Spatial Organization and Coordination of the Plant Circadian System
Abstract
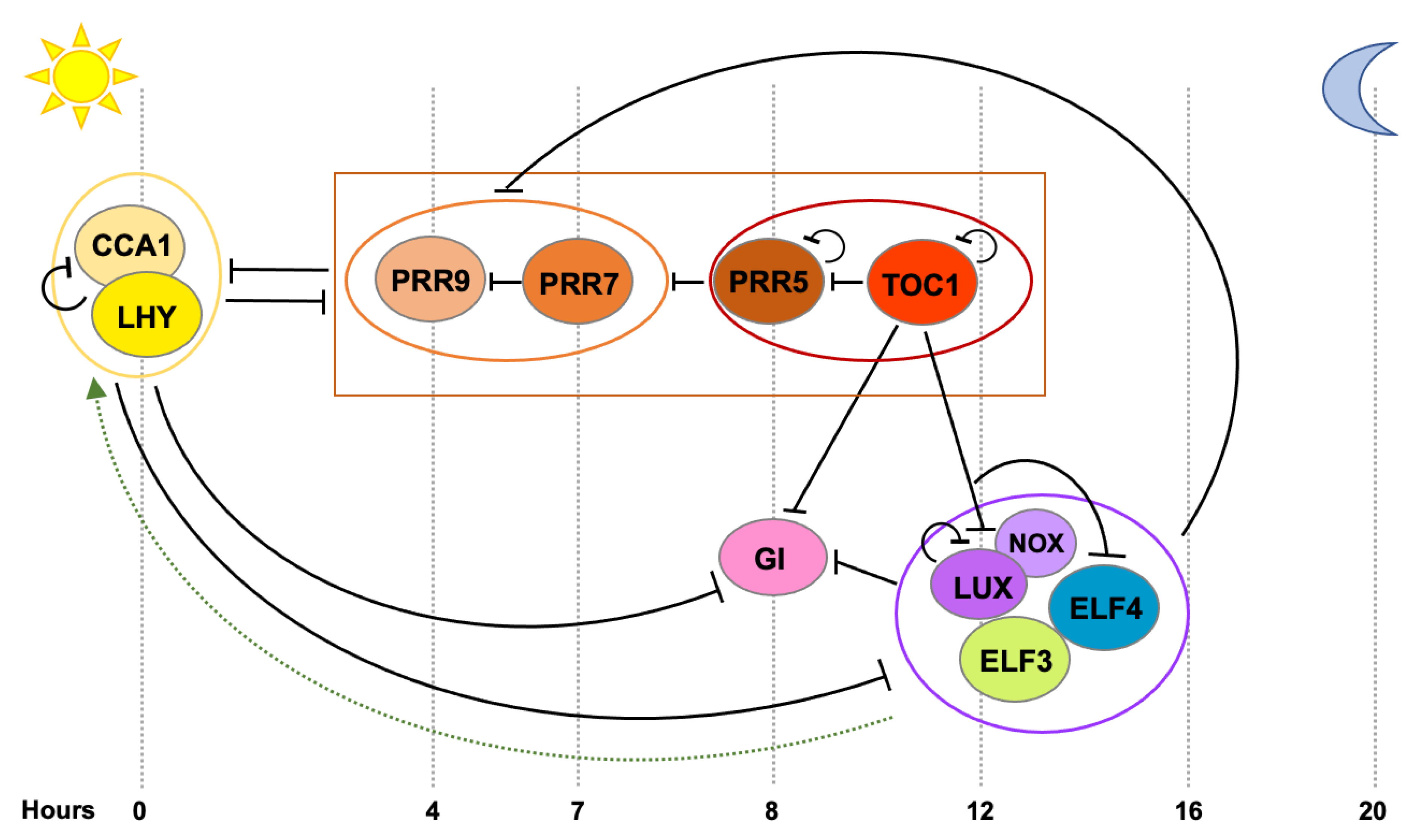
1. Introduction
2. Tissue-Specificity of the Plant Circadian Clock
2.1. Early Evidences for Tissue-Specific Clocks
2.2. Mechanisms Underlying Tissue-Specific Circadian Rhythms
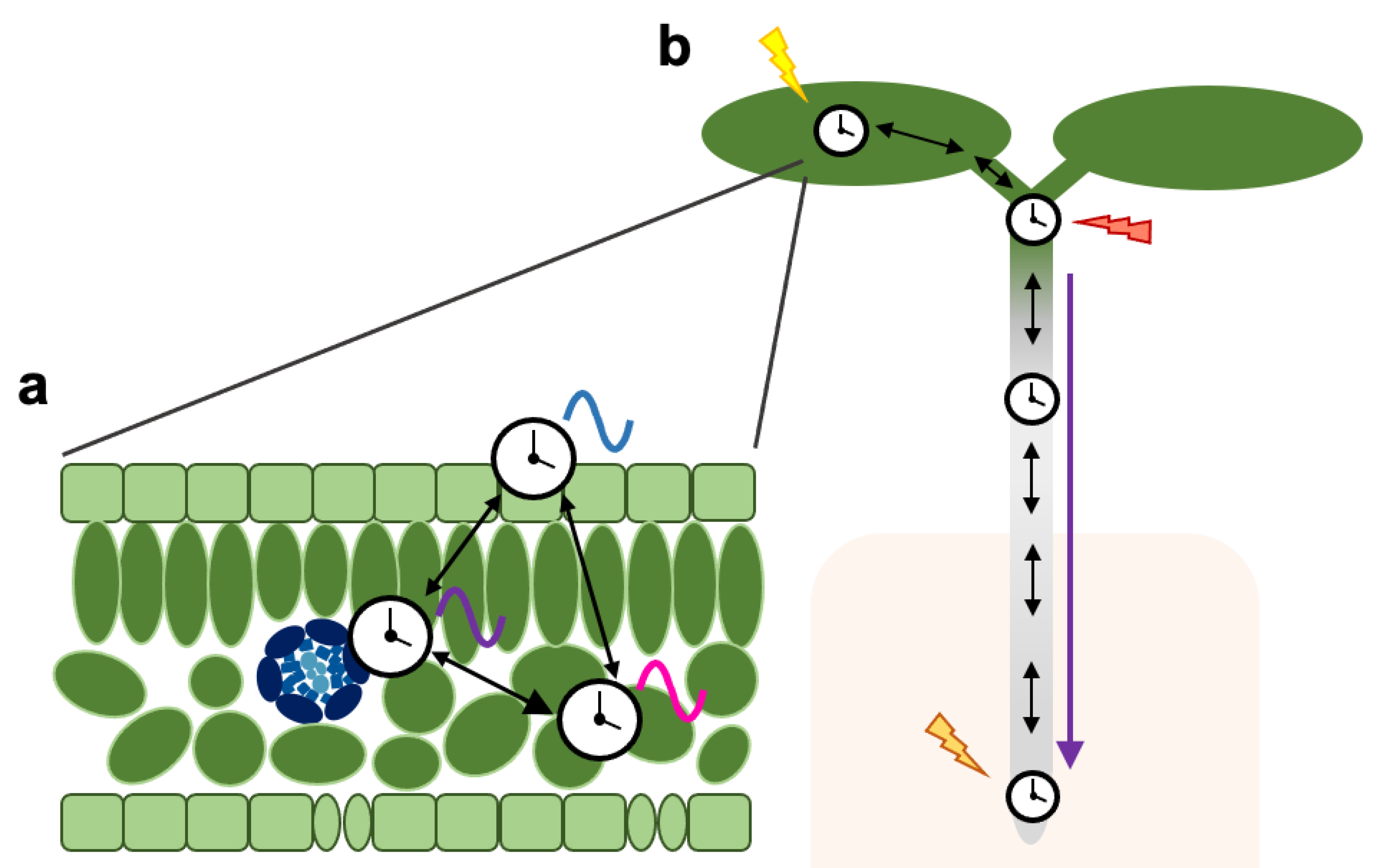
3. Coordination of Tissue-Specific Clocks across the Plant
4. Concluding Remarks
Funding
Conflicts of Interest
References
- Millar, A.J. The Intracellular Dynamics of Circadian Clocks Reach for the Light of Ecology and Evolution. Annu. Rev. Plant Biol. 2016, 67, 595–618. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Tingay, S.; Wang, Z.-Y.; Tobin, E.M. Circadian Rhythms Confer a Higher Level of Fitness to Arabidopsis Plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Webb, A.A.R.; Seki, M.; Satake, A.; Caldana, C. Continuous dynamic adjustment of the plant circadian oscillator. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Nohales, M.A.; Kay, M.A.N.S.A. Molecular mechanisms at the core of the plant circadian oscillator. Nat. Struct. Mol. Biol. 2016, 23, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Mwimba, M.; Karapetyan, S.; Liu, L.; Marqués, J.; McGinnis, E.M.; Buchler, N.E.; Dong, X. Daily humidity oscillation regulates the circadian clock to influence plant physiology. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.C.M.; Hubbard, K.E.; Gardner, M.J.; Jung, H.J.; Aubry, S.; Hotta, C.T.; Mohd-Noh, N.I.; Robertson, F.C.; Hearn, T.J.; Tsai, Y.-C.; et al. Circadian oscillations of cytosolic free calcium regulate the Arabidopsis circadian clock. Nat. Plants 2018, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Salomé, P.A.; Oliva, M.; Weigel, D.; Krämer, U. Circadian clock adjustment to plant iron status depends on chloroplast and phytochrome function. EMBO J. 2012, 32, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A.R. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nat. Cell Biol. 2013, 502, 689–692. [Google Scholar] [CrossRef]
- Litthauer, S.; Chan, K.X.; Jones, M.A. 3′-Phosphoadenosine 5′-Phosphate Accumulation Delays the Circadian System. Plant Physiol. 2018, 176, 3120–3135. [Google Scholar] [CrossRef]
- Nagel, D.H.; Kay, S.A. Complexity in the Wiring and Regulation of Plant Circadian Networks. Curr. Biol. 2012, 22, R648–R657. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Harmer, S.L. Wheels within wheels: The plant circadian system. Trends Plant Sci. 2014, 19, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated tran-scription of key pathways in Arabidopsis by the circadian clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef]
- Covington, M.F.; Maloof, J.N.; Straume, M.; A Kay, S.; Harmer, S.L. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef]
- Kamioka, M.; Takao, S.; Suzuki, T.; Taki, K.; Higashiyama, T.; Kinoshita, T.; Nakamichi, N. Direct Repression of Evening Genes by CIRCADIAN CLOCK-ASSOCIATED1 in the Arabidopsis Circadian Clock. Plant Cell 2016, 28, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Manfield, I.; Stockley, P.; Carré, I.A. Revised Morning Loops of the Arabidopsis Circadian Clock Based on Analyses of Direct Regulatory Interactions. PLoS ONE 2015, 10, e0143943. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kiba, T.; Henriques, R.; Mizuno, T.; Chua, N.-H.; Sakakibara, H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 Are Transcriptional Repressors in the Arabidopsis Circadian Clock. Plant Cell 2010, 22, 594–605. [Google Scholar] [CrossRef]
- Gendron, J.M.; Pruneda-Paz, J.L.; Doherty, C.J.; Gross, A.M.; Kang, S.E.; Kay, S.A. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Natl. Acad. Sci. USA 2012, 109, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Pérez-García, P.; Pokhilko, A.; Millar, A.J.; Antoshechkin, I.; Riechmann, J.L.; Mas, P. Mapping the Core of the Arabidopsis Circadian Clock Defines the Network Structure of the Oscillator. Science 2012, 336, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Nusinow, D.A. Into the Evening: Complex Interactions in the Arabidopsis Circadian Clock. Trends Genet. 2016, 32, 674–686. [Google Scholar] [CrossRef]
- Endo, M. Tissue-specific circadian clocks in plants. Curr. Opin. Plant Biol. 2016, 29, 44–49. [Google Scholar] [CrossRef]
- Inoue, K.; Araki, T.; Endo, M. Oscillator networks with tissue-specific circadian clocks in plants. Semin. Cell Dev. Biol. 2018, 83, 78–85. [Google Scholar] [CrossRef]
- Hennessey, T.L.; Field, C.B. Evidence of Multiple Circadian Oscillators in Bean Plants. J. Biol. Rhythm. 1992, 7, 105–113. [Google Scholar] [CrossRef]
- Sai, J.; Johnson, C.H. Different circadian oscillators control Ca2+ fluxes and Lhcb gene expression. Proc. Natl. Acad. Sci. USA 1999, 96, 11659–11663. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.T.; Haley, A.; Viry-Moussaïd, M.; Johnson, C.H.; Van Der Luit, A.H.; Trewavas, A.J. The Calcium Rhythms of Different Cell Types Oscillate with Different Circadian Phases. Plant Physiol. 2001, 125, 787–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thain, S.C.; Murtas, G.; Lynn, J.R.; McGrath, R.B.; Millar, A.J. The Circadian Clock That Controls Gene Expression in Arabidopsis Is Tissue Specific. Plant Physiol. 2002, 130, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Kozma-Bognár, L.; Bastow, R.M.; Nagy, F.; Millar, A.J. Distinct regulation of CAB and PHYB gene expression by similar circadian clocks. Plant J. 2002, 32, 529–537. [Google Scholar] [CrossRef]
- Xu, X.; Hotta, C.T.; Dodd, A.N.; Love, J.; Sharrock, R.; Lee, Y.W.; Xie, Q.; Johnson, C.H.; Webb, A.A. Distinct Light and Clock Modulation of Cytosolic Free Ca2+ Oscillations and Rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 Promoter Activity in Arabidopsis. Plant Cell 2007, 19, 3474–3490. [Google Scholar] [CrossRef]
- Fowler, S.; Lee, K.; Onouchi, H.; Samach, A.; Richardson, K.; Morris, B.; Coupland, G.; Putterill, J. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999, 18, 4679–4688. [Google Scholar] [CrossRef]
- Para, A.; Farré, E.M.; Imaizumi, T.; Pruneda-Paz, J.L.; Harmon, F.G.; Kay, S.A. PRR3 Is a Vascular Regulator of TOC1 Stability in the Arabidopsis Circadian Clock. Plant Cell 2007, 19, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Pruneda-Paz, J.L.; Breton, G.; Para, A.; Kay, S.A. A Functional Genomics Approach Reveals CHE as a Component of the Arabidopsis Circadian Clock. Science 2009, 323, 1481–1485. [Google Scholar] [CrossRef]
- Chow, B.Y.; Sanchez, S.E.; Breton, G.; Pruneda-Paz, J.L.; Krogan, N.T.; Kay, S.A. Transcriptional Regulation of LUX by CBF1 Mediates Cold Input to the Circadian Clock in Arabidopsis. Curr. Biol. 2014, 24, 1518–1524. [Google Scholar] [CrossRef]
- Fukuda, H.; Nakamichi, N.; Hisatsune, M.; Murase, H.; Mizuno, T. Synchronization of Plant Circadian Oscillators with a Phase Delay Effect of the Vein Network. Phys. Rev. Lett. 2007, 99, 098102. [Google Scholar] [CrossRef]
- Yakir, E.; Hassidim, M.; Melamed-Book, N.; Hilman, D.; Kron, I.; Green, R.M. Cell autonomous and cell-type specific circadian rhythms in Arabidopsis. Plant J. 2011, 68, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Martin, A.P.; Andriunas, F.; Offler, C.E.; Patrick, J.W.; McCurdy, D.W. GIGANTEA is a component of a regulatory pathway determining wall ingrowth deposition in phloem parenchyma transfer cells of Arabidopsis thaliana. Plant J. 2010, 63, 651–661. [Google Scholar] [CrossRef]
- Endo, M.; Shimizu, H.; Nohales, M.A.; Araki, T.; Kay, S.A. Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nat. Cell Biol. 2014, 515, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Bordage, S.; Sullivan, S.; Laird, J.; Millar, A.J.; Nimmo, H.G. Organ specificity in the plant circadian system is explained by different light inputs to the shoot and root clocks. New Phytol. 2016, 212, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Yuan, L.; Song, Y.; Sun, J.; Jia, Q.; Xie, Q.; Xu, X. Molecular investigation of organ-autonomous expression of Arabidopsis circadian oscillators. Plant Cell Environ. 2020, 43, 1501–1512. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, Y.; Li, S.; Xie, Q.; Xu, X. PRR9 and PRR7 negatively regulate the expression of EC components under warm temperature in roots. Plant Signal. Behav. 2021, 16, 1855384. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, H.G.; Laird, J.; Bindbeutel, R.; Nusinow, D.A. The evening complex is central to the difference between the circadian clocks of Arabidopsis thaliana shoots and roots. Physiol. Plant. 2020, 169, 442–451. [Google Scholar] [CrossRef]
- Chen, W.W.; Takahashi, N.; Hirata, Y.; Ronald, J.; Porco, S.; Davis, S.J.; Nusinow, D.A.; Kay, S.A.; Mas, P. A mobile ELF4 delivers circadian temperature information from shoots to roots. Nat. Plants 2020, 6, 416–426. [Google Scholar] [CrossRef]
- Greenwood, M.; Locke, J.C. The circadian clock coordinates plant development through specificity at the tissue and cellular level. Curr. Opin. Plant Biol. 2020, 53, 65–72. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light Perception: A Matter of Time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and Cryptochromes in the Entrainment of the Arabidopsis Circadian Clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef]
- Devlin, P.F.; Kay, S.A. Cryptochromes Are Required for Phytochrome Signaling to the Circadian Clock but Not for Rhythmicity. Plant Cell 2000, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.E.; Quail, P.H. Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J. 1995, 7, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Bognár, L.K.; Hall, A.; Ádám, É.; Thain, S.C.; Nagy, F.; Millar, A.J. The circadian clock controls the expression pattern of the circadian input photoreceptor, phytochrome B. Proc. Natl. Acad. Sci. USA 1999, 96, 14652–14657. [Google Scholar] [CrossRef] [PubMed]
- Tóth, R.; Kevei, É.; Hall, A.; Millar, A.J.; Nagy, F.; Kozma-Bognár, L. Circadian Clock-Regulated Expression of Phytochrome and Cryptochrome Genes in Arabidopsis. Plant Physiol. 2001, 127, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.; Domijan, M.; Gould, P.D.; Hall, A.J.W.; Locke, J.C.W. Coordinated circadian timing through the integration of local inputs in Arabidopsis thaliana. PLoS Biol. 2019, 17, e3000407. [Google Scholar] [CrossRef]
- Endo, M.; Nakamura, S.; Araki, T.; Mochizuki, N.; Nagatani, A. Phytochrome B in the Mesophyll Delays Flowering by Suppressing FLOWERING LOCUS T Expression in Arabidopsis Vascular Bundles. Plant Cell 2005, 17, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, K.; Park, E.; Kim, K.; Bae, G.; Choi, G. Epidermal Phytochrome B Inhibits Hypocotyl Negative Gravitropism Non-Cell-Autonomously. Plant Cell 2016, 28, 2770–2785. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Mochizuki, N.; Suzuki, T.; Nagatani, A. CRYPTOCHROME2 in Vascular Bundles Regulates Flowering in Arabidopsis. Plant Cell 2007, 19, 84–93. [Google Scholar] [CrossRef]
- Jang, S.; Marchal, V.; Panigrahi, K.C.S.; Wenkel, S.; Soppe, W.; Deng, X.-W.; Valverde, F.; Coupland, G. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 2008, 27, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Fiene, G.; Fackendahl, P.; Hoecker, U. The Arabidopsis repressor of light signaling SPA1 acts in the phloem to regulate seedling de-etiolation, leaf expansion and flowering time. Development 2011, 138, 1851–1862. [Google Scholar] [CrossRef]
- Kim, K.; Shin, J.; Lee, S.-H.; Kweon, H.-S.; Maloof, J.N.; Choi, G. Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2011, 108, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeong, J.; Kim, J.; Lee, N.; Kim, M.E.; Lee, S.; Kim, S.C.; Choi, G. PIF1 Regulates Plastid Development by Repressing Photosynthetic Genes in the Endodermis. Mol. Plant 2016, 9, 1415–1427. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, G.; Kim, S.; Thi, T.N.; Kim, H.; Jeong, J.; Kim, J.; Kim, J.; Choi, G.; Oh, E. The epidermis coordinates thermoresponsive growth through the phyB-PIF4-auxin pathway. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; Salomé, P.A.; McClung, C.R. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc. Natl. Acad. Sci. USA 2003, 100, 6878–6883. [Google Scholar] [CrossRef]
- Shimizu, H.; Araki, T.; Endo, M. Photoperiod sensitivity of the Arabidopsis circadian clock is tissue-specific. Plant Signal. Behav. 2015, 10, e1010933. [Google Scholar] [CrossRef]
- Shimizu, H.; Katayama, K.; Koto, T.; Torii, K.; Araki, T.; Endo, M. Decentralized circadian clocks process thermal and photoperiodic cues in specific tissues. Nat. Plants 2015, 1, 15163. [Google Scholar] [CrossRef]
- Thain, S.C.; Hall, A.; Millar, A.J. Functional independence of circadian clocks that regulate plant gene expression. Curr. Biol. 2000, 10, 951–956. [Google Scholar] [CrossRef]
- Fukuda, H.; Ukai, K.; Oyama, T. Self-arrangement of cellular circadian rhythms through phase-resetting in plant roots. Phys. Rev. E 2012, 86, 041917. [Google Scholar] [CrossRef]
- Wenden, B.; Toner, D.L.K.; Hodge, S.K.; Grima, R.; Millar, A.J. Spontaneous spatiotemporal waves of gene expression from biological clocks in the leaf. Proc. Natl. Acad. Sci. USA 2012, 109, 6757–6762. [Google Scholar] [CrossRef]
- Takahashi, N.; Hirata, Y.; Aihara, K.; Mas, P. A Hierarchical Multi-oscillator Network Orchestrates the Arabidopsis Circadian System. Cell 2015, 163, 148–159. [Google Scholar] [CrossRef]
- Muranaka, T.; Oyama, T. Heterogeneity of cellular circadian clocks in intact plants and its correction under light-dark cycles. Sci. Adv. 2016, 2, e1600500. [Google Scholar] [CrossRef]
- Gould, P.D.; Domijan, M.; Greenwood, M.; Tokuda, I.T.; Rees, H.; Kozma-Bognar, L.; Hall, A.J.; Locke, J.C. Coordination of robust single cell rhythms in the Arabidopsis circadian clock via spatial waves of gene expression. eLife 2018, 7, e31700. [Google Scholar] [CrossRef] [PubMed]
- James, A.B.; Monreal, J.A.; Nimmo, G.A.; Kelly, C.L.; Herzyk, P.; Jenkins, G.I.; Nimmo, H.G. The Circadian Clock inArabidopsisRoots Is a Simplified Slave Version of the Clock in Shoots. Science 2008, 322, 1832–1835. [Google Scholar] [CrossRef]
- Nimmo, H.G. Entrainment of Arabidopsis roots to the light:dark cycle by light piping. Plant Cell Environ. 2018, 41, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Dalchau, N.; Baek, S.J.; Briggs, H.M.; Robertson, F.C.; Dodd, A.N.; Gardner, M.J.; Stancombe, M.A.; Haydon, M.J.; Stan, G.-B.; Gonçalves, J.M.; et al. The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc. Natl. Acad. Sci. USA 2011, 108, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Bendix, C.; Marshall, C.M.; Harmon, F.G. Circadian Clock Genes Universally Control Key Agricultural Traits. Mol. Plant 2015, 8, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Kay, S.A. The Plant Circadian Clock: From a Simple Timekeeper to a Complex Developmental Manager. Cold Spring Harb. Perspect. Biol. 2016, 8, a027748. [Google Scholar] [CrossRef] [PubMed]
- Hanano, S.; Domagalska, M.A.; Nagy, F.; Davis, S.J. Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 2006, 11, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Katsir, L.; Davies, K.A.; Bergmann, D.C.; Laux, T. Peptide Signaling in Plant Development. Curr. Biol. 2011, 21, R356–R364. [Google Scholar] [CrossRef]
- Stahl, Y.; Simon, R. Gated communities: Apoplastic and symplastic signals converge at plasmodesmata to control cell fates. J. Exp. Bot. 2013, 64, 5237–5241. [Google Scholar] [CrossRef]
- Han, X.; Kumar, D.; Chen, H.; Wu, S.; Kim, J.-Y. Transcription factor-mediated cell-to-cell signalling in plants. J. Exp. Bot. 2013, 65, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Miñambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohales, M.A. Spatial Organization and Coordination of the Plant Circadian System. Genes 2021, 12, 442. https://doi.org/10.3390/genes12030442
Nohales MA. Spatial Organization and Coordination of the Plant Circadian System. Genes. 2021; 12(3):442. https://doi.org/10.3390/genes12030442
Chicago/Turabian StyleNohales, Maria A. 2021. "Spatial Organization and Coordination of the Plant Circadian System" Genes 12, no. 3: 442. https://doi.org/10.3390/genes12030442
APA StyleNohales, M. A. (2021). Spatial Organization and Coordination of the Plant Circadian System. Genes, 12(3), 442. https://doi.org/10.3390/genes12030442




