TYK2 Promoter Variant Is Associated with Impaired Insulin Secretion and Lower Insulin Resistance in Japanese Type 2 Diabetes Patients
Abstract
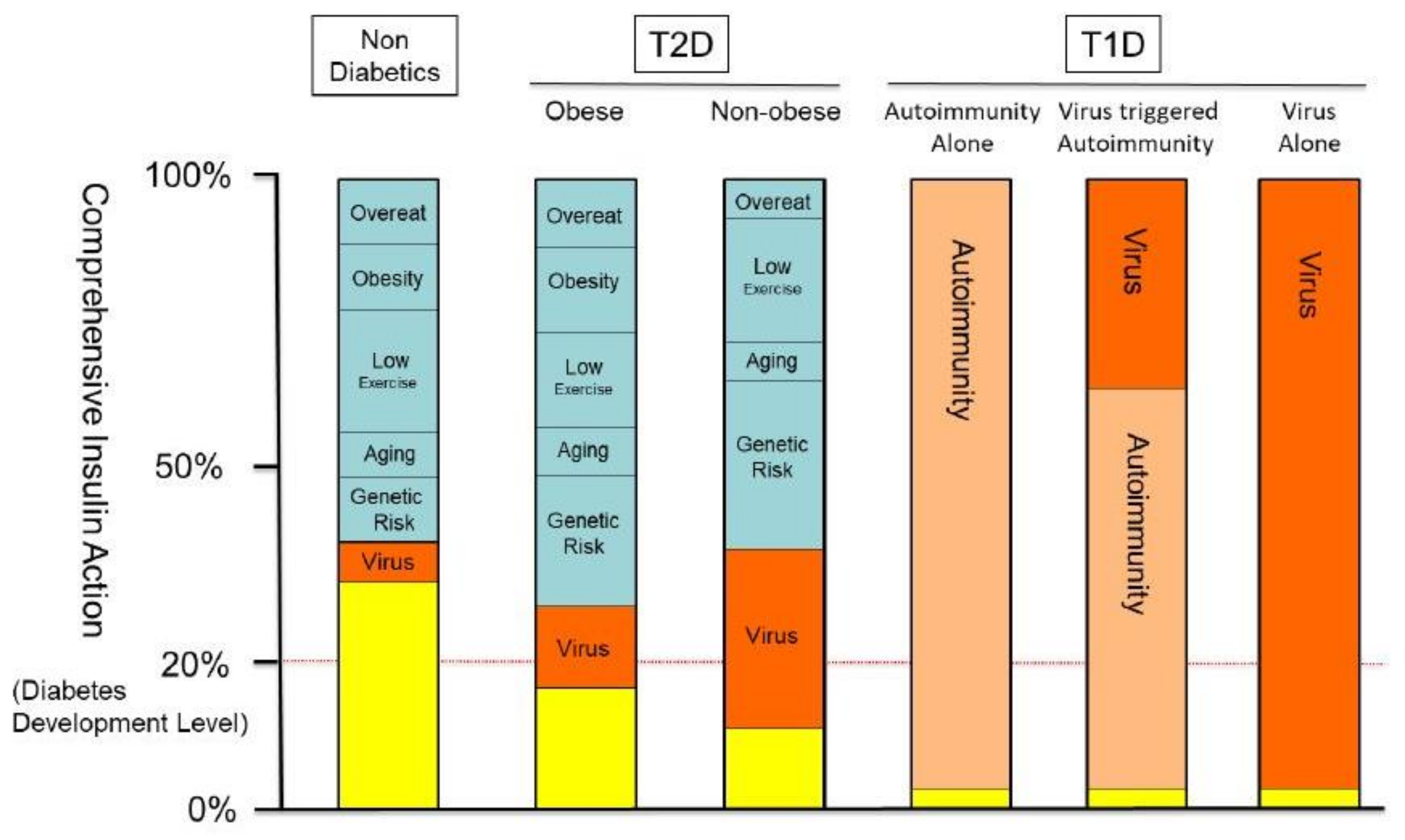
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Physical Examination and Serum Biochemical Measurements
2.3. DNA Extraction and Genotyping of TYK2PV
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
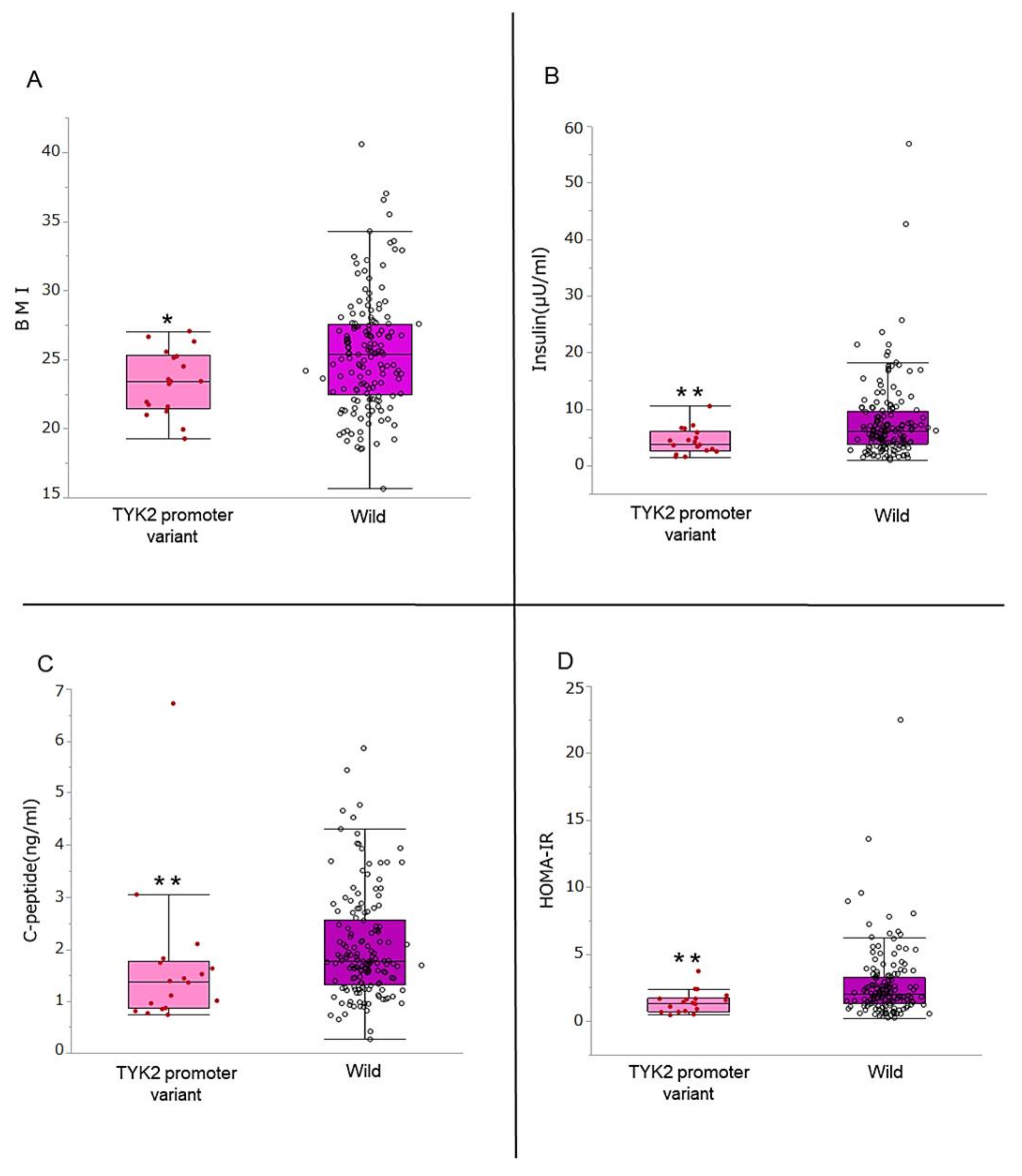
3.2. Comparison of BMI, Insulin Secretion Ability, and Insulin Resistance
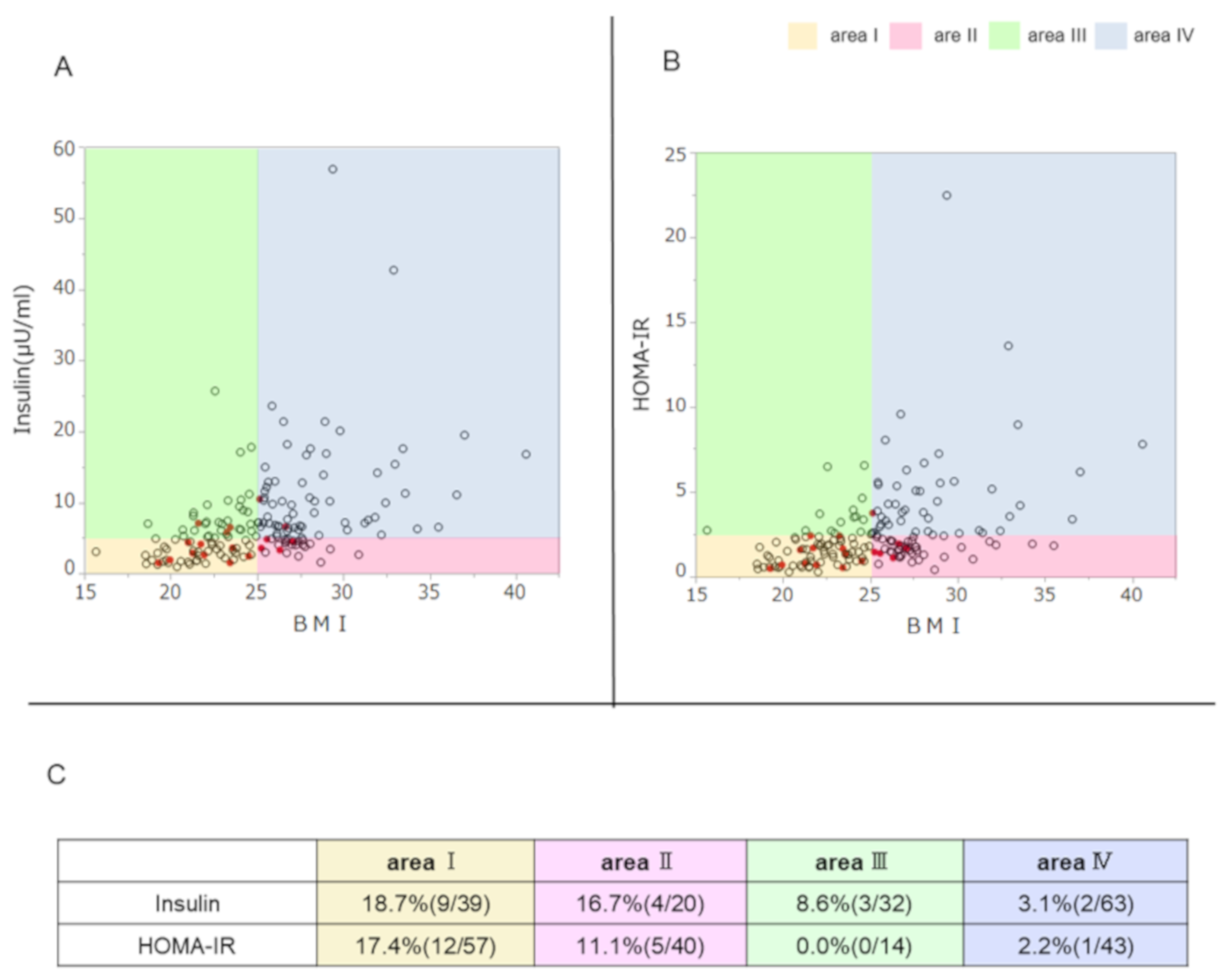
3.3. Association of TYK2PV with the Ability to Secrete Insulin, Insulin Resistance, and BMI
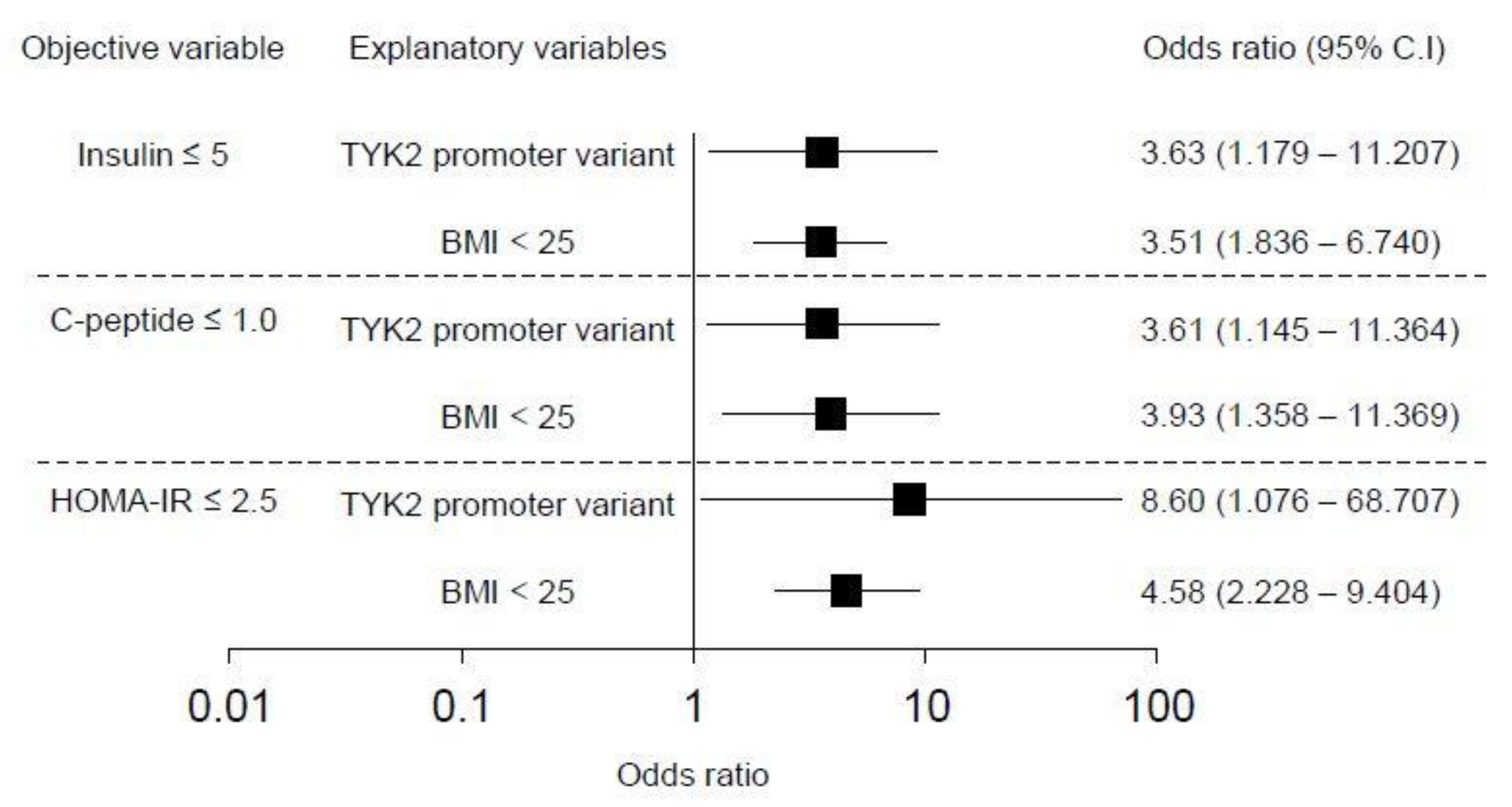
3.4. Effect of TYK2PV on Insulin Secretion Ability and Insulin Resistance: Multivariate Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Prior Presentation
References
- IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussel, Belgium, 2019; Available online: https://www.diabetesatlas.org/en/ (accessed on 3 November 2019).
- Kota, S.K.; Uqale, S.; Gupta, N.; Modi, K.D. Laparoscopic ileal interposition with diverted sleeve gastrectomy for treatment of type 2 diabetes. Diabetes Metab. Syndr. 2012, 6, 125–131. [Google Scholar] [CrossRef]
- Onodera, T.; Yoon, J.W.; Brown, K.S.; Notkins, A.L. Evidence for a single locus controlling susceptibility to virus-induced diabetes mellitus. Nature 1978, 5672, 693–696. [Google Scholar] [CrossRef]
- Jun, H.S.; Yoon, J.W. A new look at virus in type 1 diabetes. Diabetes 2003, 19, 8–31. [Google Scholar]
- Clements, G.B.; Galbraith, D.N.; Taylor, K.W. Coxsackie B virus infection and onset of childhood diabetes. Lancet 1995, 346, 221–223. [Google Scholar] [CrossRef]
- Tauriainen, S.; Oikarinen, S.; Oikarinen, M.; Hyöty, H. Enteroviruses in the pathogenesis of type 1 diabetes. Immunopathology 2011, 33, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Krogbold, L.; Edwin, B.; Buanes, T.; Frisk, G.; Skog, O.; Anagandula, M.; Korsgren, O.; Undlien, D.; Eike, C.M.; Richardson, J.S.; et al. Detection of a low grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015, 64, 1682–1687. [Google Scholar] [CrossRef]
- Vehik, K.; Lynch, K.F.; Wong, M.; Tian, X.; Ross, C.M.; Gibbs, A.R.; Ajami, J.N.; Petrosino, F.J.; Rewers, M.; Toppari, J.; et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. Lett. 2019, 25, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Lancaster, A.; Wilkins, C.; Sutar, S.M.; Uang, A.; Vick, C.; Clepper, L.; Thackray, L.; Brassil, M.M.; Virgin, W.H.; et al. IRF-3, IRF-5 and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Hober, D.; Sauter, P. Pathogenesis of type 1 diabetes mellitus: Interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010, 6, 279–289. [Google Scholar] [CrossRef]
- Trinchieri, G. Type 1 interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- Nakamura, R.; Shibata, K.; Yamada, H.; Shimoda, K.; Nakayama, K.; Yoshikai, Y. Tyk2-Signaling plays an important role in host defense against Escherichia coli through IL-23-Induced IL-17 Production by gammadelta T. Cells. J. Immunol. 2008, 1181, 2071–2075. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mine, K.; Inoue, Y.; Teshima, M.; Oqawa, S.; Kai, Y.; Kurafuji, T.; Hirakawa, K.; Miyakawa, D.; Ikeda, H.; et al. Reduced Tyk2 gene expression in β-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat. Commun. 2015, 6, 6748. [Google Scholar] [CrossRef]
- Nagafuchi, S.; Kamada-Hibio, Y.; Hirakawa, K.; Tsutsu, N.; Minami, M.; Okada, A.; Kai, K.; Teshima, M.; Moroishi, A.; Murakami, Y.; et al. TYK2 promoter variant and diabetes mellitus in the Japanese. EBioMedicine 2015, 2, 744–749. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2016, 39 (Suppl. 1), S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. Performance of GFR equations in Japanese subjects. Clin. Exp. Nephrol. 2013, 17, 352–358. [Google Scholar] [CrossRef]
- Steiner, D.F.; Cunningham, D.; Spigelman, L.; Aten, B. Insulin biosynthesis: Evidence for a precursor. Science 1967, 157, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and Beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Meier, J.J.; Menge, B.A.; Breuer, T.G.K.; Muller, C.A.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Schrader, H. Functional assessment of pancreatic Beta-cell area in humans. Diabetes 2009, 58, 1595–1603. [Google Scholar] [CrossRef]
- Richardson, S.J.; Willcox, A.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009, 52, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Gkrania-Klotsas, E.; Langenberg, C.; Tauriainen, S.; Sharp, S.J.; Luben, R.; Forouhi, N.G.; Khaw, K.T.; Hyöty, H.; Wareham, N.J. The association between prior infection with five serotypes of Coxsackievirus B and incident type 2 diabetes mellitus in the EPIC-Norfolk study. Diabetologia 2012, 55, 967–970. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Legoff, J.; Nguewa, J.L.; Boudou, P.; Balti, E.V.; Noubiap, J.J.; Kamwa, V.; Atogho-Tiedeu, B.; Azabji-Kenfack, M.; Djahmeni, E.N.; et al. Human herpesvirus 8 infection DNA positivity is associated with low insulin secretion: A case-control study in a sub-Saharan African population with diabetes. J. Diabetes 2018, 10, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, L. Metabolic Manifestations of Hepatitis C Virus: Diabetes Mellitus, Dyslipidemia. Clin Liver Dis. 2017, 21, 475–486. [Google Scholar] [CrossRef] [PubMed]
- DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014, 46, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Hut, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Shojima, N.; Hosoe, J.; Kadowaki, T. Genetic architecture of type 2 diabetes. BBRC 2014, 452, 213–220. [Google Scholar] [CrossRef]
- Keaton, J.M.; Hellwege, J.N.; Ng, M.C.; Palmer, N.D.; Pankow, J.S.; Fornage, M.; Wilson, J.G.; Correa, A.; Rasmussen-Torvik, L.J.; Rotter, J.I.; et al. Genome-wide interaction with selected type 2 diabetes loci reveals novel loci for type 2 diabetes in African Americans. Pac. Symp. Biocomput. 2017, 22, 242–253. [Google Scholar]
- Keaton, J.M.; Gao, C.; Guan, M.; Hellwage, J.N.; Palmer, N.D.; Pankow, J.S.; Fornage, M.; Wilson, J.G.; Correa, A. Rasmussen-Torvik LJ; et al. Genome-wide interaction with the insulin secretion locus MTNR1B reveals CMIP as a novel type 2 diabetes susceptibility gene in African Americans. Genet. Epidemiol. 2018, 42, 559–570. [Google Scholar] [CrossRef]
- Davis, T.M.; Cull, C.A.; Holman, R.R. Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the first 9 years of type 2 diabetes: UK Prospective Diabetes Study (UKPDS 55). Diabetes Care 2001, 24, 1167–1174. [Google Scholar] [CrossRef][Green Version]
- Sone, H.; Katagiri, A.; Ishibashi, S.; Abe, R.; Saito, Y.; Murase, T.; Yamashita, H.; Yajima, Y.; Ito, H.; Ohashi, Y.; et al. Effects of lifestyle modifications on patients with type 2 diabetes: The Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three-year interim report. Horm. Metab. Res. 2002, 34, 509–515. [Google Scholar] [CrossRef]
- Sone, H.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N. Japan Diabetes Complication Study Group. Obesity and type 2 diabetes in Japanese patients. Lancet 2003, 361, 85. [Google Scholar] [CrossRef]
- Matsumoto, K.; Miyake, S.; Yano, M.; Ueki, Y.; Yamaguchi, Y.; Akazawa, S.; Tominaga, Y. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 1997, 20, 1562–1568. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- American Diabetes Association 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S73–S85. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Xu, W.; Shi, L.; Zhang, Q.; Zhu, D.; Hu, Y.; Zhou, Z.; Yan, X.; Tian, H.; et al. Effect of intensive insulin therapy on the Beta-cell function and glycemic control in patients with newly diagnosed type 2 diabetes. Lancet 2008, 371, 1753–1760. [Google Scholar] [CrossRef]
- Kendall, D.M.; Cuddihy, R.M.; Berqenstal, R.M. Clinical application of incretin-based therapy: Therapeutic potential, patient selection and clinical use. Eur. J. Intern. Med. 2009, 20, S329–S339. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Vieira, E.; Gylfe, E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 2006, 55, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–843. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; Williams, K.; Hanley, A.J.; Haffner, S.M. Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: Comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes 2008, 57, 1638–1644. [Google Scholar] [CrossRef][Green Version]
- Nagafuchi, S.; Mine, K.; Takahashi, H.; Anzai, K.; Yoshikai, Y. Viruses with masked pathogenicity and genetically susceptible hosts-How to discover potentially pathogenic virus. J. Med. Virol. 2019, 91, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Hyöty, H.; Leon, F.; Knip, M. Developing a vaccine for type 1 diabetes by targeting coxsackievirus B. Expert Rev. Vaccines 2018, 17, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.N.; Hankanemi, M.M.; Svedin, E.; Sioofy-Khojine, A.; Oikarinen, S.; Hyöty, H.; Laitinen, O.H.; Hytönen, V.P.; Flodström-Tullberg, M. A Coxsakievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia 2018, 61, 476–481. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (n = 172) | TYK2PV (n = 18) | Wild Type (n = 154) | p-Value * |
|---|---|---|---|---|
| Age, yr. | 65.5 (57–71) | 62.5 (52.7–73.2) | 66 (57–71) | 0.783 |
| Female sex, no. (%) | 64 (37.2) | 4 (22.2) | 60 (38.9) | 0.164 |
| Duration of diabetes, yr. | 9 (4–15.5) | 10.0 (6.5–15.0) | 9 (4.0–15.7) | 0.93 |
| Body weight, kg | 63.6 (56.2–71.9) | 61.4 (54.5–70.4) | 64 (56.2–72.9) | 0.457 |
| BMI, kg/m2 | 25.2 (22.3–27.4) | 23.4 (21.5–25.3) | 25.4 (22.5–27.6) | 0.025 |
| Hypertension, no. (%) | 82 (47.6) | 5 (27) | 77 (50.0) | 0.074 |
| Dyslipidemia, no. (%) | 89 (51.7) | 8 (44.4) | 81 (52.5) | 0.512 |
| Hyperuricemia, no. (%) | 10 (5.8) | 2 (11.1) | 8 (5.2) | 0.310 |
| Dietetic therapy, no. (%) | 17 (10.0) | 3 (16.6) | 14 (9.0) | 0.318 |
| α-Glucosidase inhibitors, no. (%) | 30 (17.4) | 3 (16.6) | 27 (17.5) | 0.927 |
| Meglitinides, no. (%) | 7 (4.1) | 1 (5.5) | 7 (4.5) | 0.355 |
| Thiazolidinediones, no. (%) | 9 (5.2) | 6 (33.3) | 8 (5.2) | 0.948 |
| Metformin, no. (%) | 76 (44.1) | 6 (33.3) | 70 (45.4) | 0.327 |
| DPP-4inhibitors, no. (%) | 106 (61.6) | 11 (61.1) | 95 (61.0) | 0.962 |
| GLP-1Ra, no. (%) | 7 (4.0) | 2 (11.1) | 5 (3.2) | 0.110 |
| SGLT-2 inhibitor, no. (%) | 32 (19.1) | 2 (11.1) | 31 (20.1) | 0.357 |
| White blood cell, /μl | 6080 (4940–7100) | 5600 (4790–6350) | 6100 (4993–7210) | 0.271 |
| Hemoglobin, g/dl | 14.4 (13.6–15.5) | 14.7 (13.95–15.85) | 14.4 (13.5–15.5) | 0.320 |
| Platelet, x104/μl | 21.8 (18.1–26.0) | 23.9 (18.4–25.8) | 21.6 (17.8–26.0) | 0.545 |
| Neutrophils, % | 58.1 (52.1–64.2) | 60.0 (54.5–65.0) | 58.1 (52.0–64.2) | 0.672 |
| Lymphocytes, % | 31.8 (26.3–38.5) | 32.1 (25.7–36.7) | 31.8 (26.5–38.7) | 0.583 |
| Monocytes, % | 5.3 (4.4–6.1) | 5.0 (4.35–5.85) | 5.4 (4.4–6.2) | 0.456 |
| Eosinophils, % | 2.4 (1.6–3.9) | 2.0 (1.5–4.2) | 2.4 (1.6–3.6) | 0.869 |
| Basophils, % | 0.5 (0.4–0.9) | 0.5 (0.2–0.7) | 0.5 (0.4–0.9) | 0.246 |
| Cr, mg/dl | 0.74 (0.61–0.84) | 0.76 (0.67–0.85) | 0.72 (0.60–0.84) | 0.230 |
| eGFR, ml/min/1.73m2 | 77.0 (67.0–88.2) | 79.6 (68.6–90.5) | 76.9 (67.0–87.5) | 0.779 |
| AST, U/l | 22 (18–27) | 24.0 (15.7–27.2) | 22.0 (18.0–27.3) | 0.580 |
| ALT, U/l | 22 (16–33) | 21.5 (14.7–28.2) | 22.5 (16–33.3) | 0.604 |
| HDL, mmol/L | 1.48 (1.24–1.68) | 1.42 (1.22–1.67) | 1.48 (1.24–1.70) | 0.491 |
| LDL, mmol/L | 2.85 (2.25–3.39) | 2.67 (2.21–3.16) | 2.87 (2.25–3.42) | 0.302 |
| Triglyceride, mmol/L | 1.23 (0.88–1.67) | 1.03 (0.75–1.27) | 1.28 (0.92–1.69) | 0.069 |
| Fasting plasma glucose, mg/dl | 137 (117–153) | 139 (116–149) | 136 (116–154) | 0.924 |
| HbA1c, mmol/mol (%) | 52(7.0) {46(6.4)-59(7.6)} | 50(6.8) {46(6.4)-57(7.4)} | 52(7.0) {46(6.4)-59(7.6)} | 0.741 |
| Insulin, μU/ml | 6.0 (3.6–8.9) | 3.9 (2.6–6.0) | 6.2 (3.9–9.7) | 0.007 |
| HOMA-β | 29.9 (17.3–47.1) | 19.1 (12.3–34.7) | 30.5 (18.7–48.3) | 0.021 |
| HOMA-IR | 1.91 (1.23–3.06) | 1.39 (0.73–1.73) | 2.05 (1.28–3.30) | 0.006 |
| C-peptide, ng/ml | 1.72 (1.26–2.44) | 1.37 (0.86–1.76) | 1.76 (1.32–2.55) | 0.008 |
| C-peptide index | 1.28 (0.92–1.74) | 0.90 (0.70–1.38) | 1.32 (0.96–1.77) | 0.016 |
| Anti-GAD-Ab positive, no. (%) | 0 (0) | 0 (0) | 0 (0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, H.; Takahashi, H.; Mine, K.; Higashimoto, K.; Inoue, K.; Kojima, M.; Kuroki, S.; Eguchi, T.; Ono, Y.; Inuzuka, S.; et al. TYK2 Promoter Variant Is Associated with Impaired Insulin Secretion and Lower Insulin Resistance in Japanese Type 2 Diabetes Patients. Genes 2021, 12, 400. https://doi.org/10.3390/genes12030400
Mori H, Takahashi H, Mine K, Higashimoto K, Inoue K, Kojima M, Kuroki S, Eguchi T, Ono Y, Inuzuka S, et al. TYK2 Promoter Variant Is Associated with Impaired Insulin Secretion and Lower Insulin Resistance in Japanese Type 2 Diabetes Patients. Genes. 2021; 12(3):400. https://doi.org/10.3390/genes12030400
Chicago/Turabian StyleMori, Hitoe, Hirokazu Takahashi, Keiichiro Mine, Ken Higashimoto, Kanako Inoue, Motoyasu Kojima, Shigetaka Kuroki, Takahisa Eguchi, Yasuhiro Ono, Sadataka Inuzuka, and et al. 2021. "TYK2 Promoter Variant Is Associated with Impaired Insulin Secretion and Lower Insulin Resistance in Japanese Type 2 Diabetes Patients" Genes 12, no. 3: 400. https://doi.org/10.3390/genes12030400
APA StyleMori, H., Takahashi, H., Mine, K., Higashimoto, K., Inoue, K., Kojima, M., Kuroki, S., Eguchi, T., Ono, Y., Inuzuka, S., Soejima, H., Nagafuchi, S., & Anzai, K. (2021). TYK2 Promoter Variant Is Associated with Impaired Insulin Secretion and Lower Insulin Resistance in Japanese Type 2 Diabetes Patients. Genes, 12(3), 400. https://doi.org/10.3390/genes12030400







