Abstract
Osteoporosis, characterized by reduced bone mass and increased bone fragility, is a disease prevalent in women. Likewise, breast cancer is a multifactorial disease and considered the major cause of mortality in premenopausal and postmenopausal women worldwide. Our data demonstrated the association of the MYLK gene and PTGS1 gene variants with osteoporosis and benign breast tumor risk and the impact of ovariectomy on osteoporosis in Korean women. We performed a genome-wide association study (GWAS) of women with osteoporosis and benign breast tumors. There were 60 single nucleotide polymorphisms (SNPs) and 12 SNPs in the MYLK and PTGS1 genes, associated with benign breast tumors and osteoporosis. Our study showed that women with homozygous MYLK rs12163585 major alleles had an increased risk of osteoporosis following ovariectomy compared to those with minor alleles. Women carrying the minor PTGS1 rs1213265 allele and not treated via ovariectomy carried a higher risk of osteoporosis than those who underwent ovariectomy with a homozygous genotype at the major alleles. Our results suggest that both the MYLK and PTGS1 genes are genetic factors associated with the phenotypes, and these associations appear to be modulated by ovariectomy.
Keywords:
osteoporosis; benign breast tumor; ovariectomy; MYLK; PTGS1; genome-wide association study 1. Introduction
Osteoporosis is defined by low bone mass and deterioration in bone architecture [1,2]. It is mainly caused by factors such as increasing age, postmenopausal status, deficiencies in sex hormones like estrogen and androgen, premature ovarian failure, ethnic background, low body mass index, and vitamin D deficiency [3]. Previous studies have shown that early or premature menopause and ovarian resection were associated with the risk of osteoporosis due to the effect of estrogen deficiency on osteoclasts [4,5,6]. The Korean National Health and Nutrition Examination Survey (KNHANES) reported that the incidence of osteoporosis in Korean females aged 50 years and older was 38% [7].
Benign breast disease, which proliferates in epithelial tissue, is a breast cancer precursor associated with an increased risk of breast cancer [8,9]. Women carrying benign breast tumors had a two-fold increased risk of breast cancer and a five-fold increased risk of an atypical hyperplasia [8]. Breast cancer is a multifactorial disease and the major cause of mortality in premenopausal and postmenopausal women worldwide [10]. The accumulation of adipocytes in postmenopausal women can influence breast cancer development by increasing estrogen and insulin levels [11,12,13].
Thus, biochemical and genetic links between postmenopausal osteoporosis and breast disease are of great interest. Both bone and breast tissue are regulated not only by estrogen, which is a hormone that controls bone density, but also receptor activator of nuclear factor-κB ligand (RANKL), thereby restoring the equilibrium between bone formation and resorption [14,15,16,17]. In postmenopausal women, breast cancer and osteoporosis are common, and although both are dependent on estrogens this leads to conflicting implications for the diagnosis and treatment, that is, estrogens reduce the risk of fractures but increase the risk of breast cancer. In particular, the RANKL/RANK pathway, regulating osteoclast differentiation and activation, is also involved in breast carcinogenesis [18].
To prevent and treat heavy menstrual bleeding, dysmenorrhea, chronic pelvic pain, endometriosis, uterine prolapse, and gynecologic cancer, ovariectomy, a major gynecologic procedure, has been performed in premenopausal women [4]. Breast cancer and osteoporosis are affected by estrogen levels, which complicate the diagnosis and treatment. Estrogens, which are secreted by the ovary, reduce the risk of fractures but increase the risk of breast cancer [19]. Therefore, ovariectomy prevents breast cancer but is one of the risk factors for bone loss [20]. One study investigated the risk of osteoporosis in Korean women who underwent hysterectomy, which increased the risk of osteoporosis regardless of age or bilateral ovariectomy [4].
To the best of our knowledge, no study has demonstrated an association between both MYLK and PTGS1 genes in the risk of osteoporosis and benign breast tumor. Furthermore, few/no study has examined the impact of ovariectomy on gene-disease risk for osteoporosis among Korean women. We identified the SNPs in our genome-wide association study (GWAS) that simultaneously increased the risk of osteoporosis and benign breast tumors.
2. Materials and Methods
2.1. Study Population
The present study was performed with data obtained from the Korean Genome and Epidemiology Study (KoGES) [21], which was a large-scale cohort study conducted in a Korean population. KoGES is composed of six types cohorts, including subjects from the Health Examinee (HEXA) study used to determine the association between benign breast tumors, osteoporosis, and ovariectomy. Details of the KoGES and HEXA studies are described elsewhere [21]. Briefly, a total of 173,357 participants aged 40–79 years were recruited between 2004 and 2013. Following informed consent, the participants were examined at health examination centers in Korea. We performed a series of cross-sectional analyses in the present study by the baseline data from the HEXA study [21].
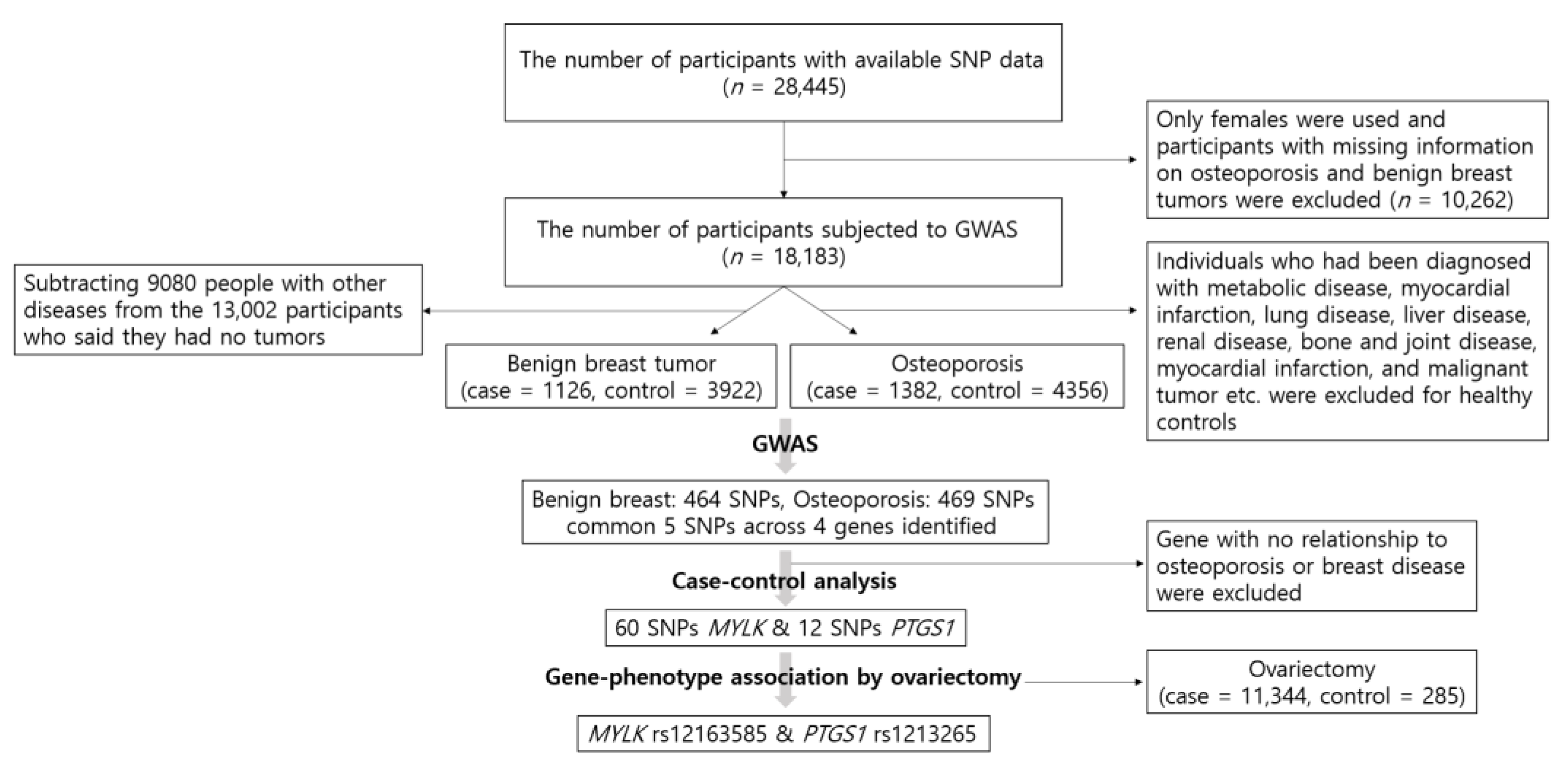
Figure 1 is a schematic illustration depicting the participant selection process for this study and the process from GWAS.
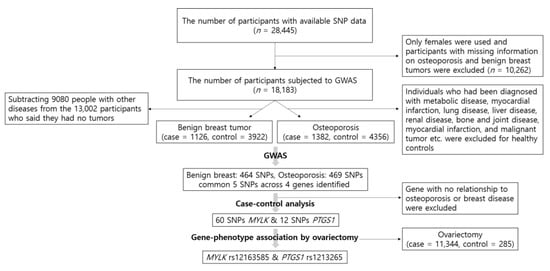
Figure 1.
Flow chart of exclusion criteria in the present study population and the process from genome-wide association study (GWAS).
We used GWAS to test the association between SNPs and the genetic risk of benign breast tumors and osteoporosis. Participants with missing information on osteoporosis and benign breast tumors were excluded from the 28,445 participants with accessible SNP information. In addition, since we analyzed the association between osteoporosis and ovariectomy, only females were evaluated in this study (n = 18,183). A control group of 6518 healthy participants was used in the present study, which consisted of individuals who were not diagnosed with hypertension, diabetes mellitus, hyperlipidemia, transient ischemic attack, myocardial infarction, chronic gastritis, gastric ulcer, intestinal polyps, acute liver disease, fatty liver, cholelithiasis, chronic bronchitis, chronic obstructive pulmonary disease (COPD), asthma, allergy, thyroid disease, arthritis, osteoporosis, gout, cataracts, glaucoma, periodontal disease, chronic nephritis, renal failure, malignant tumor, or fractures. Since the disease history was included in the KoGES project, it was selected as the criterion for healthy controls. Of the 6518 healthy controls, 2162 males were excluded and a total of 4356 healthy female controls were used. However, because benign breast tumors were not considered when the healthy controls were identified, the healthy control group for benign breast tumors included a final number of 3922 after subtracting 9080 subjects with other diseases from the 13,002 participants who stated the absence of tumors. The diagnosis of benign breast tumors and/or osteoporosis was made by a medical doctor. Consequently, 4356 healthy controls and 1382 osteoporosis cases were identified, and 3922 healthy controls and 1126 benign breast tumor cases were analyzed.
2.2. Assignment of Ovariectomy
Information on the ovariectomies was obtained through self-report during a trained interviewer administered survey. The questionnaire was composed of four options: 1 = no, did not undergo ovariectomy, 2 = yes, removed only one ovary, 3 = yes, removed both of them but partially, and 4 = yes, removed both of them entirely. To ensure accurate results, the participants who answered 1–3 were defined as the “no ovariectomy” group and the participants with responses of 4 were defined as the ovariectomy group. Of a total of 18,183 women, 11,344 answered the questionnaire, 11,629 women were defined as the “no ovariectomy” group, and 285 women were defined as the ovariectomy group. The subjects’ age based on a response of 4, indicating both ovaries were entirely removed, was considered the surgical age.
2.3. Genome-Wide Genotyping and Selection of SNPs
Genotype data were obtained from the Center for Genome Science, Korea National Institute of Health. DNA samples isolated from the participants were genotyped with an Axiom® 2.0 Reagent Kit (Affymetrix Axiom® 2.0 Assay User Guide). The genotype data were obtained from the K-CHIP designed by the Center for Genome Science at the Korea National Institute of Health. Additional information regarding this protocol has been presented elsewhere [22,23]. Subjects with a high missing call rate (>10%), high missing genotype rates (>5%), minor allele frequency <0.01, or gender inconsistency were excluded during the quality control process. We performed GWAS and selected SNPs with a p-value of less than 0.001. The location of the genes and SNPs was identified using the genome reference consortium human build 37 (GRCh37).
2.4. Statistical Analysis
PLINK version 1.90 beta (https://www.cog-genomics.org/plink2, accessed on 3 March 2021) and predictive analytics software (PASW) version 18.0 (SPSS Inc., Chicago, IL, USA) were used for most statistical analyses. We investigated the interaction between SNP and the risk of diseases using logistic regression models with the additive genetic model. The multivariable model was adjusted for age and residence [24,25] to investigate the effect of complex factors. The residential areas initially consisted of 16 areas indicated by administrative district codes. However, we reorganized them into rural (Gyeonggi, Chungcheongbuk, Chungcheongnam, Sejong city, Gangwon, Jeollabuk, Jeollanam, Gyeongsangbuk, and Gyeongsangnam) and urban (Seoul, Busan, Ulsan, Daegu, Daejeon, Incheon, and Gwangju) areas. The association between SNPs and the risk of diseases was computed by the odds ratio (OR) and 95% confidence interval (95% CI). Statistical significance was determined by the two-tailed Student’s t-test, and p-values < 0.05 were considered significant.
HaploReg database (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php, accessed on 3 March 2021) was also used to predict the potential functional effects of the MYLK rs12163585 and PTGS1 rs1213265 genotypes. The geography of genetic variants (GGV) browser (https://popgen.uchicago.edu/ggv, accessed on 3 March 2021) was utilized to report the worldwide frequency of the minor alleles of SNPs.
2.5. Ethical Review
This study was approved by the Institutional Review Board of the Korean National Institute of Health (KNIH, KBN-2019-004) and Hoseo University (IRB approval no.: 1041231-150811-BR-034-03). Written informed consent was obtained from all subjects.
3. Results
3.1. Subject Characteristics
In this study, 18,183 females from the HEXA cohort were included in the association study. Age and the number of subjects with each disease are listed in Table 1. Healthy controls were filtered from 18,183 females in the HEXA cohort and categorized into 4356 healthy controls and 1382 women with osteoporosis (cases). Similarly, there were 3922 healthy controls and 1126 women with benign breast tumors (cases). There were 285 ovariectomy cases in the total HEXA females, and 61 and 45 females had ovariectomies in the healthy controls and cases in the osteoporosis group, respectively. In the benign breast tumor group, 54 healthy controls and 38 subjects with benign breast tumors underwent ovariectomies. The osteoporotic patients were older (average 59.6 years) than the subjects in the control group (average 50.5 years). In addition, the age at ovariectomy in the osteoporotic group was older (average 47 years) than that of the healthy controls (average 45.33 years). There was no significant difference between the cases and the controls in the benign breast tumor group.

Table 1.
Characteristics of women in the Health Examinee (HEXA) study cohort.
3.2. Selection of SNPs from Genome-Wide Association Study Based on the HEXA Data
In the GWAS, the SNPs (p < 0.001) were filtered based on the HEXA data. Of them, 464 and 469 SNPs associated with benign breast tumor and osteoporosis were found respectively. Only five SNPs were common between the two diseases (rs3732486, rs3732487, rs58154051, rs2293973, and rs1213265). Two (rs3732486 and rs3732487) of the five SNPs were found in the MYLK gene, rs58154051 in the RPS6KA2 gene, rs2293973 in DLGAP2, and rs1213265 in PTGS1 (Table 2). In this study, after excluding genes unrelated to osteoporosis or breast disease, we focused on the MYLK and PTGS1 genes, which presented the least p-value and the highest odds ratio for both diseases. The rs3732487 SNP in MYLK showed associations with both benign breast tumor (OR = 1.20, 95% CI: 1.09–1.32, p = 1.94 × 10−4) and osteoporosis (OR = 1.21, 95% CI: 1.09–1.35, p = 2.57 × 10−4). In addition, rs1213265 in PTGS1 showed significant associations with benign breast tumors (OR = 1.81, 95% CI: 1.29–2.53, p = 6.03 × 10−4) and osteoporosis (OR = 2.03, 95% CI: 1.36–3.03, p = 4.90 × 10−4).

Table 2.
Significant association of SNPs in both benign breast tumors and osteoporosis in the HEXA women cohort.
3.3. Association of SNPs with Benign Breast Tumor and Osteoporosis
We analyzed the association between benign breast tumors and osteoporosis and MYLK and PTGS1 SNPs. Sixty SNPs in the MYLK gene and 12 SNPs in the PTGS1 gene were found. Among the 60 SNPs in the MYLK gene, nine and six SNPs were significantly associated with benign breast tumors and osteoporosis, respectively (Table 3 and Supplementary Table S1). In addition, three common SNPs (rs3732487, rs3732486, and rs12163585) were associated with both diseases. In the case of the PTGS1 gene, of the 12 SNPs, two common SNPs (rs1213265 and rs3119773) were associated with benign breast tumors and osteoporosis (Supplementary Table S1). The association of these five SNPs in the two genes with benign breast tumors and osteoporosis in the HEXA cohort females was analyzed using the additive model. While the MYLK rs3732487 and rs3732486 variants showed similar patterns of increased risk of benign breast tumors (OR = 1.20, 95% CI: 1.09–1.32, p = 1.94 × 10−4; OR = 1.19, 95% CI: 1.08–1.31, p = 3.52 × 10−4, respectively) and osteoporosis (OR = 1.21, 95% CI: 1.09–1.35, p = 2.57 × 10−4; OR = 1.21, 95% CI: 1.09–1.34, p = 3.23 × 10−4, respectively), the rs12163585 variant was associated with a decreased risk of benign breast tumors and osteoporosis (OR = 0.87, 95% CI: 0.79–0.96, p = 5.69 × 10−3; OR = 0.88, 95% CI: 0.80–0.98, p = 1.98 × 10−2, respectively) (Table 3). In the case of the PTGS1 gene, the rs1213265 and rs3119773 variants significantly increased the risk of benign breast tumors (OR = 1.89, 95% CI: 1.23–2.88, p = 3.38 × 10−3; OR = 1.88, 95% CI: 1.23–2.88, p = 3.41 × 10−3, respectively) and osteoporosis (OR = 2.88, 95% CI: 1.36–3.85, p = 1.93 × 10−3; OR = 2.28, 95% CI: 1.35–3.84, p = 1.99 × 10−3, respectively) (Table 3). Nine other SNPs in the MYLK gene showed association with benign breast tumors or osteoporosis (p < 0.05), but the association was significant in only one of the two diseases.

Table 3.
Association of SNPs in the MYLK and PTGS1 genes with benign breast tumor and osteoporosis in the HEXA women included in the additive genetic model.
3.4. MYLK rs12163585 Variant and Ovariectomy with Osteoporosis
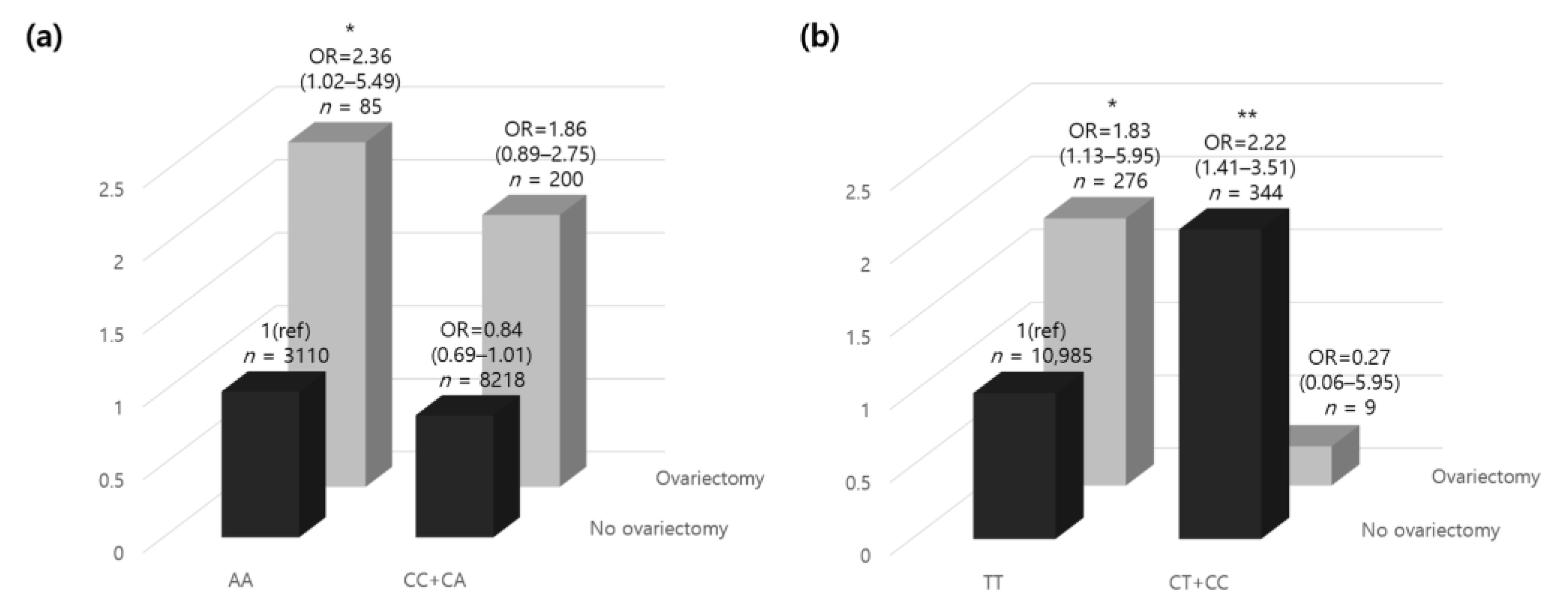
Excluding the participants with missing genotype data (n = 16), among the 11,613 participants, there were 200 ovariectomized participants with minor alleles and 85 with homozygous genotypes in the major alleles. In contrast, in the non-ovariectomized participants, the number of minor allele carriers was 8218, and 3110 carried a homozygous genotype in the major allele. In the presence of a minor rs12163585 allele, the risk of osteoporosis was increased 1.86-fold in women who underwent an ovariectomy. However, in individuals carrying the homozygous genotype in the major alleles, the risk of osteoporosis was significantly increased by 2.36-fold (Figure 2a). The results showed that those who underwent ovariectomy, which increased the risk of osteoporosis and was minor allele carriers, carried a decreased risk of developing osteoporosis than those without minor alleles. Those who had minor alleles and underwent ovariectomy had a lower risk of developing osteoporosis than those carrying homozygous genotypes in the major alleles. Our results confirmed that having a minor rs12163585 allele lowered the risk for osteoporosis, which can be interpreted in the same context as the GWA study results.
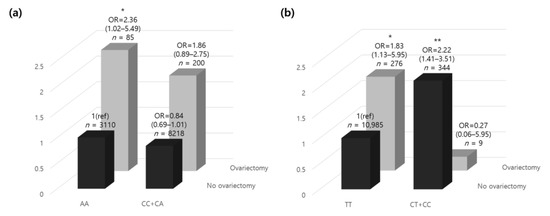
Figure 2.
The relative odds ratio of osteoporosis according to oophorectomy and (a) MYLK rs12163585 and (b) PTGS1 rs1213265 genotypes. The ORs (95% CI) of the genetic correlation between dominant/homozygous genotypes of each gene and ovariectomy are shown. The homozygous model was set as the reference allele. p-values were adjusted for age and residential area by analysis of covariance. * p < 0.05, ** p < 0.01. Abbreviations: OR, odds ratio; CI, confidence interval.
3.5. PTGS1 Variant rs1213165 and Ovariectomy with Osteoporosis
Excluding participants with missing genotype data (n = 15), the 11,614 participants included nine minor allele carriers and 276 homozygous genotype major allele carriers who had ovariectomies. In contrast, in the non-ovariectomized participants, the number of minor allele carriers was 344, and 10,985 carried homozygous genotypes in the major allele. With a homozygous rs1213165 genotype in the major allele, the risk of osteoporosis was increased 1.83-fold in women who underwent ovariectomy (Figure 2b). In contrast, in individuals carrying a minor allele, the risk of osteoporosis was decreased 0.27-fold, but there was no statistical significance. In addition, those who did not undergo ovariectomy and were minor allele carriers had a 2.22-fold higher risk of developing osteoporosis than those without minor alleles. Interestingly, those who carried minor alleles and did not undergo ovariectomy had a higher risk of osteoporosis than those with a homozygous genotype in the major allele who underwent ovariectomy.
3.6. Functional Annotations of MYLK and PTGS1
The HaploReg database was used to predict the potential functional effects of MYLK rs12163585 and PTGS1 rs1213265 (Supplementary Table S2). Both MYLK rs12163585 and PTGS1 rs1213265 were found to change the motif factor-binding site, as shown in Supplementary Table S2. We also performed the analysis of eQTL for MYLK and PTGS1 based on GTEx databases (Supplementary Figure S1). Gene expression for MYLK was high in the female genital organs, especially breast, cervix, ovary, and uterus.
4. Discussion
In this prospective GWAS, we identified an association between MYLK rs12163585 and PTGS1 rs1213265 variants, ovariectomy, and the risk of osteoporosis using HEXA Korean women data. Our results showed that (1) women with a homozygous genotype in the MYLK rs12163585 major alleles had an increased risk of osteoporosis following ovariectomy than those with minor alleles, and (2) women who had the minor PTGS1 rs1213265 allele and were not ovariectomized carried a higher risk of osteoporosis than those with homozygous genotype of the major alleles and undergoing ovariectomy. The GWAS of osteoporosis and benign breast tumors revealed that five common SNPs in four genes were significantly associated with the two diseases. SNP rs58154051, located on chromosome 6 and belonging to the RPS6KA2 gene, did not show a statistically significant relationship with ovariectomy or osteoporosis (Supplementary Figure S2). SNP rs2293973, which is located on chromosome 8 and belongs to DLGAP2, was considered a gene variant unrelated to osteoporosis or breast disease. From the present study, following genetic signals, we can prevent osteoporosis and breast cancer, and suggest ovariectomy or not. However, further studies with greater age of cases and large sample size, especially in stratified analysis, are required in the future. To the best of our knowledge, no study has reported the association between ovariectomies and osteoporosis with MYLK and PTGS1 genes until now, and consequently, the MYLK and PTGS1 genes were selected in the present study.
Although the genetic variations in the MYLK gene were selected as the targets in our study, its association with breast disease or osteoporosis has yet to be reported. MYLK is an element of the actin cytoskeleton and is involved in foundational cellular processes such as cell adhesions, migration, and survival [26]. It is included in the oxytocin signaling pathway and hence, RhoA/Rho kinase pathways are also activated, contributing to the invasion of cancer cells [27]. Expression of MYLK is downregulated in breast cancer and loss of MYLK leads to disruption of cell–cell adhesion and invasive behavior of breast epithelial cells [28].
Meanwhile, MLCK is well known as a molecular target in lung inflammation, a defining feature of sepsis and acute lung injury (ALI) [29]. Gao et al. speculated that MYLK was a candidate gene engaged in acute lung injury susceptibility and disease [30]. Another study showed that the thoracic aortic disease phenotype was associated with MYLK pathogenic variants [31]. Recently, Dai et al. reported the higher expression of circular RNA (circRNA) MYLK in human prostate cancer tissue and suggested using circRNA-MYLK as a tool to diagnose and determine treatments for prostate cancer [32]. The MYLK gene significantly influenced the progression of prostate cancer, which is a sex hormone-dependent disease in males, and the MYLK variants found in this study were associated with benign breast tumors related to female hormones, in line with the previous study. Furthermore, androgen, involved in maintaining bone mass density and preventing osteoporosis, is transformed into endogenous estrogen. The ovaries generate enormous amounts of androgen for years in postmenopausal women, helping to retain bone mass. Ovariectomy, which reduces androgen production, may increase the risk of osteoporosis [33]. Our results show the correlation between the MYLK gene and ovariectomy and demonstrate that the risk of osteoporosis in women who underwent ovariectomy was significantly higher and nearly double the risk of those without (Figure 2a).
PTGS1, also known as cyclooxygenase 1 (COX1), is ubiquitously found in tissues and involved in the biosynthesis of prostaglandin (PG), which regulates renal, gastrointestinal, and platelet function [34]. Besides, PTGS1 has been connected with multiple pathological disorders including inflammation, arthritis, and cancer. One study had compared whole-genome expression data of breast tissue samples with serum hormone levels using data from healthy women and breast cancer patients using microarrays. PTGS1 was found differentially expressed dependent on estradiol levels, which was downregulated in breast samples from women with high serum estradiol [35]. In both ex vivo and in vivo studies, PTGS1 that controls osteogenesis of adipose-derived stem cells via regulating the NF-κB signaling pathway is required for the osteogenic differentiation of adipose-derived stem cells [36]. Nagao et al. performed a meta-analysis to determine the association between PTGS variants and nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the biosynthesis of PG by PTGS and reduce inflammation [37]. Another study confirmed wide genomic regions that caused inflammatory arthritis in a heterogeneous [10] mice cohort and identified PTGS1 as a key candidate based on the differential expression in arthritis [38]. Wang et al. reported that the depletion of PTGS1 promoted osteogenesis in adipose-derived stem cells and suppressed the NF-κB pathway. Additionally, the knockdown of PTGS1 may regulate the inflammatory microenvironment during bone remodeling [36].
Most hysterectomies performed for gynecologically benign conditions, preserve the ovaries. However, occasionally, some physicians suggest bilateral ovariectomies (BOs) along with hysterectomy to prevent the development of cancer [39]. The hysterectomy rates for benign disease were 1.48, 1.49, and 1.52/1000 Korean women aged over 16 years in 2007, 2008, and 2009, respectively, which showed an increasing trend [40]. In our present study, we considered a case of ovariectomy when bilateral ovariectomy was performed, and the rate of ovariectomies for benign breast tumors was 3.37/1000 women. The data used in the study were followed up from 2004 to 2013, with increasing trend similar to that of the previous study.
We analyzed the minor allele frequency of MYLK rs12163585 and PTGS1 rs1213265 with a geographic genome variants (GGV) browser. The GGV browser uses maps of allelic frequencies in populations distributed across the globe based on 1000 genomes (hg19). While rs12163585 was mostly seen in Southeast Asia, rs1213265 was mostly detected in Africa (Supplementary Figure S1). The MYLK rs12163585 minor allele was associated with a decreased risk of osteoporosis and benign breast tumors in Asia, while the PTGS1 rs1213265 minor allele detected mostly in Africa was associated with an increase in both diseases. In the Haploview results, MYLK rs12163585 was predicted to change the motif factor binding site of GATA (Supplementary Table S2). GATAs (GATA-DNA-binding protein) are known as a transcription factor in osteoblasts and functions in transducing cell survival signaling. GATAs are expressed in osteoblasts and control osteoblast survival and functions [41]. Above all, GATA-3 has been reported that its expression is induced during fracture healing and many studies have reported the correlation between the GATA-3 and estrogen receptor in breast cancers as well [42,43,44]. Additionally, SOX, which was predicted to change the motif factor binding site by MYLK, was suggested to sensitive triple-negative breast cancer marker along with GATA-3 [45]. Expression of HAND1, which was predicted to changed motif factor binding site by PTGS1, develops long bones and involves in their morphogenesis [46]. Smad4 gene that has interaction with estrogen receptor α is required for TGF-β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells [47,48,49].
5. Conclusions
Genetic variants in MYLK and PTGS1 are associated with both benign breast tumors and osteoporosis. Analysis of the differences in the risk of osteoporosis and ovariectomy in the MYLK rs12163585 and PTGS1 rs1213265 genotypes showed significant associations with each genotype. Consequently, pathological factors such as ovariectomies substantially affect the association between gene variants and osteoporosis in Korean women.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/12/3/378/s1, Figure S1: Geographic distribution of (a) MYLK rs12163585 and (b) PTGS1 rs1213265. The position of the SNPs is shown at the top (chr3:123416351 and chr9:125136447) and the blue quarter indicates a 0.25 minor allele global frequency based on 1000 genomes (hg19). In Korea, the minor allele frequencies of rs12163585 and rs1213265 were 0.48 and 0.015, respectively. Figure S2: Relative odds ratio of osteoporosis according to the ovariectomy and RPS6KA2 rs58154051. The OR (95% CI) of genetic interaction of dominant /homozygous genotypes of each gene with variable ovariectomy. The homozygous model was set as the reference allele. p values were adjusted for age and residential area by analysis of covariance. Abbreviations: OR, odds ratio; CI, confidence interval. Table S1: Association of the SNPs in MYLK and PTGS1 with benign breast tumors and osteoporosis in the HEXA women cohort in the additive genetic model. Table S2: Results of HaploReg analysis of MYLK rs12163585 and PTGS1 rs1213265. Abbreviations: A1, minor allele; A2, major allele; IPSC, Ips DF 6.9 cells.
Author Contributions
Conceptualization, H.-W.C. and H.-S.J.; methodology, H.-S.J.; software, H.-W.C.; validation, H.-W.C., H.-S.J. and Y.-B.E.; investigation, H.-W.C.; resources, Y.-B.E.; data curation, H.-W.C. and H.-S.J.; writing—original draft preparation, H.-W.C. and H.-S.J.; writing—review and editing, H.-S.J. and Y.-B.E.; supervision, H.-S.J. and Y.-B.E.; project administration, Y.-B.E.; funding acquisition, Y.-B.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Soonchunhyang University Research Fund and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [NRF-2020R1F1A1071977].
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Korean National Institute of Health (KNIH) and Hoeso University (IRB approval no.: 1041231-150811-BR-034-03).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethnical concerns.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Ramaswamy, B.; Shapiro, C.L. Osteopenia and osteoporosis in women with breast cancer. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2003; pp. 763–775. [Google Scholar]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis: Definition and clinical presentation. Spine 1997, 22, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current concepts. Joints 2018, 6, 122. [Google Scholar] [CrossRef]
- Choi, H.G.; Jung, Y.J.; Lee, S.W. Increased risk of osteoporosis with hysterectomy: A longitudinal follow-up study using a national sample cohort. Am. J. Obstet. Gynecol. 2019, 220, 571–573. [Google Scholar] [CrossRef]
- Faubion, S.S.; Kuhle, C.L.; Shuster, L.T.; Rocca, W.A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015, 18, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Joo, I.W.; Jang, M.-J.; Kim, Y.T.; Oh, K.; Oh, H.J. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2011. Yonsei Med. J. 2014, 55, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Fraumeni, J. Cancer Precursors: Epidemiology, Detection, and Prevention; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Silvera, S.A.N.; Rohan, T.E. Benign proliferative epithelial disorders of the breast: A review of the epidemiologic evidence. Breast Cancer Res. Treat. 2008, 110, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Catsburg, C.; Gunter, M.J.; Chen, C.; Cote, M.L.; Kabat, G.C.; Nassir, R.; Tinker, L.; Wactawski-Wende, J.; Page, D.L.; Rohan, T.E. Insulin, estrogen, inflammatory markers, and risk of benign proliferative breast disease. Cancer Res. 2014, 74, 3248–3258. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Gagliardi, J.; Bouhan, D.; Spollen, W.G.; Givan, S.A.; Franklin, C.L. The influence of caging, bedding, and diet on the composition of the microbiota in different regions of the mouse gut. Sci. Rep. 2018, 8, 4065. [Google Scholar] [CrossRef] [PubMed]
- Trémollieres, F.A. Screening for osteoporosis after breast cancer: For whom, why and when. Maturitas 2014, 79, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Sigl, V.; Penninger, J.M. RANKL/RANK–from bone physiology to breast cancer. Cytokine Growth Factor Rev. 2014, 25, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Fontanges, E.; Fontana, A.; Delmas, P. Osteoporosis and breast cancer. Joint Bone Spine 2004, 71, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, H.; Yun, R.; Lee, S.; Lee, J. Estrogen-related receptor γ gene (ESRRG) rs1890552 A> G polymorphism in a Korean population: Association with urinary prostaglandin F2α concentration and impaired fasting glucose or newly diagnosed type 2 diabetes. Diabetes. Metab. J. 2017, 43, 385–388. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.; Huang, L.; Jee, S.H.; Lee, J.H. Genetic risk score of common genetic variants for impaired fasting glucose and newly diagnosed type 2 diabetes influences oxidative stress. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, X.; Li, C.; Li, J.; Zhao, Y.; Chen, L.; Qi, X.; Qiao, L.; Da, W. Urban–Rural Differences in Bone Mineral Density and its Association with Reproductive and Menstrual Factors Among Older Women. Calcif. Tissue Int. 2020, 106, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, S.; Timsina, L.; Bhattacharyya, O.; Fisher, C.S.; Haggstrom, D.A. Breast Cancer Presentation, Surgical Management and Mortality Across the Rural–Urban Continuum in the National Cancer Database. Ann. Surg. Oncol. 2020, 27, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Surma, M.; Shi, S.; Lambert-Cheatham, N.; Shi, J. Novel insights into the roles of Rho kinase in cancer. Arch. Immunol. Ther. Exp. 2016, 64, 259–278. [Google Scholar] [CrossRef]
- Tahara, M.; Morishige, K.-i.; Sawada, K.; Ikebuchi, Y.; Kawagishi, R.; Tasaka, K.; Murata, Y. RhoA/Rho-kinase cascade is involved in oxytocin-induced rat uterine contraction. Endocrinology 2002, 143, 920–929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.; Helfman, D.M. Loss of MLCK leads to disruption of cell–cell adhesion and invasive behavior of breast epithelial cells via increased expression of EGFR and ERK/JNK signaling. Oncogene 2016, 35, 4495–4508. [Google Scholar] [CrossRef]
- Parker, J.C. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J. Appl. Physiol. 2000, 89, 2241–2248. [Google Scholar] [CrossRef]
- Gao, L.; Grant, A.; Halder, I.; Brower, R.; Sevransky, J.; Maloney, J.P.; Moss, M.; Shanholtz, C.; Yates, C.R.; Meduri, G.U. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am. J. Respir. 2006, 34, 487–495. [Google Scholar] [CrossRef]
- Wallace, S.E.; Regalado, E.S.; Gong, L.; Janda, A.L.; Guo, D.-c.; Russo, C.F.; Kulmacz, R.J.; Hanna, N.; Jondeau, G.; Boileau, C. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet. Med. 2019, 21, 144–151. [Google Scholar] [CrossRef]
- Dai, Y.; Li, D.; Chen, X.; Tan, X.; Gu, J.; Chen, M.; Zhang, X. Circular RNA myosin light chain kinase (MYLK) promotes prostate cancer progression through modulating Mir-29a expression. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018, 24, 3462. [Google Scholar] [CrossRef]
- Davey, D.A. Androgens in women before and after the menopause and post bilateral oophorectomy: Clinical effects and indications for testosterone therapy. Women’s Health 2012, 8, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and− 2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef]
- Haakensen, V.D.; Bjøro, T.; Lüders, T.; Riis, M.; Bukholm, I.K.; Kristensen, V.N.; Troester, M.A.; Homen, M.M.; Ursin, G.; Børresen-Dale, A.-L. Serum estradiol levels associated with specific gene expression patterns in normal breast tissue and in breast carcinomas. BMC Cancer 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, M.; Lv, L.; Zhang, X.; Zhang, P.; Zhou, Y. Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling. Stem. Cell. Res. Ther. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Nagao, M.; Sato, Y.; Yamauchi, A. A meta-analysis of PTGS1 and PTGS2 polymorphisms and NSAID intake on the risk of developing cancer. PLoS ONE 2013, 8, e71126. [Google Scholar]
- Johnsen, A.K.; Valdar, W.; Golden, L.; Ortiz-Lopez, A.; Hitzemann, R.; Flint, J.; Mathis, D.; Benoist, C. Genome-wide and species-wide dissection of the genetics of arthritis severity in heterogeneous stock mice. Arthritis. Rheum. 2011, 63, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Chalas, E.; Edelson, M.; Moore, D.H.; Burke, W.M.; Cliby, W.A.; Berchuck, A.; Society of Gynecologic Oncologists Clinical Practice Committee. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: Recommendations based on risk of ovarian cancer. Obstet. Gynecol. 2010, 116, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Park, H.M. Trends in laparoscopic surgery for hysterectomy in K orea between 2007 and 2009. J. Obstet. Gynaecol. Res. 2014, 40, 1695–1699. [Google Scholar] [CrossRef]
- Wu, G.-J.; Wang, W.; Lin, Y.-L.; Liu, S.H.; Chen, R.-M. Oxidative stress-induced apoptotic insults to rat osteoblasts are attenuated by nitric oxide pretreatment via GATA-5-involved regulation of Bcl-X L gene expression and protein translocation. Arch. Toxicol. 2016, 90, 905–916. [Google Scholar] [CrossRef]
- Liao, M.-H.; Lin, P.-I.; Ho, W.-P.; Chan, W.P.; Chen, T.-L.; Chen, R.-M. Participation of GATA-3 in regulation of bone healing through transcriptional upregulation of bcl-x L expression. Exp. Mol. Med. 2017, 49, 398. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Thompson, D.A.; Baker, R.J.; Weigel, R.J. GATA-3 is expressed in association with estrogen receptor in breast cancer. Int. J. Cancer 1999, 84, 122–128. [Google Scholar] [CrossRef]
- Baa, A.K.; Naik, R.D.; Vanidassane, I.; Arora, S.; Shamim, S.A.; Mallick, S.; Batra, A. Unusual gastric metastasis in triple-negative (estrogen receptor/progesterone receptor/HER2neu negative) GATA-binding protein 3-positive breast cancer. IJNM 2020, 35, 82. [Google Scholar]
- Jamidi, S.K.; Hu, J.; Aphivatanasiri, C.; Tsang, J.Y.; Poon, I.K.; Li, J.J.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Sry-related high-mobility-group/HMG box 10 (SOX10) as a sensitive marker for triple-negative breast cancer. Histopathology 2020, 77, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Funato, N.; Taga, Y.; Laurie, L.E.; Tometsuka, C.; Kusubata, M.; Ogawa-Goto, K. The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression. Int. J. Mol. Sci. 2020, 21, 8638. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.; van Dinther, M.; Buijs, J.; Que, I.; Löwik, C.; van der Pluijm, G.; ten Dijke, P. The tumor suppressor Smad4 is required for transforming growth factor β–induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006, 66, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, W.; Tulley, S.; Gupta, G.P.; Serganova, I.; Chen, C.-R.; Manova-Todorova, K.; Blasberg, R.; Gerald, W.L.; Massagué, J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13909–13914. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, Y.; Gathings, B.; Wan, M.; Li, X.; Grizzle, W.; Liu, Z.; Lu, C.; Mao, Z.; Cao, X. Smad4 as a transcription corepressor for estrogen receptor α. J. Biol. Chem. 2003, 278, 15192–15200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).