A Network Medicine Approach for Drug Repurposing in Duchenne Muscular Dystrophy
Abstract
1. Introduction
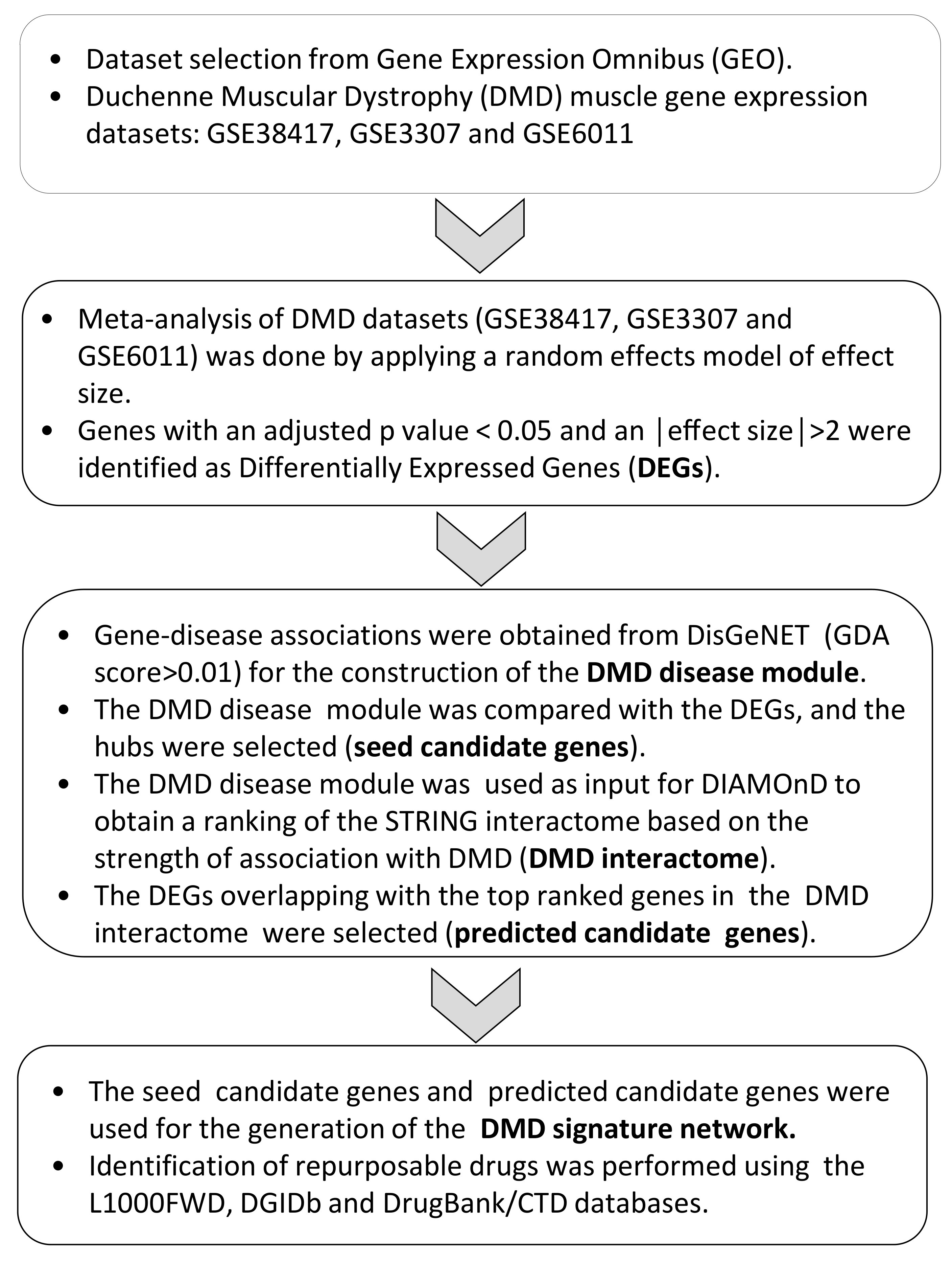
2. Materials and Methods
2.1. Dataset Selection and Analysis
2.2. Enrichment Analysis and Sub-Cluster Identification
2.3. Network Construction, Seed Gene Identification, and Disease Propagation Algorithms
2.4. Drug Identification
2.5. Statistical Analysis
3. Results
3.1. Meta-Analysis of Gene Expression in DMD
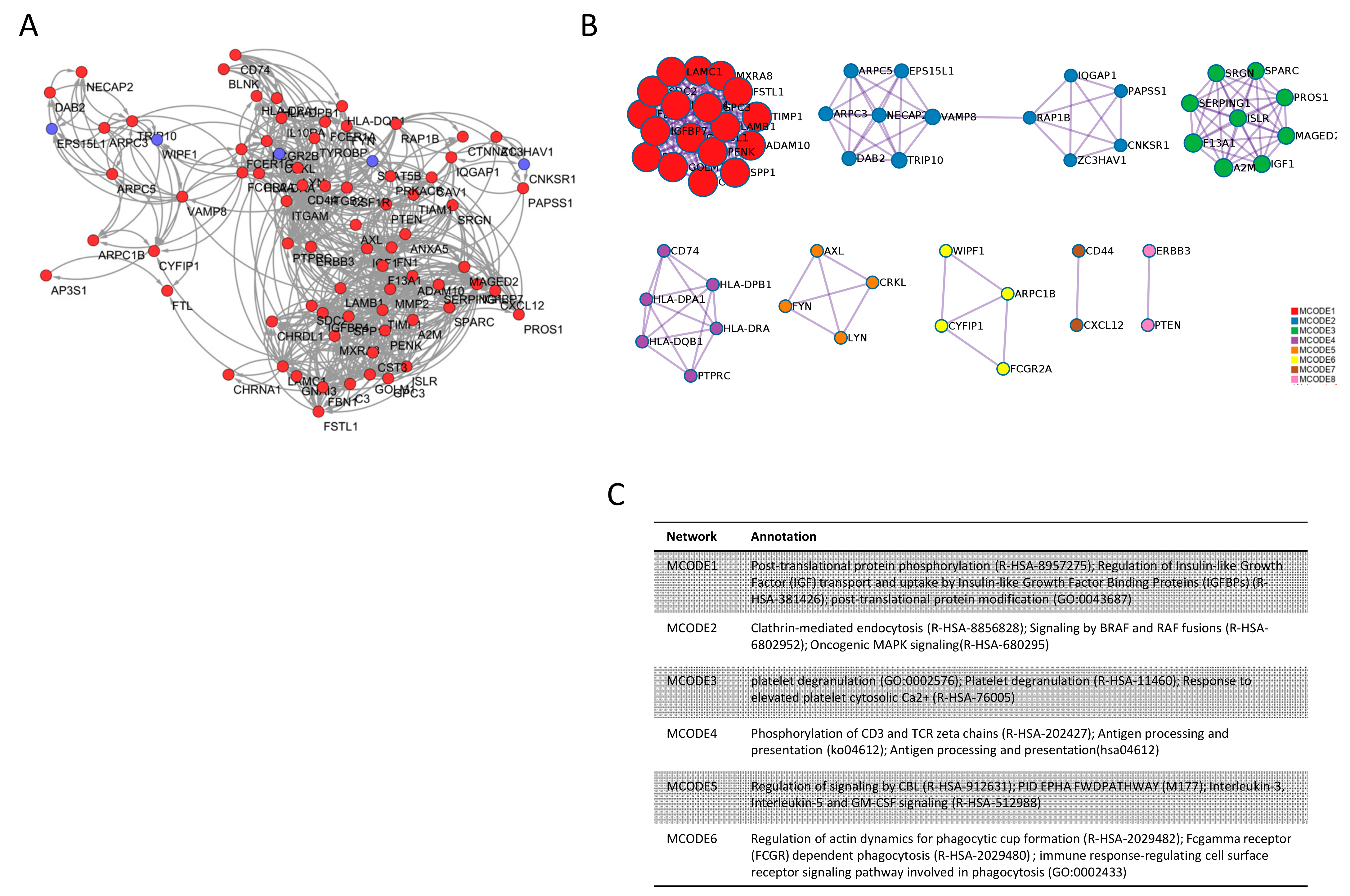
3.2. DMD Module Identification and New Associated Gene Prediction
3.3. Candidate Gene Identification
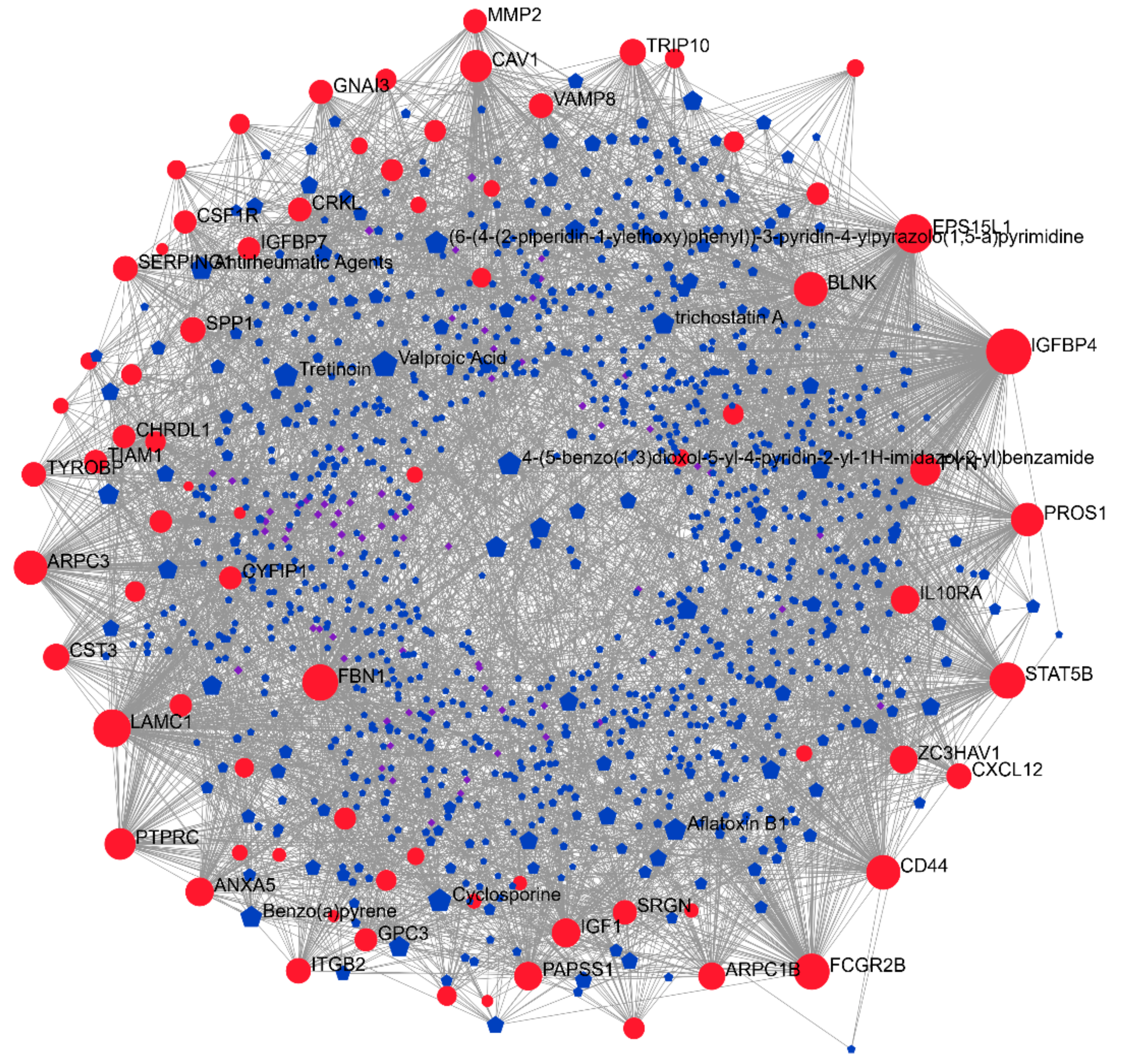
3.4. Drugs Associated with DMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darras, B.T.; Menache-Starobinski, C.C.; Hinton, V.; Kunkel, L.M. Dystrophinopathies. In Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician’s Approach; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124171275. [Google Scholar]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidbal, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, K.; Liang, F.; Giordano, C.; Lemaire, C.; Danialou, G.; Okazaki, T.; Bourdon, J.; Rafei, M.; Galipeau, J.; Divangahi, M.; et al. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR 2. EMBO Mol. Med. 2014, 6, 1476–1492. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Scotton, C.; Passarelli, C.; Ferlini, A. Duchenne muscular dystrophy: From diagnosis to therapy. Molecules 2015, 20, 18168–18184. [Google Scholar] [CrossRef]
- Burns, D.P.; O’Halloran, K.D. Evidence of hypoxic tolerance in weak upper airway muscle from young mdx mice. Respir. Physiol. Neurobiol. 2016, 226, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Werneck, L.C.; Lorenzoni, P.J.; Ducci, R.D.P.; Fustes, O.H.; Kay, C.S.K.; Scola, R.H. Duchenne muscular dystrophy: An historical treatment review. Arq. Neuropsiquiatr. 2019, 77, 579–589. [Google Scholar] [CrossRef]
- Shimizu-Motohashi, Y.; Murakami, T.; Kimura, E.; Komaki, H.; Watanabe, N. Exon skipping for Duchenne muscular dystrophy: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Simmons, T.R.; Wein, N.; Vulin-Chaffiol, A.; Heller, K.; Rutherford, A.; Rodino-Klapac, L.; Flanigan, K.M. Treatment of DMD 5′ mutations through two different EXON2 skipping strategies: Intramuscular delivery of RAAV9.snrna mediated skipping and antisense morpholino oligomers. Mol. Ther. 2015, 23, S202. [Google Scholar] [CrossRef][Green Version]
- Okubo, M.; Noguchi, S.; Hayashi, S.; Nakamura, H.; Komaki, H.; Matsuo, M.; Nishino, I. Exon skipping induced by nonsense/frameshift mutations in DMD gene results in Becker muscular dystrophy. Qual. Life Res. 2020, 139, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Motohashi, Y.; Komaki, H.; Motohashi, N.; Takeda, S.; Yokota, T.; Aoki, Y. Restoring Dystrophin Expression in Duchenne Muscular Dystrophy: Current Status of Therapeutic Approaches. J. Pers. Med. 2019, 9, 1. [Google Scholar] [CrossRef]
- Korinthenberg, R. A new era in the management of Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2019, 61, 292–297. [Google Scholar] [CrossRef]
- Duan, D. Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther. 2018, 26, 2337–2356. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2010, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Nabirotchkin, S.; Peluffo, A.E.; Rinaudo, P.; Yu, J.; Hajj, R.; Cohen, D. Next-generation drug repurposing using human genetics and network biology. Curr. Opin. Pharmacol. 2020, 51, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Desai, R.J.; Handy, D.E.; Wang, R.; Schneeweiss, S.; Barabási, A.-L.; Loscalzo, J. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Ghiassian, S.D.; Menche, J.; Barabási, A.-L. A DIseAse MOdule Detection (DIAMOnD) Algorithm Derived from a Systematic Analysis of Connectivity Patterns of Disease Proteins in the Human Interactome. PLoS Comput. Biol. 2015, 11, e1004120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lachmann, A.; Keenan, A.B.; Ma’Ayan, A. L1000FWD: Fireworks visualization of drug-induced transcriptomic signatures. Bioinformatics 2018, 34, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Cotto, K.C.; Wagner, A.H.; Feng, Y.-Y.; Kiwala, S.; Coffman, A.C.; Spies, G.; Wollam, A.; Spies, N.C.; Griffith, O.L.; Griffith, M. DGIdb 3.0: A redesign and expansion of the drug–gene interaction database. Nucleic Acids Res. 2018, 46, D1068–D1073. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef]
- Bakay, M.; Wang, Z.; Melcon, G.; Schiltz, L.; Xuan, J.; Zhao, P.; Sartorelli, V.; Seo, J.; Pegoraro, E.; Angelini, C.; et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb–MyoD pathways in muscle regeneration. Brain 2006, 129, 996–1013. [Google Scholar] [CrossRef]
- Dadgar, S.; Wang, Z.; Johnston, H.; Kesari, A.; Nagaraju, K.; Chen, Y.-W.; Hill, D.A.; Partridge, T.A.; Giri, M.; Freishtat, R.J.; et al. Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy. J. Cell Biol. 2014, 207, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Pescatori, M.; Broccolini, A.; Minetti, C.; Bertini, E.; Bruno, C.; D’Amico, A.; Bernardini, C.; Mirabella, M.; Silvestri, G.; Giglio, V.; et al. Gene expression profiling in the early phases of DMD: A constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007, 21, 1210–1226. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Marot, G.; Foulley, J.-L.; Mayer, C.-D.; Jaffrézic, F. Moderated effect size and P-value combinations for microarray meta-analyses. Bioinformatics 2009, 25, 2692–2699. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Mah, J.K.; Korngut, L.; Dykeman, J.; Day, L.; Pringsheim, T.; Jette, N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 2014, 24, 482–491. [Google Scholar] [CrossRef]
- Sharma, A.; Menche, J.; Huang, C.C.; Ort, T.; Zhou, X.; Kitsak, M.; Sahni, N.; Thibault, D.; Voung, L.; Guo, F.; et al. A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes in asthma. Hum. Mol. Genet. 2015, 24, 3005–3020. [Google Scholar] [CrossRef]
- Sonawane, A.R.; Weiss, S.T.; Glass, K.; Sharma, A. Network Medicine in the Age of Biomedical Big Data. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Fiscon, G.; Conte, F.; Farina, L.; Paci, P. Network-Based Approaches to Explore Complex Biological Systems towards Network Medicine. Genes 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.D.; Mazzon, E.; Mangano, K.; Basile, M.S.; Cavalli, E.; Mammana, S.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Transcriptomic Analysis Reveals Involvement of the Macrophage Migration Inhibitory Factor Gene Network in Duchenne Muscular Dystrophy. Genes 2019, 10, 939. [Google Scholar] [CrossRef]
- Bindoff, L.; Cullen, M.J. Experimental (−) emetine myopathy. J. Neurol. Sci. 1978, 39, 1–15. [Google Scholar] [CrossRef]
- Kuntzer, T.; Reichmann, H.; Bogousslavsky, J.; Regli, F. Emetine-induced myopathy and carnitine deficiency. J. Neurol. 1990, 237, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Wagner, B.K.; Ma, Y.; Chinsomboon, J.; Laznik, D.; Spiegelman, B.M. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1 and oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 4721–4726. [Google Scholar] [CrossRef]
- Wang, T.-H.; Wang, H.-S.; Ichijo, H.; Giannakakou, P.; Foster, J.S.; Fojo, T.; Wimalasena, J. Microtubule-interfering Agents Activate c-Jun N-terminal Kinase/Stress-activated Protein Kinase through Both Ras and Apoptosis Signal-regulating Kinase Pathways. J. Biol. Chem. 1998, 273, 4928–4936. [Google Scholar] [CrossRef]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; De Bardi, M.; Bicciato, S.; et al. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Madaro, L.; Torcinaro, A.; De Bardi, M.; Contino, F.F.; Pelizzola, M.; Diaferia, G.R.; Imeneo, G.; Bouchè, M.; Puri, P.L.; De Santa, F. Macrophages fine tune satellite cell fate in dystrophic skeletal muscle of mdx mice. PLoS Genet. 2019, 15, e1008408. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.; Piggott, R.W.; Emmerson, T.; Winder, S.J. Dasatinib as a treatment for Duchenne muscular dystrophy. Hum. Mol. Genet. 2015, 25, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sanarica, F.; Mantuano, P.; Conte, E.; Cozzoli, A.; Capogrosso, R.; Giustino, A.; Cutrignelli, A.; Cappellari, O.; Rolland, J.; De Bellis, M.; et al. Proof-of-concept validation of the mechanism of action of Src tyrosine kinase inhibitors in dystrophic mdx mouse muscle: In vivo and in vitro studies. Pharmacol. Res. 2019, 145. [Google Scholar] [CrossRef] [PubMed]
- Bajanca, F.; Vandel, L. Epigenetic Regulators Modulate Muscle Damage in Duchenne Muscular Dystrophy Model. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Teveroni, E.; Pellegrino, M.; Sacconi, S.; Calandra, P.; Cascino, I.; Farioli-Vecchioli, S.; Puma, A.; Garibaldi, M.; Morosetti, R.; Tasca, G.; et al. Estrogens enhance myoblast differentiation in facioscapulohumeral muscular dystrophy by antagonizing DUX4 activity. J. Clin. Investig. 2017, 127, 1531–1545. [Google Scholar] [CrossRef]
- Ma, Z.; Zhong, Z.; Zheng, Z.; Shi, X.-M.; Zhang, W. Inhibition of Glycogen Synthase Kinase-3β Attenuates Glucocorticoid-Induced Suppression of Myogenic Differentiation In Vitro. PLoS ONE 2014, 9, e105528. [Google Scholar] [CrossRef]
- Verhees, K.J.P.; Pansters, N.A.M.; Baarsma, H.A.; Remels, A.H.V.; Haegens, A.; de Theije, C.C.; Schols, A.M.W.J.; Gosens, R.; Langen, R.C.J. Pharmacological inhibition of GSK-3 in a guinea pig model of LPS-induced pulmonary inflammation: II. Effects on skeletal muscle atrophy. Respir. Res. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Salam, E.A.; Abdel-Meguid, I.E.; Shatla, R.; Korraa, S. Evaluation of neural damage in Duchenne muscular dystrophy patients. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2014, 33, 13–18. [Google Scholar]
- Sharma, K.R.; Mynhier, M.A.; Miller, R.G. Cyclosporine increases muscular force generation in Duhenne muscular dystrophy. Neurology 1993, 43. [Google Scholar] [CrossRef]
- Kirschner, J.; Schessl, J.; Schara, U.; Reitter, B.; Stettner, G.M.; Hobbiebrunken, E.; Wilichowski, E.; Bernert, G.; Weiss, S.; Stehling, F.; et al. Treatment of Duchenne muscular dystrophy with ciclosporin A: A randomised, double-blind, placebo-controlled multicentre trial. Lancet Neurol. 2010, 9, 1053–1059. [Google Scholar] [CrossRef]
- Wood, C.L.; Cheetham, T.D.; Hollingsworth, K.G.; Guglieri, M.; Ailins-Sahun, Y.; Punniyakodi, S.; Mayhew, A.; Straub, V. Observational study of clinical outcomes for testosterone treatment of pubertal delay in Duchenne muscular dystrophy. BMC Pediatr. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Accession Number | Tissue | Samples | Platform |
|---|---|---|---|
| GSE38417 | Muscle | 16 DMD patients and 6 healthy controls | Affymetrix Human Genome U133 Plus 2.0 |
| GSE3307 | Muscle | 10 DMD patients and 17 healthy controls | Affymetrix Human Genome U133A and U133B |
| GSE6011 | Muscle | 23 DMD patients and 14 healthy controls | Affymetrix Human Genome U133A |
| Sig_ID | Drug | Drug Category | Indication | Similarity Score | p-Value | q-Value | Z-Score | Combined Score |
|---|---|---|---|---|---|---|---|---|
| CPC007_A375_24H:BRD-K03067624-003-19-3:10 | emetine | emetine alkaloids | anti-protozoal | −0.1948 | 1.40 × 10−11 | 2.85 × 10−8 | 1.78 | −19.35 |
| CPC017_A375_6H:BRD-K76674262-001-01-7:10 | homoharringtonine | cephalotaxus alkaloids | CML | −0.1818 | 1.32 × 10−10 | 1.49 × 10−7 | 1.71 | −16.86 |
| CPC004_VCAP_24H:BRD-A01643550-001-03-1:10 | prednisolone | synthetic glucocorticoid | anti-inflammatory or immunosuppressive agent | −0.1688 | 2.09 × 10−9 | 1.01 × 10−6 | 1.81 | −15.74 |
| CPC002_VCAP_24H:BRD-K90553655-001-03-6:10 | testosterone | anabolic steroid | hypogonadism | −0.1688 | 2.42 × 10−9 | 1.12 × 10−6 | 1.83 | −15.77 |
| CPC017_HT29_6H:BRD-K07691486-001-04-9:10 | roscovitine | synthetic organic | kinase inhibitor | −0.1558 | 1.58 × 10−8 | 4.10 × 10−6 | 1.67 | −13.04 |
| CPD002_PC3_24H:BRD-K08547377-394-01-9:10 | irinotecan | DNA replication inhibitor | colon cancer | −0.1429 | 1.39 × 10−7 | 2.06 × 10−5 | 1.66 | −11.38 |
| CPC006_PC3_6H:BRD-K82135108-001-01-9:10 | elesclomol | sulfur compounds | anti-cancer activity | −0.1429 | 1.36 × 10−7 | 2.06 × 10−5 | 1.77 | −12.16 |
| PCLB002_A375_24H:BRD-K02130563:1.11 | panobinostat | non-selective histone deacetylase (HDAC) inhibitor | multiple myeloma and other cancers | −0.1429 | 2.04 × 10−7 | 2.55 × 10−5 | 1.59 | −10.64 |
| MUC.CP006_MCF7_6H:BRD-K77987382-001-08-2:10 | mebendazole | benzimidazole | antihelmintic | −0.1429 | 1.22 × 10−7 | 1.97 × 10−5 | 1.63 | −11.26 |
| CPC005_PC3_24H:BRD-A07000685-001-03-6:10 | hydrocortisone | adrenal glucocorticoid | immune and allergic disorders, adrenal insufficiency disorders | −0.1429 | 1.92 × 10−7 | 2.45 × 10−5 | 1.78 | −11.93 |
| Drugs | Indication | Number of Target Genes among the Predicted Candidate Genes | Predicted Candidate Genes | Number of Total Target Genes | p-Value | FDR |
|---|---|---|---|---|---|---|
| ROVELIZUMAB | Hemorrhagic shock, multiple sclerosis, stroke | 2 | ITGB2, ITGAM | 3 | 1.37 × 10−4 | 0.012558 |
| DASATINIB | Chronic myelogenous leukemia, acute lymphoblastic leukemia | 3 | LYN, CSF1R, FYN | 23 | 4.86 × 10−4 | 0.014904 |
| ILORASERTIB | Phase II Study for CDKN2A deficient solid tumors | 3 | LYN, CSF1R, FYN | 21 | 3.69 × 10−4 | 0.014904 |
| NINTEDANIB | Idiopathic Pulmonary Fibrosis, NSCLC | 2 | LYN, FYN | 19 | 0.007 | 0.029273 |
| JNJ-26483327 | Phase I study for solid tumors | 2 | LYN, FYN | 9 | 0.002 | 0.029273 |
| ACALABRUTINIB | Mantle cell lymphoma | 2 | LYN, FYN | 14 | 0.004 | 0.029273 |
| IBRUTINIB | B cell cancers | 2 | LYN, FYN | 16 | 0.005 | 0.029273 |
| XL-228 | Phase I study for chronic myeloid leukemia | 2 | LYN, FYN | 16 | 0.005 | 0.029273 |
| PEXMETINIB | Hematological cancers | 2 | LYN, FYN | 12 | 0.003 | 0.029273 |
| TG100-801 | Diabetic macular edema and proliferative diabetic retinopathy | 2 | LYN, FYN | 16 | 0.005 | 0.029273 |
| ENMD-981693 | Phase II study for solid cancers | 2 | LYN, FYN | 19 | 0.007 | 0.029273 |
| GALLAMINE | Non-depolarizing muscle relaxant | 1 | CHRNA1 | 1 | 0.007 | 0.029273 |
| AME-133V | Phase III study for follicular lymphoma and Phase I for rheumatoid arthritis | 1 | ITGB2 | 1 | 0.007 | 0.029273 |
| ERLIZUMAB | Heart attack, stroke, and traumatic shock | 1 | ITGB2 | 1 | 0.007 | 0.029273 |
| BEMCENTINIB | Phase II study for solid and hematological tumors | 1 | AXL | 1 | 0.007 | 0.029273 |
| MILATUZUMAB | Multiple myeloma and other hematological tumors | 1 | CD74 | 1 | 0.007 | 0.029273 |
| ARRY-382 | Phase II study for advanced solid tumors | 1 | CSF1R | 1 | 0.007 | 0.029273 |
| MM-121 | Phase II study for lung and breast cancer | 1 | ERBB3 | 1 | 0.007 | 0.029273 |
| PATRITUMAB | Phase II study for squamous cell cancer of the head and neck | 1 | ERBB3 | 1 | 0.007 | 0.029273 |
| BOSUTINIB | Chronic myelogenous leukemia | 2 | LYN, FYN | 23 | 0.011 | 0.044 |
| BAFETINIB | Phase II study for chronic myelogenous leukemia | 1 | LYN | 2 | 0.014 | 0.046 |
| BACITRACIN | Polypeptide antibiotic | 1 | A2M | 2 | 0.014 | 0.046 |
| EFALIZUMAB | psoriasis | 1 | ITGB2 | 2 | 0.014 | 0.046 |
| LIFITEGRAST | Keratoconjunctivitis sicca | 1 | ITGB2 | 2 | 0.014 | 0.046 |
| BPI-9016 | Phase I study for NSCLC | 1 | AXL | 2 | 0.014 | 0.046 |
| Rank | Drug | Degree | FDR |
|---|---|---|---|
| 1 | Cytarabine | 12 | 0 |
| 2 | Entinostat | 32 | 0 |
| 3 | Isotretinoin | 21 | 0 |
| 4 | Paclitaxel | 15 | 0 |
| 5 | Tretinoin | 48 | 0 |
| 6 | Mitoxantrone | 8 | 8.45 × 10−9 |
| 7 | Raloxifene Hydrochloride | 14 | 2.54 × 10−8 |
| 8 | Doxorubicin | 15 | 4.6 × 10−8 |
| 9 | Indomethacin | 8 | 2.15 × 10−7 |
| 10 | Simvastatin | 8 | 2.7 × 10−7 |
| 11 | Decitabine | 20 | 1.01 × 10−6 |
| 12 | Curcumin | 12 | 1.53 × 10−6 |
| 13 | Cisplatin | 24 | 2.53 × 10−6 |
| 14 | Dexamethasone | 15 | 2.96 × 10−6 |
| 15 | Ethinyl Estradiol | 10 | 3.51 × 10−6 |
| 16 | Azacitidine | 9 | 4.2 × 10−6 |
| 17 | Trichostatin A | 37 | 4.82 × 10−6 |
| 18 | Aspirin | 10 | 6.71 × 10−6 |
| 19 | Vorinostat | 25 | 9.95 × 10−6 |
| 20 | Ascorbic Acid | 8 | 1.25 × 10−5 |
| 21 | Zoledronic acid | 19 | 1.25 × 10−5 |
| 22 | Resveratrol | 24 | 2.22 × 10−5 |
| 23 | Sulforafan | 13 | 2.3 × 10−5 |
| 24 | Tamoxifen | 12 | 2.82 × 10−5 |
| 25 | Tamibarotene | 19 | 4.06 × 10−5 |
| 26 | Carmustine | 8 | 5.77 × 10−5 |
| 27 | Vitamin E | 17 | 5.77 × 10−5 |
| 28 | Cyclosporine | 44 | 0.000133 |
| 29 | Etoposide | 9 | 0.000134 |
| 30 | Carbamazepine | 20 | 0.000136 |
| 31 | Methotrexate | 22 | 0.000136 |
| 32 | Panobinostat | 14 | 0.000136 |
| 33 | Phenobarbital | 14 | 0.000136 |
| 34 | Calcitriol | 21 | 0.000243 |
| 35 | Valproic Acid | 60 | 0.000383 |
| 36 | Genistein | 14 | 0.000496 |
| 37 | Testosterone enanthate | 9 | 0.00079 |
| 38 | Fulvestrant | 7 | 0.0013 |
| 39 | Progesterone | 18 | 0.0013 |
| 40 | Acetylcysteine | 8 | 0.002457 |
| 41 | Catechin | 8 | 0.002534 |
| 42 | Rosiglitazone | 9 | 0.00257 |
| 43 | Estradiol | 30 | 0.003208 |
| 44 | Quercetin | 20 | 0.006024 |
| 45 | Afimoxifene | 8 | 0.013992 |
| 46 | Troglitazone | 8 | 0.034244 |
| 47 | Vincristine | 8 | 0.037712 |
| 48 | Bortezomib | 7 | 0.037951 |
| 49 | Fluorouracil | 12 | 0.040236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.D.; Basile, M.S.; Ciurleo, R.; Bramanti, A.; Arcidiacono, A.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P. A Network Medicine Approach for Drug Repurposing in Duchenne Muscular Dystrophy. Genes 2021, 12, 543. https://doi.org/10.3390/genes12040543
Lombardo SD, Basile MS, Ciurleo R, Bramanti A, Arcidiacono A, Mangano K, Bramanti P, Nicoletti F, Fagone P. A Network Medicine Approach for Drug Repurposing in Duchenne Muscular Dystrophy. Genes. 2021; 12(4):543. https://doi.org/10.3390/genes12040543
Chicago/Turabian StyleLombardo, Salvo Danilo, Maria Sofia Basile, Rosella Ciurleo, Alessia Bramanti, Antonio Arcidiacono, Katia Mangano, Placido Bramanti, Ferdinando Nicoletti, and Paolo Fagone. 2021. "A Network Medicine Approach for Drug Repurposing in Duchenne Muscular Dystrophy" Genes 12, no. 4: 543. https://doi.org/10.3390/genes12040543
APA StyleLombardo, S. D., Basile, M. S., Ciurleo, R., Bramanti, A., Arcidiacono, A., Mangano, K., Bramanti, P., Nicoletti, F., & Fagone, P. (2021). A Network Medicine Approach for Drug Repurposing in Duchenne Muscular Dystrophy. Genes, 12(4), 543. https://doi.org/10.3390/genes12040543








