Molecular Cloning and Functional Analysis of 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase from Santalum album
Abstract
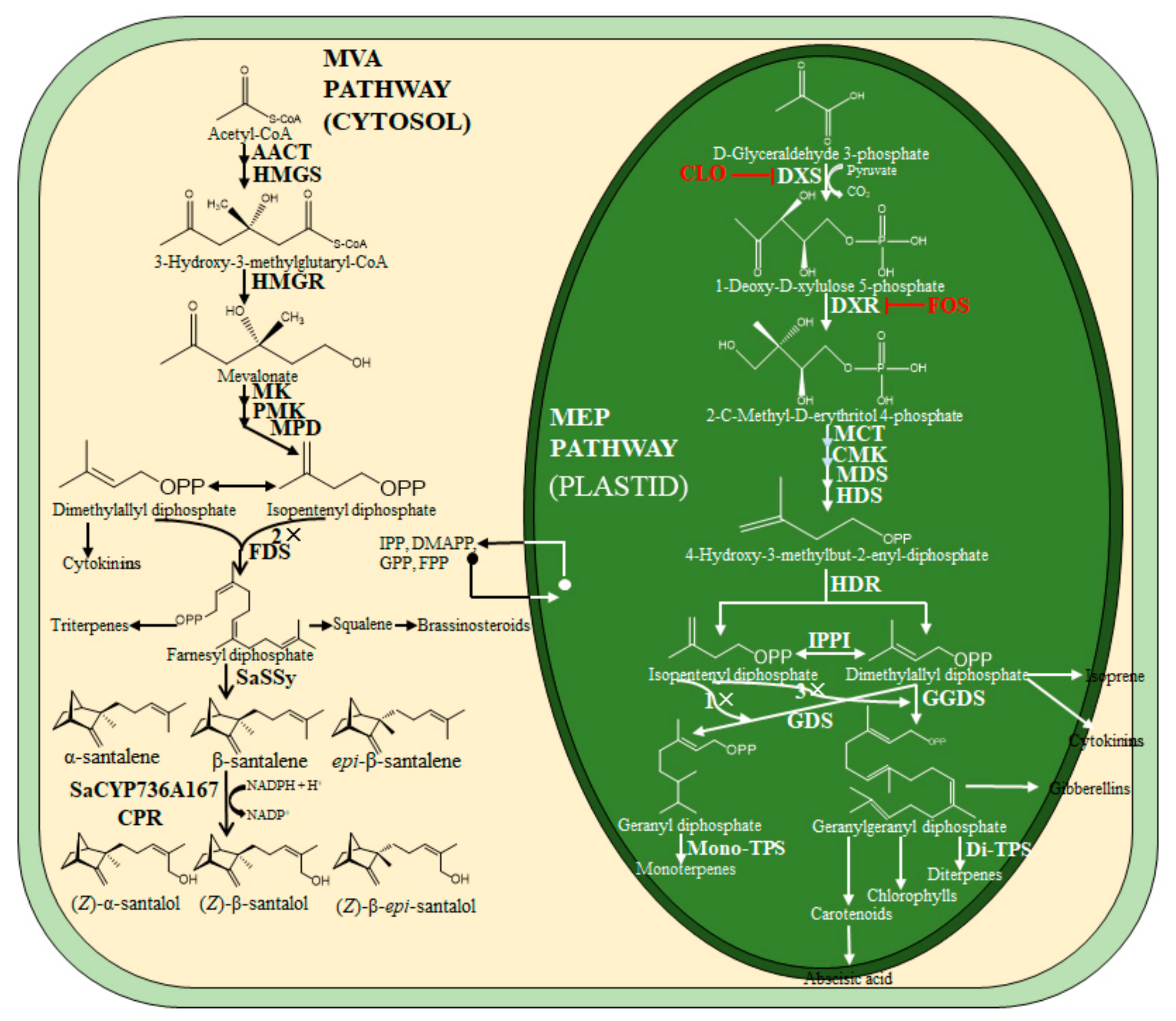
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Isolation Full-Length cDNA and Genomic DNA of SaDXR from S. album
2.3. Sequence, Protein Structure and Phylogenetics Analysis
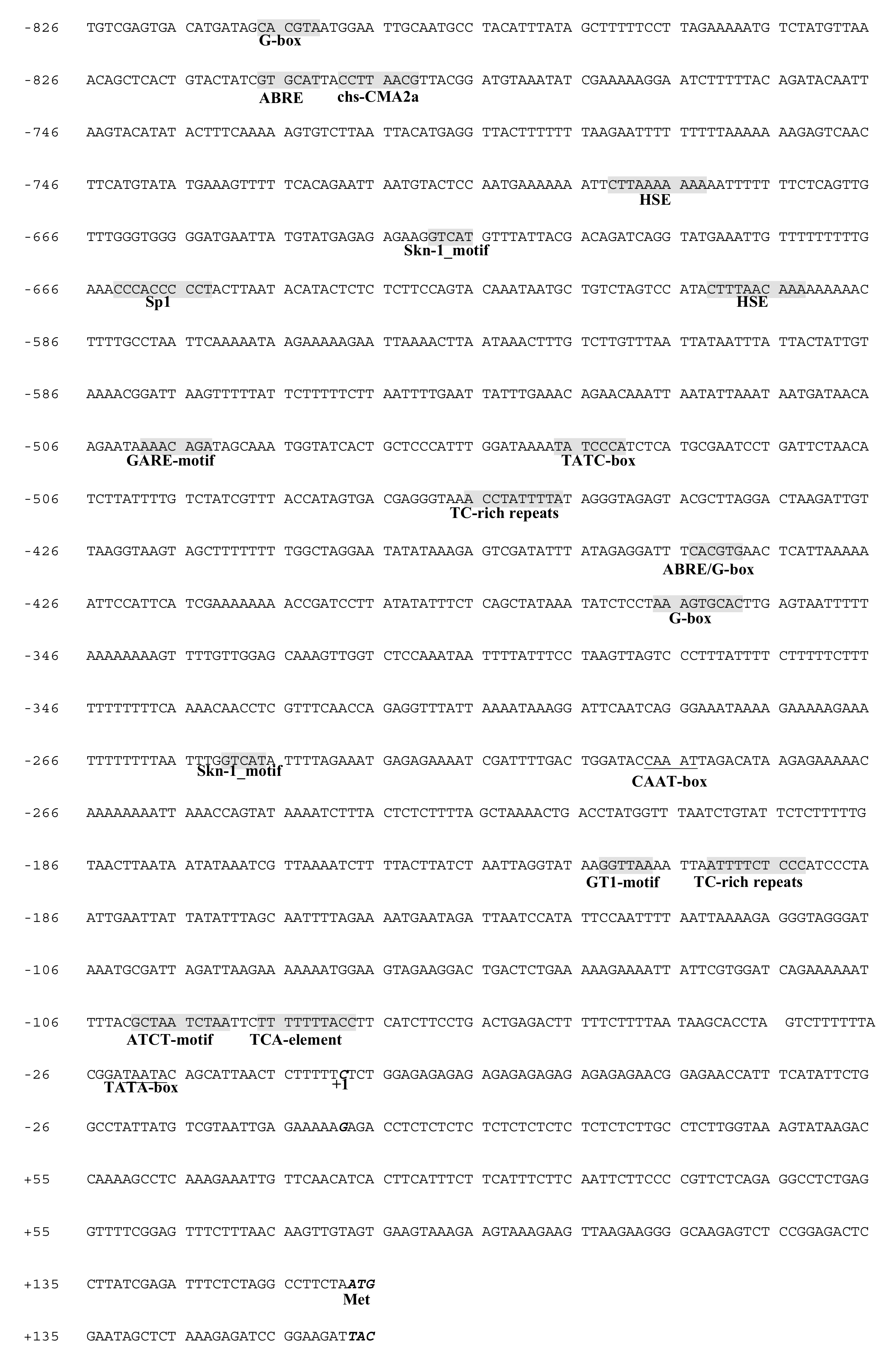
2.4. Cloning of the SaDXR Promoter and Putative cis-Element Analysis
2.5. Subcellular Localization of SaDXR
2.6. Quantitative Real-Time PCR (qRT-PCR) and Semi-Quantitative RT-PCR Analysis of SaDXR Gene Expression Profiles
2.7. Metabolite Extraction and Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
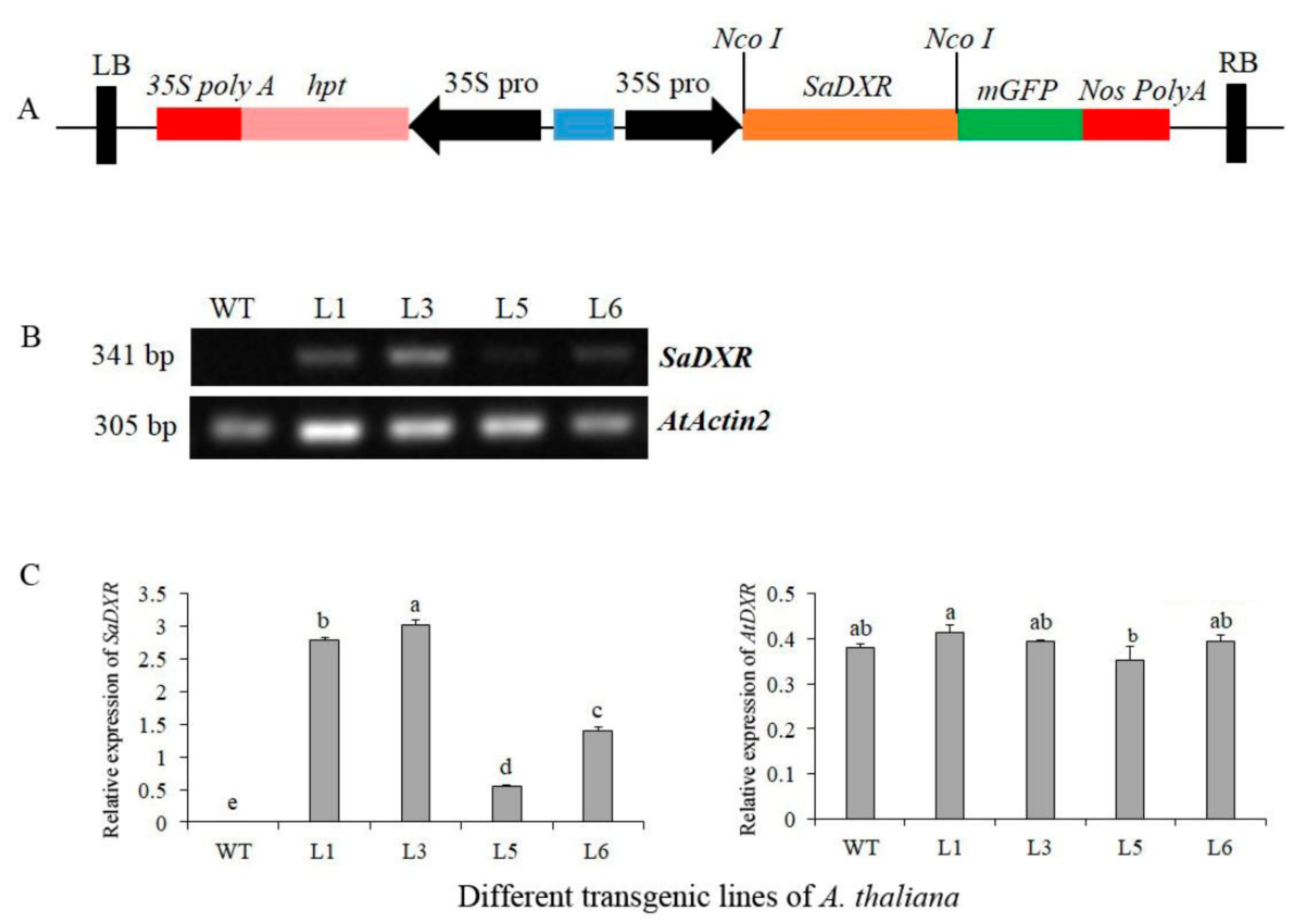
2.8. Construction of Plant Overexpression Vector, Transformation and Regeneration of A. thaliana
2.9. Contents of Chlorophyll and Carotenoids in Transgenic A. thaliana Lines
2.10. Statistical Analysis
3. Results
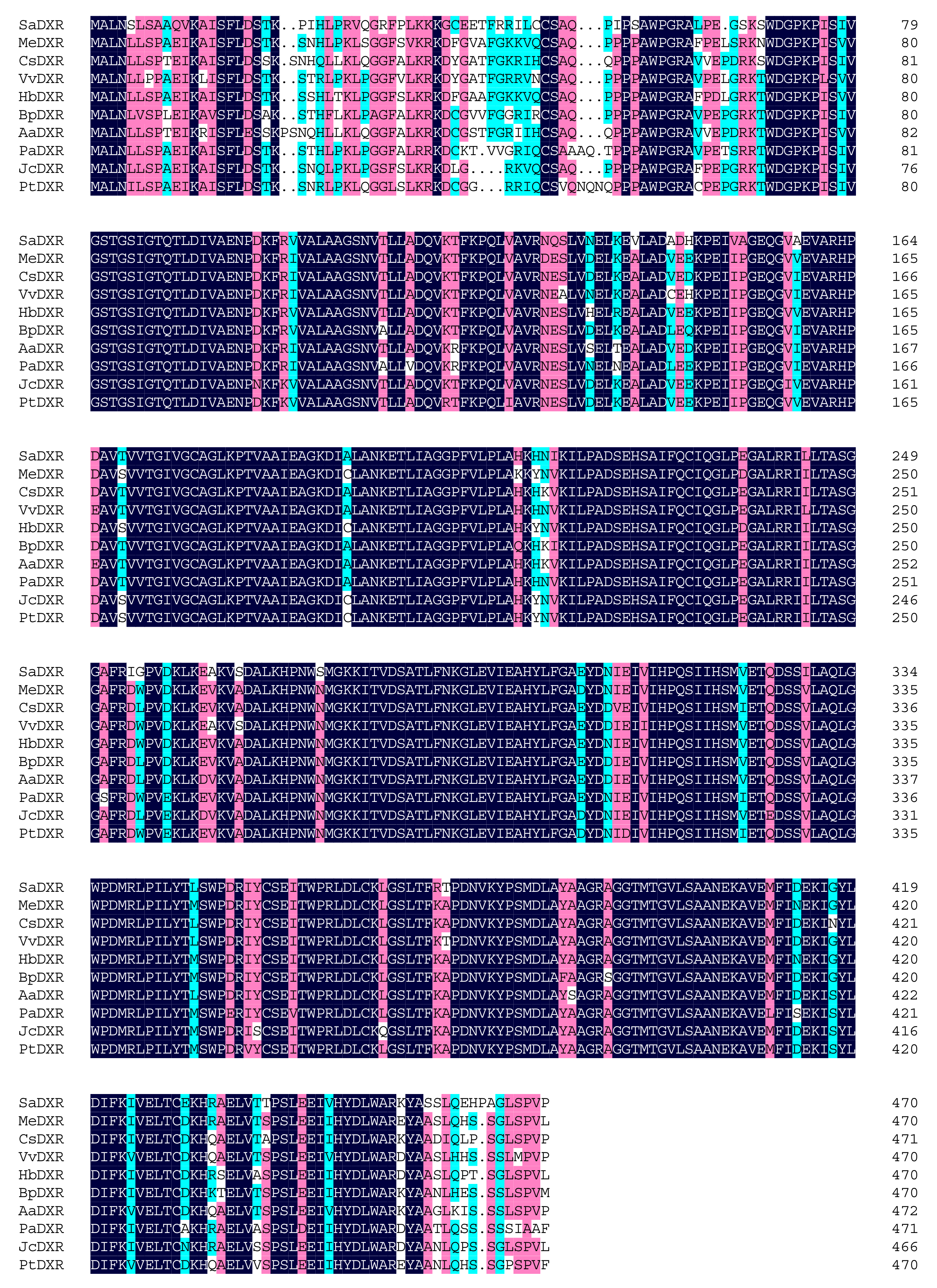
3.1. Molecular Cloning and Sequence Analysis of the Full Length SaDXR
3.2. Analysis of the SaDXR Gene
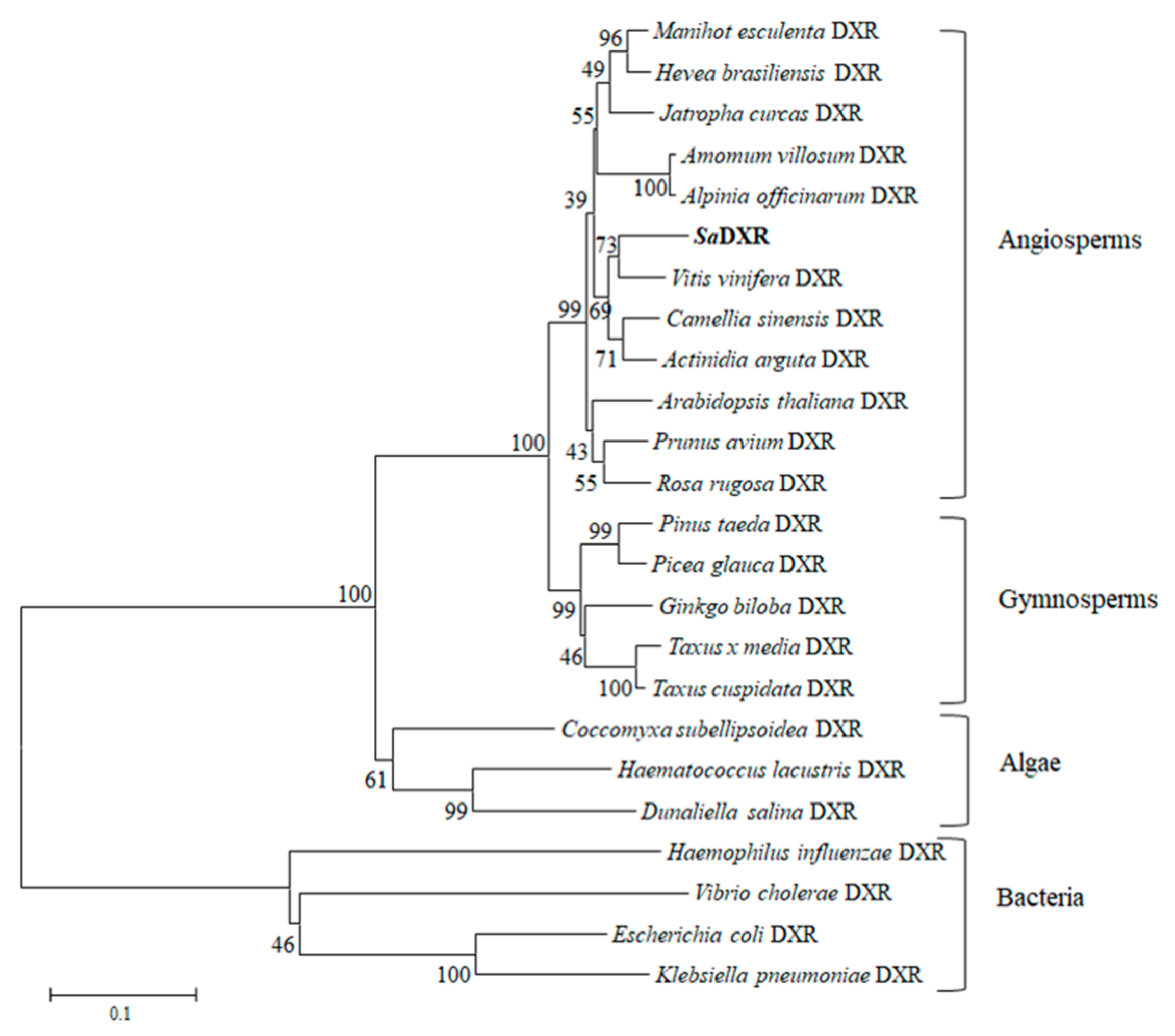
3.3. Protein Structure and Evolutionary Analysis of SaDXR
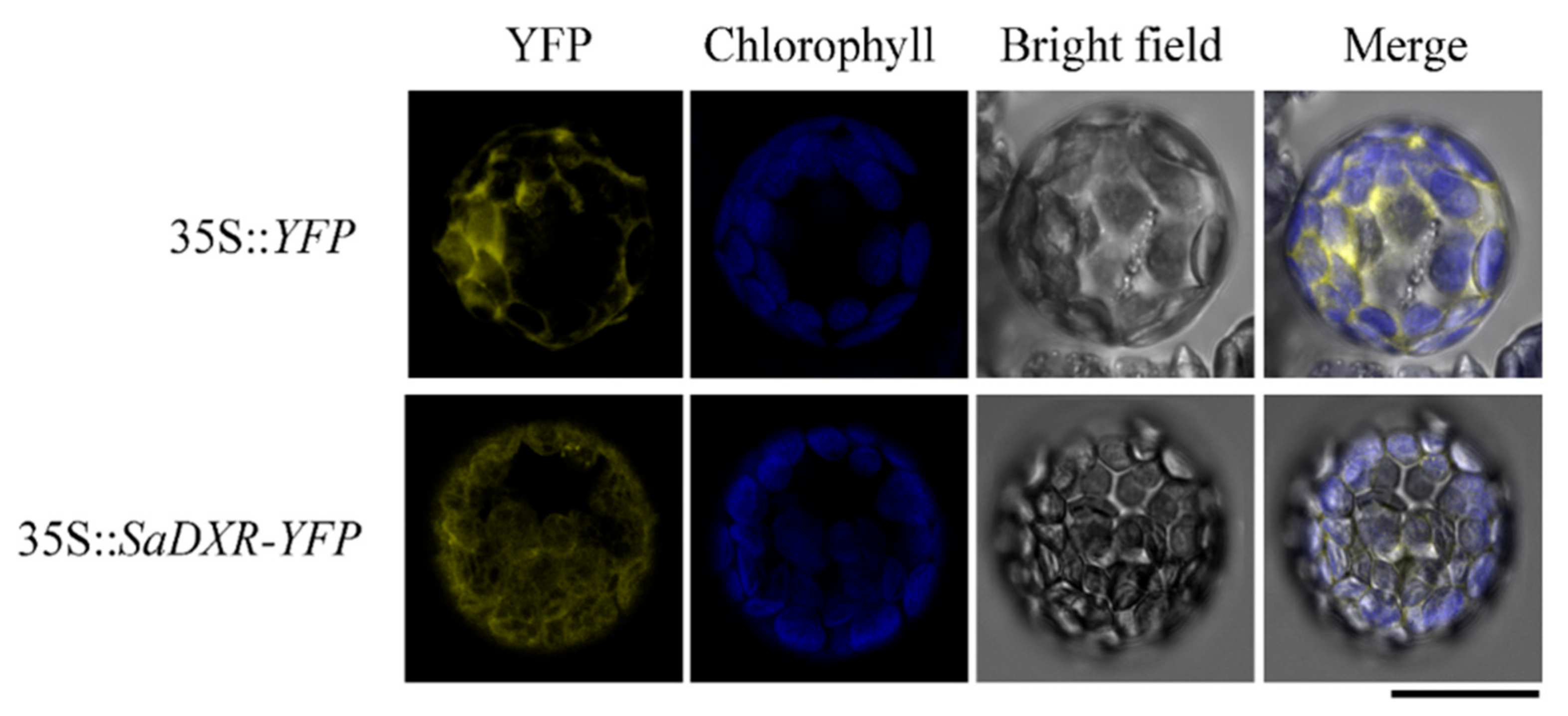
3.4. SaDXR Is Localized in Chloroplasts
3.5. Isolation and Characterization of the SaDXR Promoter
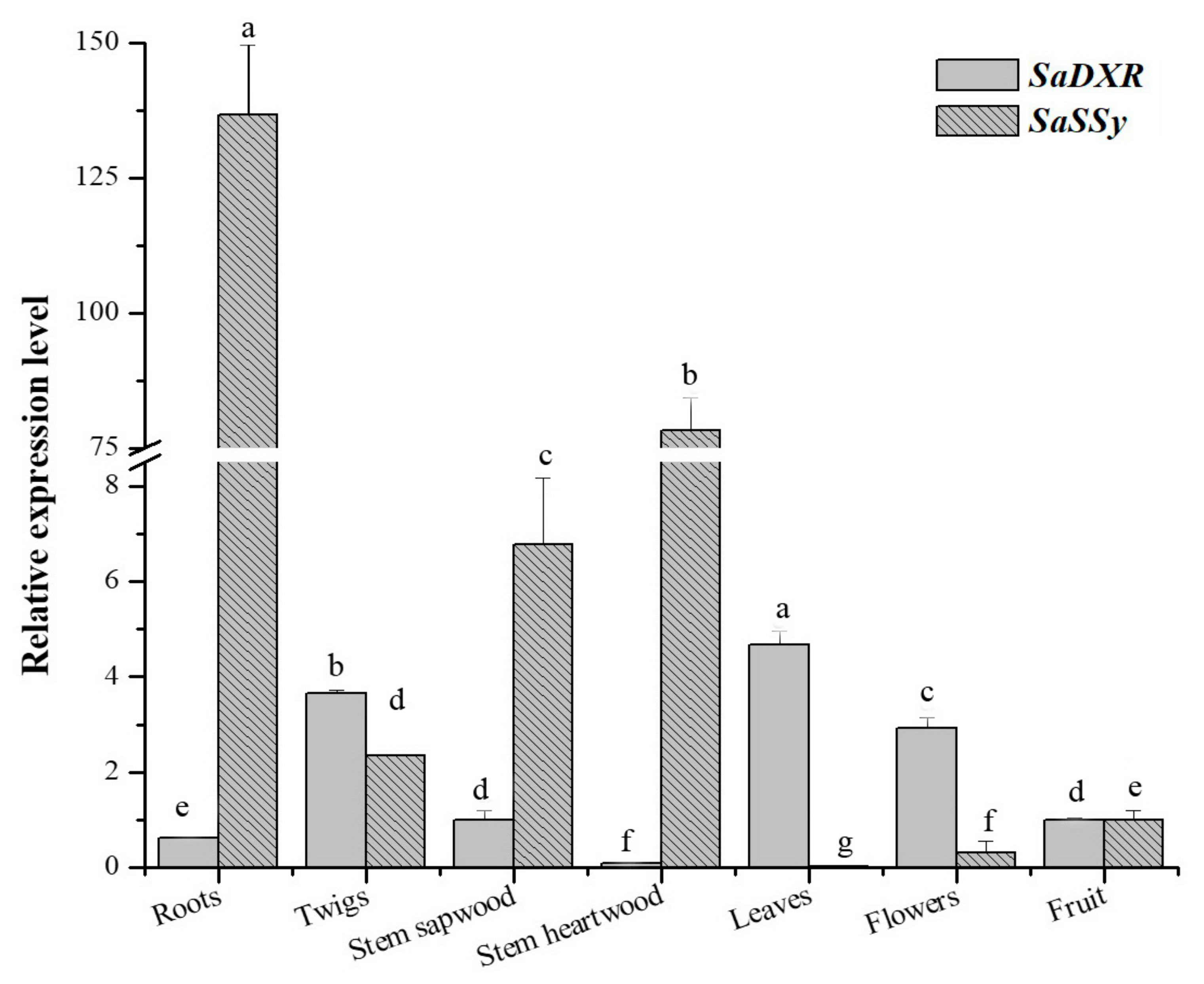
3.6. Tissue-Specific Expression of SaDXR
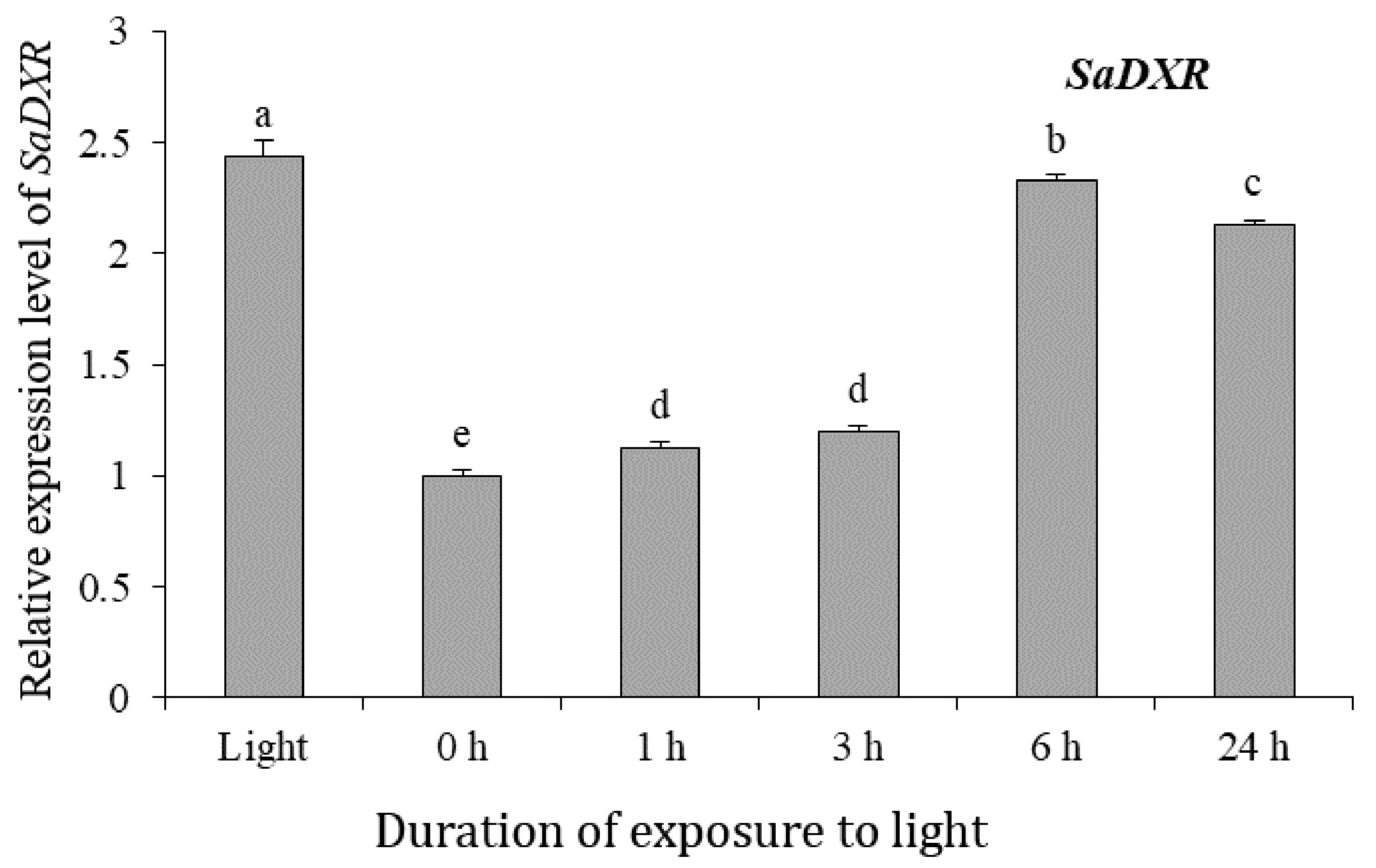
3.7. Expression of SaDXR in Response to Light
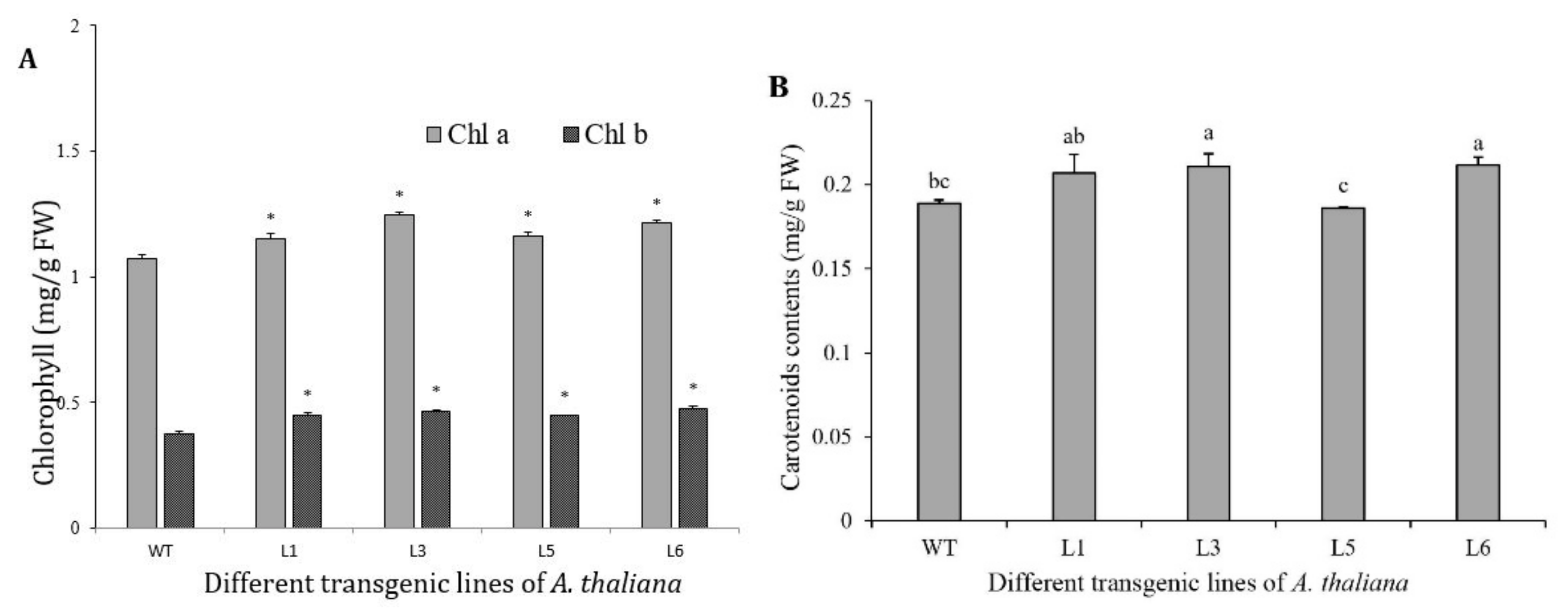
3.8. Chlorophylls and Carotenoids Increased in 35S::SaDXR Overexpression Arabidopsis Lines
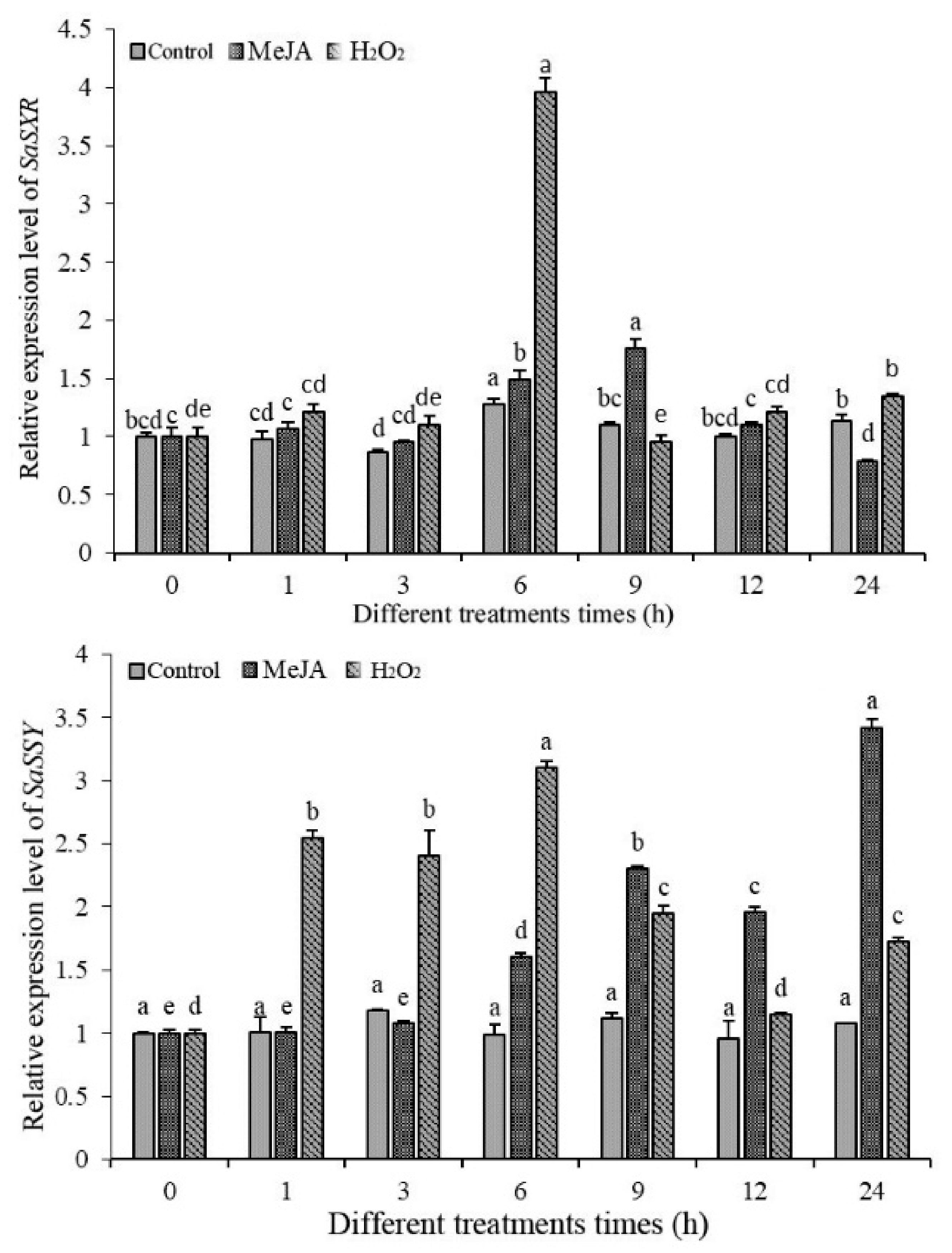
3.9. Transcript Profiles of SaDXR under MeJA, H2O2
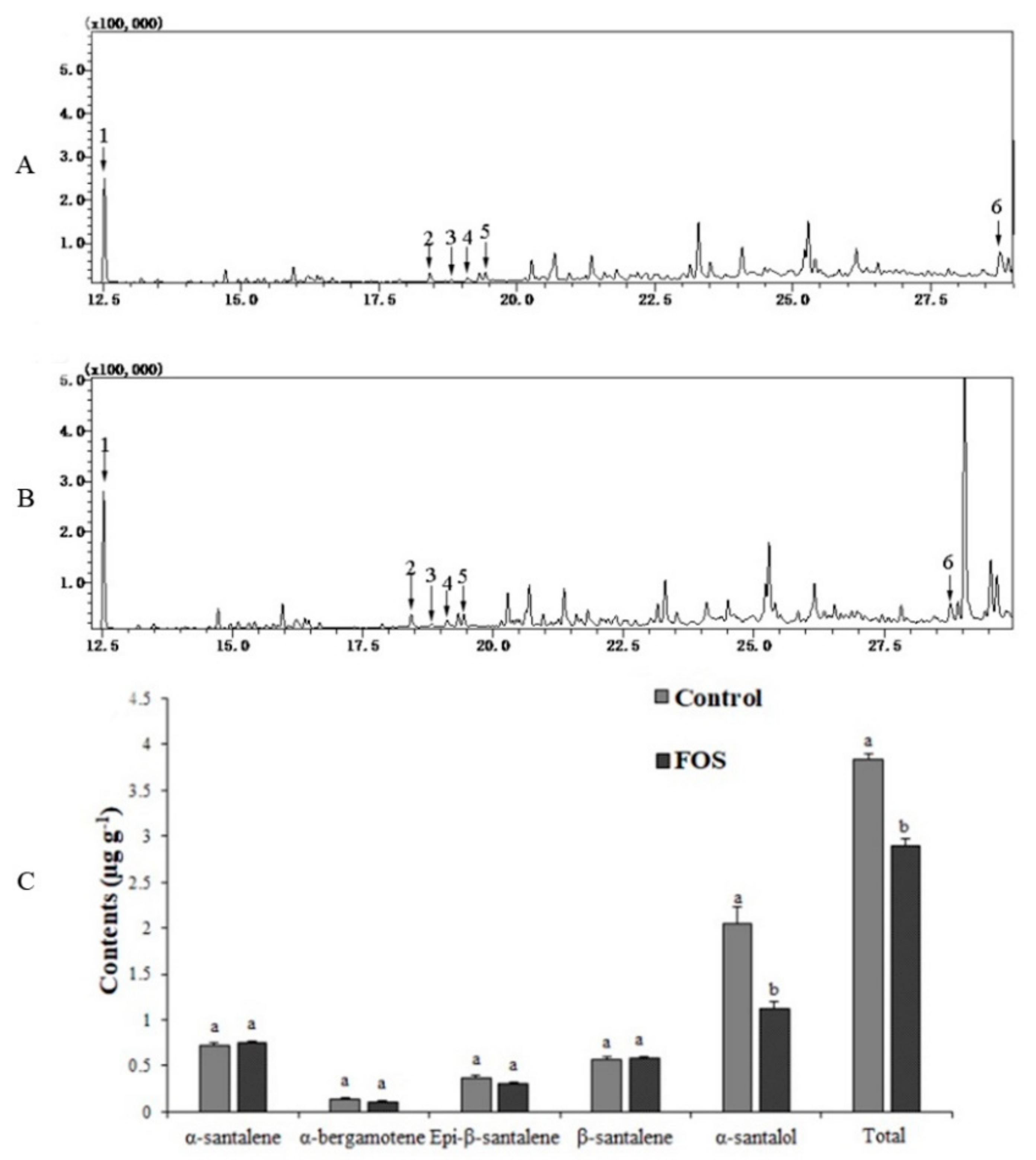
3.10. Metabolite Analysis in the FOS Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misra, B.B.; Dey, S. Developmental variations in sesquiterpenoid biosynthesis in East Indian sandalwood tree (Santalum album L.). Trees 2013, 27, 1071–1086. [Google Scholar] [CrossRef]
- Baldovini, N.; Delasalle, C.; Joulain, D. Phytochemistry of the heartwood from fragrant Santalum species: A review. Flav. Frag. J. 2011, 26, 7–26. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yan, H.F.; Niu, M.Y.; Cheng, Q.W.; Zhang, X.H.; Teixeira da Silva, J.A.; Ma, G.H. Multiple strategies for increasing yields of essential oil and obtaining sandalwood terpenoids by biotechnological methods in sandalwood. Trees 2018, 32, 17–28. [Google Scholar] [CrossRef]
- Wang, C.L.; Kim, S.W. Shaking up ancient scents: Insights into santalol synthesis in engineered Escherichia coli. Proc. Biochem. 2015, 50, 1177–1183. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, Y.H.; Chen, D.F.; Simonsen, H.T. Metabolic engineering of the moss Physcomitrella patens to produce the sesquiterpenoids patchoulol and alpha/beta-santalene. Front. Plant Sci. 2014, 5, 636. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Ann. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotech. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Perello, C.; Llamas, E.; Burlat, V.; Ortiz-Alcaide, M.; Phillips, M.A.; Pulido, P.; Rodriguez-Concepcion, M. Differential subplastidial localization and turnover of enzymes involved in isoprenoid biosynthesis in chloroplasts. PLoS ONE 2016, 11, e0150539. [Google Scholar]
- Hemmerlin, A.; Hoeffler, J.F.; Meyer, O.; Tritsch, D.; Kagan, I.A.; Grosdemange-Billiard, C.; Rohmer, M.; Bach, T.J. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 2003, 278, 26666–26676. [Google Scholar] [CrossRef]
- Jones, C.G.; Keeling, C.I.; Ghisalberti, E.L.; Barbour, E.L.; Plummer, J.A.; Bohlmann, J. Isolation of cDNAs and functional characterisation of two multi-product terpene synthase enzymes from sandalwood, Santalum album L. Arch. Biochem. Biophys. 2008, 477, 121–130. [Google Scholar] [CrossRef]
- Jones, C.G.; Moniodis, J.; Zulak, K.G.; Scaffidi, A.; Plummer, J.A.; Ghisalberti, E.L.; Barbour, E.L.; Bohlmann, J. Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J. Biol. Chem. 2011, 286, 17445–17454. [Google Scholar] [CrossRef]
- Rani, A.; Ravikumar, P.; Reddy, M.D.; Kush, A. Molecular regulation of santalol biosynthesis in Santalum album L. Gene 2013, 527, 642–648. [Google Scholar] [CrossRef]
- Diaz-Chavez, M.L.; Moniodis, J.; Madilao, L.L.; Jancsik, S.; Keeling, C.I.; Barbour, E.L.; Ghisalberti, E.L.; Plummer, J.A.; Jones, C.G.; Bohlmann, J. Biosynthesis of sandalwood oil: Santalum album CYP76F cytochromes P450 produce santalols and bergamotol. PLoS ONE 2013, 8, e75083. [Google Scholar] [CrossRef] [PubMed]
- Moniodis, J.; Jones, C.G.; Barbour, E.L.; Plummer, J.A.; Ghisalberti, E.L.; Bohlmann, J. The transcriptome of sesquiterpenoid biosynthesis in heartwood xylem of Western Australian sandalwood (Santalum spicatum). Phytochemistry 2015, 113, 79–86. [Google Scholar] [CrossRef]
- Srivastava, P.L.; Daramwar, P.P.; Krithika, R.; Pandreka, A.; Shankar, S.S.; Thulasiram, H.V. Functional characterization of novel sesquiterpene synthases from Indian sandalwood, Santalum album. Sci. Rep. 2015, 5, 10095. [Google Scholar] [CrossRef]
- Celedon, J.M.; Chiang, A.; Yuen, M.M.S.; Diaz-Chavez, M.L.; Madilao, L.L.; Finnegan, P.M.; Barbour, E.L.; Bohlmann, J. Heartwood-specific transcriptome and metabolite signatures of tropical sandalwood (Santalum album) reveal the final step of (z)-santalol fragrance biosynthesis. Plant J. 2016, 86, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.S.; Chang, W.C.; Xiao, Y.L.; Liu, H.W.; Liu, P.H. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Ann. Rev. Biochem. 2013, 82, 497. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef]
- Zhang, X.H.; Teixeira da Silva, J.A.; Niu, M.Y.; Li, M.Z.; He, C.M.; Zhao, J.H.; Zeng, S.J.; Duan, J.; Ma, G.H. Physiological and transcriptomic analyses reveal a response mechanism to cold stress in Santalum album L. leaves. Sci. Rep. 2017, 7, 42165. [Google Scholar] [CrossRef]
- Zhang, X.H.; Berkowitz, O.; Teixeira da Silva, J.A.; Zhang, M.H.; Ma, G.H.; Whelan, J.; Duan, J. RNA-seq analysis identifies key genes associated with haustorial development in the root hemiparasite Santalum album. Front. Plant Sci. 2015, 6, 00661. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, S.F.; Li, J.J.; Zheng, B.; Bao, M.Z.; Ning, G.G. Fusion primer and nested integrated PCR (FPNI-PCR): A new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotech. 2011, 11, 109. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, R.G.; Devisetty, U.K.; Maloof, J.N.; Zuo, Y.; Li, J.J.; Shen, Y.X.; Zhao, J.; Bao, M.Z.; Ning, G.G. The divergence of flowering time modulated by FT/TFL1 is independent to their interaction and binding activities. Front. Plant Sci. 2017, 8, 697. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef]
- Celedon, J.M.; Bohlmann, J. Genomics-based discovery of plant genes for synthetic biology of terpenoid fragrances: A case study in sandalwood oil biosynthesis. Methods Enzymol. 2016, 576, 47–67. [Google Scholar]
- Clough, S.J.; Bent, A.F. A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Henriquez, M.A.; Soliman, A.; Li, G.Y.; Hannoufa, A.; Ayele, B.T.; Daayf, F. Molecular cloning, functional characterization and expression of potato (Solanum tuberosum) 1-deoxy-d-xylulose 5-phosphate synthase 1 (StDXS1) in response to Phytophthora infestans. Plant Sci. 2016, 243, 71–83. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids—Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Kuzuyama, T.; Takahashi, S.; Takagi, M.; Seto, H. Characterization of 1-deoxy-d-xylulose 5-phosphate reductoisomerase, an enzyme involved in isopentenyl diphosphate biosynthesis, and identification of its catalytic amino acid residues. J. Biol. Chem. 2000, 275, 19928–19932. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.Y.; Gong, Y.F.; Zuo, K.J.; Ling, H.; Qiu, C.X.; Zhang, F.; Wang, Y.C.; Pi, Y.; Liu, X.; Sun, X.F. Molecular cloning, expression profiling and functional analysis of a DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase from Camptotheca acuminata. J. Plant Physiol. 2008, 165, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Diao, J.; Cai, G.; Deng, L.; Zheng, B.; Yao, Y.; Song, Y. Antimalarial and structural studies of pyridine-containing inhibitors of 1-deoxyxylulose-5-phosphate reductoisomerase. ACS Med. Chem. Lett. 2013, 4, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Hashimoto, T. Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant Cell Physiol. 2011, 52, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Hedhili, S.; Montiel, G.; Zhang, Y.X.; Chatel, G.; Pré, M.; Gantet, P.; Memelink, J. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011, 67, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Lu, X.; Yan, T.X.; Fu, X.Q.; Lv, Z.Y.; Zhang, F.Y.; Pan, Q.F.; Wang, G.F.; Sun, X.F.; Tang, K.X. The jasmonate-responsive AaMYC2 transcription factor positively regulates artemisinin biosynthesis in Artemisia annua. New Phytol. 2016, 210, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Adhikari, M.N.; Liu, H.; Xu, H.; He, G.Z.; Zhan, R.T.; Wei, J.S.; Chen, W.W. Characterization and functional analysis of the genes encoding 1-deoxy-d-xylulose-5-phosphate reductoisomerase and 1-deoxy-d-xylulose-5-phosphate synthase, the two enzymes in the MEP pathway, from Amomum villosum Lour. Mol. Biol. Rep. 2012, 39, 8287–8296. [Google Scholar] [CrossRef]
- Zhang, M.; Li, K.; Liu, J.Y.; Yu, D.Y. Identification and differential expression of two isogenes encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase in Glycine max. Plant Biotech. Rep. 2012, 6, 363–371. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Singh, N.; Bansal, S.; Mishra, B.; Sangwan, R.S. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: Active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 2017, 159, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Kher, M.M.; Soner, D.; Page, T.; Zhang, X.H.; Nataraj, M.; Ma, G.H. Sandalwood: Basic biology, tissue culture, and genetic transformation. Planta 2016, 243, 847–887. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.H.; Cheng, Q.W.; Teixeira da Silva, J.A.; Fang, L.; Ma, G.H. Elicitors modulate young sandalwood (Santalum album L.) growth, heartwood formation, and concrete oil synthesis. Plants 2021, 10, 339. [Google Scholar] [CrossRef]
- Liu, X.J. Studies on Essential Oil Composition, Distribution and Inducing Heartwood Formation of Sandal (Santalum album). Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2012; pp. 65–180. [Google Scholar]
- Seetang-Nun, Y.; Sharkey, T.D.; Suvachittanont, W. Molecular cloning and characterization of two cDNAs encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase from Hevea brasiliensis. J. Plant Physiol. 2008, 165, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Paulet, L.; Cairó, A.; Botella-Pavía, P.; Besumbes, O.; Campos, N.; Boronat, A.; Rodríguez-Concepción, M. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 2006, 62, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.F.; Miao, J.; Li, S.A.; Qin, G.J.; Tang, S.; Li, H.N.; Gu, H.Y.; Qu, L.J. Disruption of the 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR) gene results in albino, dwarf and defects in trichome initiation and stomata closure in Arabidopsis. Cell Res. 2010, 20, 688–700. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takeno, S.; Hayashi, S.; Sendai, M.; Bamba, T.; Yoshimura, S.; Tomizawa, K.I.; Fukusaki, E.; Miyake, C. Overexpression of 1-deoxy-d-xylulose-5-phosphate reductoisomerase gene in chloroplast contributes to increment of isoprenoid production. J. Biosci. Bioeng. 2008, 105, 518–526. [Google Scholar] [CrossRef]
- Engprasert, S.; Taura, F.; Shoyama, Y. Molecular cloning, expression and characterization of recombinant 1-deoxy-d-xylulose-5-phosphate reductoisomerase from Coleus forskohlii Briq. Plant Sci. 2005, 169, 287–294. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep. 2007, 26, 2129–2136. [Google Scholar] [CrossRef]
- Xiang, L.; Zeng, L.X.; Yuan, Y.; Chen, M.; Wang, F.; Liu, X.Q.; Zeng, L.J.; Lan, X.Z.; Liao, Z.H. Enhancement of artemisinin biosynthesis by overexpressing DXR, CYP71AV1 and CPR in the plants of Artemisia annua L. Plant Omics 2012, 5, 503–507. [Google Scholar]
- Wu, S.J.; Shi, M.; Wu, J.Y. Cloning and characterization of the 1-deoxy-d-xylulose 5-phosphate reductoisomerase gene for diterpenoid tanshinone biosynthesis in Salvia miltiorrhiza (Chinese sage) hairy roots. Biotech. Appl. Biochem. 2009, 52, 89–95. [Google Scholar] [CrossRef]
- Yang, D.F.; Du, X.H.; Liang, X.; Han, R.L.; Liang, Z.S.; Liu, Y.; Liu, F.H.; Zhao, J.J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.Q.; Ju, G.H.; Yu, X.H.; Hao, X.L.; Huang, Q.; Xiao, J.B.; Cui, L.J.; Kai, G.Y. Increased accumulation of the cardio-cerebrovascular disease treatment drug tanshinone in Salvia miltiorrhiza hairy roots by the enzymes 3-hydroxy-3-methylglutaryl CoA reductase and 1-deoxy-d-xylulose 5-phosphate reductoisomerase. Funct. Integrat. Genom. 2014, 14, 603–615. [Google Scholar] [CrossRef]
- Vaccaro, M.; Malafronte, N.; Alfieri, M.; De Tommasi, N.; Leone, A. Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tissue Org. Cult. 2014, 119, 65–77. [Google Scholar] [CrossRef]
- Singh, S.; Pal, S.; Shanker, K.; Chanotiya, C.S.; Gupta, M.M.; Dwivedi, U.N.; Shasany, A.K. Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol. Plant. 2014, 152, 617–633. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhao, Y.J.; Wang, J.D.; Hu, T.Y.; Tong, Y.R.; Zhou, J.W.; Song, Y.D.; Gao, W.; Huang, L.Q. Overexpression and RNA interference of TwDXR regulate the accumulation of terpenoid active ingredients in Tripterygium wilfordii. Biotech. Lett. 2018, 40, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, E.; Porta, H.; Arroyo, A.; Román, C.S.; Medina, L.; Rodríguez-Concepción, M.; León, P. Functional characterization of the three genes encoding 1-deoxy-d-xylulose 5-phosphate synthase in maize. J. Exp. Bot. 2011, 62, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Zhang, P.; Zhang, M.; Fu, C.H.; Zhao, C.F.; Dong, Y.S.; Guo, A.Y.; Yu, L.J. Transcriptional profile of Taxus chinensis cells in response to methyl jasmonate. BMC Genom. 2012, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Zhang, Z.; Wang, M.X.; Wei, J.H.; Chen, H.J.; Gao, Z.H.; Sui, C.; Luo, H.M.; Zhang, X.L.; Yang, Y. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013, 14, 227. [Google Scholar] [CrossRef]
- Xiang, L.; Zhu, S.Q.; Zhao, T.F.; Zhang, M.; Liu, W.H.; Chen, M.; Lan, X.Z.; Liao, Z.H. Enhancement of artemisinin content and relative expression of genes of artemisinin biosynthesis in Artemisia annua by exogenous MeJA treatment. Plant Growth Regul. 2015, 75, 435–441. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Ahumada, I.; Diez-Juez, E.; Sauret-Güeto, S.; Lois, L.M.; Gallego, F.; Carretero-Paulet, L.; Campos, N.; Boronat, A. 1-Deoxy-d-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J. 2001, 27, 213–222. [Google Scholar] [CrossRef]
- Patra, B.; Schluttenhofer, C.; Wu, Y.M.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim. Bioph. Acta Gene Regul. Mechan. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef]
- Zhou, M.L.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotech. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Zhang, H.B.; Bokowiec, M.T.; Rushton, P.J.; Han, S.C.; Timko, M.P. Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Mol. Plant. 2012, 5, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Liao, Y.C.; Lv, F.F.; Zhang, Z.; Sun, P.W.; Gao, Z.H.; Hu, K.P.; Sui, C.; Jin, Y.; Wei, J.H. Transcription factor AsMYC2 controls the jasmonate-responsive expression of ASS1 regulating sesquiterpene biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant Cell Physiol. 2017, 58, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, H.; Li, Y.; Xiong, Y.; Niu, M.; Zhang, X.; Teixeira da Silva, J.A.; Ma, G. Molecular Cloning and Functional Analysis of 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase from Santalum album. Genes 2021, 12, 626. https://doi.org/10.3390/genes12050626
Zhang Y, Yan H, Li Y, Xiong Y, Niu M, Zhang X, Teixeira da Silva JA, Ma G. Molecular Cloning and Functional Analysis of 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase from Santalum album. Genes. 2021; 12(5):626. https://doi.org/10.3390/genes12050626
Chicago/Turabian StyleZhang, Yueya, Haifeng Yan, Yuan Li, Yuping Xiong, Meiyun Niu, Xinhua Zhang, Jaime A. Teixeira da Silva, and Guohua Ma. 2021. "Molecular Cloning and Functional Analysis of 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase from Santalum album" Genes 12, no. 5: 626. https://doi.org/10.3390/genes12050626
APA StyleZhang, Y., Yan, H., Li, Y., Xiong, Y., Niu, M., Zhang, X., Teixeira da Silva, J. A., & Ma, G. (2021). Molecular Cloning and Functional Analysis of 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase from Santalum album. Genes, 12(5), 626. https://doi.org/10.3390/genes12050626





