, W.; Lin, C.; Lin, Z.; Ma, Y. First identification of Kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus Populations from Haikou City, Hainan Island, China. Infect. Dis. Poverty 2016, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Bonizzoni, M.; Zhong, D.; Zhou, G.; Cai, S.; Li, Y.; Wang, X.; Lo, E.; Lee, R.; Sheen, R.; et al. Multi-country survey revealed prevalent and novel F1534S mutation in Voltage-Gated Sodium Channel (VGSC) gene in Aedes albopictus. PLoS Negl. Trop. Dis. 2016, 10, e0004696. [Google Scholar] [CrossRef] [Green Version]
- Vera-Maloof, F.Z.; Saavedra-Rodriguez, K.; Elizondo-Quiroga, A.E.; Lozano-Fuentes, S.; Iv, W.C.B. Coevolution of the Ile1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl. Trop. Dis. 2015, 9, e0004263. [Google Scholar] [CrossRef]
- Stevenson, B.J.; Pignatelli, P.; Nikou, D.; Paine, M.J.I. Pinpointing p450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: Developing new tools to combat insecticide resistance. PLoS Negl. Trop. Dis. 2012, 6, e1595. [Google Scholar] [CrossRef] [Green Version]
- Pavlidi, N.; Monastirioti, M.; Daborn, P.; Livadaras, I.; Van Leeuwen, T.; Vontas, J. Transgenic expression of the Aedes aegypti CYP9J28 confers pyrethroid resistance in Drosophila Melanogaster. Pestic. Biochem. Physiol. 2012, 104, 132–135. [Google Scholar] [CrossRef]

| Yaoundé | Douala | Dschang | |
|---|---|---|---|
| Altitude above sea level | 726 m | 1 m | 1500 m |
| Population size | 2,765,568 | 2,768,436 | 301,385 |
| Surface area | 180 km2 | 210 km2 | 225 km2 |
| Landscape | Congo Guinean equatorial forest | Coastal area | Highland area |
| Annual rainfall | 1700 mm | 4000 to 5000 mm | 1364 mm |
| Population | Sample Size (Alleles) | Resistant Mutation Allelic Frequencies (Heterozygous/Homozygous Mosquitoes) | |||
|---|---|---|---|---|---|
| Pyrethroids/DDT | |||||
| % F1534C | %V1016G | % V1016I | % S989P | ||
| Yaoundé | 64 | 0.0 (0/0) | 0.0 (0/0) | 0.0 (0/0) | 0.0 (0/0) |
| Douala | 60 | 90.0 (6/24) | 1.7 (1/0) | 26.7 (14/1) | 0.0 (0/0) |
| Dschang | 20 | 60.0 (2/2) | 0.0 (0/0) | 60.0 (2/2) | 0.0 (0/0) |
| Cameroon Ae. aegypti susceptible strain | 40 | 0.0 (0/0) | 0.0 (0/0) | 0.0 (0/0) | 0.0 (0/0) |
| Population | Sample Size (Alleles) | Resistant Mutation Allelic Frequencies (Heterozygous/Homozygous Mosquitoes) |
|---|---|---|
| Pyrethroids/DDT | ||
| % F1534C | ||
| Yaoundé | 48 | 0.0 (0/0) |
| Douala | 48 | 2.08 (1/0) |
| Dschang | 48 | 0.0 (0/0) |
| Cameroon Ae. albopictus susceptible strain | 40 | 0.0 (0/0) |
| Populations | Detoxification Gene Fold Changes (95% CI), p Value | ||||||
|---|---|---|---|---|---|---|---|
| Cyp6BB2 | Cyp9J26 | GSTD4 | CCEae3a | Cyp9J28 | Cyp9M6 | Cyp9J32 | |
| Yaoundé | 1.09 (0.773–1.48) p = 0.509 | 1.91 (0.936–3.88) p= 0.075 | 10.1 (0.630–39.0) p = 0.101 | 1.59 (0.961–2.61) p = 0.071 | 4.67 * (2.84–7.55) p < 0.001 | 1.56 * (1.19–2.13) p < 0.001 | 3.58 * (2.82–4.72) p = 0.032 |
| Douala | 0.556 (0.364–0.853) p < 0.001 | 1.621 (0.697–3.48) p = 0.298 | 9.34 * (1.34–55.3) p = 0.030 | 0.503 (0.251–1.01) p = 0.052 | 3.57 * (2.23–7.03) p < 0.001 | 1.88 * (1.49–2.59) p < 0.001 | 1.98 * (1.29–3.75) p < 0.001 |
| Dschang | 0.335 (0.122–0.890) p < 0.001 | 0.368 (0.235–0.649) p < 0.001 | 12.1 (0.840–40) p = 0.198 | 1.04 (0.900–1.183) p = 0.507 | 0.762 (0.251–2.27) p = 0.695 | 0.851 (0.655–1.14) p = 0.285 | 1.37 (0.518–3.74) p = 0.714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djiappi-Tchamen, B.; Nana-Ndjangwo, M.S.; Mavridis, K.; Talipouo, A.; Nchoutpouen, E.; Makoudjou, I.; Bamou, R.; Mayi, A.M.P.; Awono-Ambene, P.; Tchuinkam, T.; et al. Analyses of Insecticide Resistance Genes in Aedes aegypti and Aedes albopictus Mosquito Populations from Cameroon. Genes 2021, 12, 828. https://doi.org/10.3390/genes12060828
Djiappi-Tchamen B, Nana-Ndjangwo MS, Mavridis K, Talipouo A, Nchoutpouen E, Makoudjou I, Bamou R, Mayi AMP, Awono-Ambene P, Tchuinkam T, et al. Analyses of Insecticide Resistance Genes in Aedes aegypti and Aedes albopictus Mosquito Populations from Cameroon. Genes. 2021; 12(6):828. https://doi.org/10.3390/genes12060828
Chicago/Turabian StyleDjiappi-Tchamen, Borel, Mariette Stella Nana-Ndjangwo, Konstantinos Mavridis, Abdou Talipouo, Elysée Nchoutpouen, Idene Makoudjou, Roland Bamou, Audrey Marie Paul Mayi, Parfait Awono-Ambene, Timoléon Tchuinkam, and et al. 2021. "Analyses of Insecticide Resistance Genes in Aedes aegypti and Aedes albopictus Mosquito Populations from Cameroon" Genes 12, no. 6: 828. https://doi.org/10.3390/genes12060828
APA StyleDjiappi-Tchamen, B., Nana-Ndjangwo, M. S., Mavridis, K., Talipouo, A., Nchoutpouen, E., Makoudjou, I., Bamou, R., Mayi, A. M. P., Awono-Ambene, P., Tchuinkam, T., Vontas, J., & Antonio-Nkondjio, C. (2021). Analyses of Insecticide Resistance Genes in Aedes aegypti and Aedes albopictus Mosquito Populations from Cameroon. Genes, 12(6), 828. https://doi.org/10.3390/genes12060828







