The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Strains and Genetics
2.2. Clone Generation
2.3. Pupae Staging
2.4. Antibodies and Immunofluorescence
2.5. Measurement of Fluorescence Intensity
2.6. EC Purification, RNA Isolation, and Quantitive PCR
2.7. Statistical Analysis
3. Results
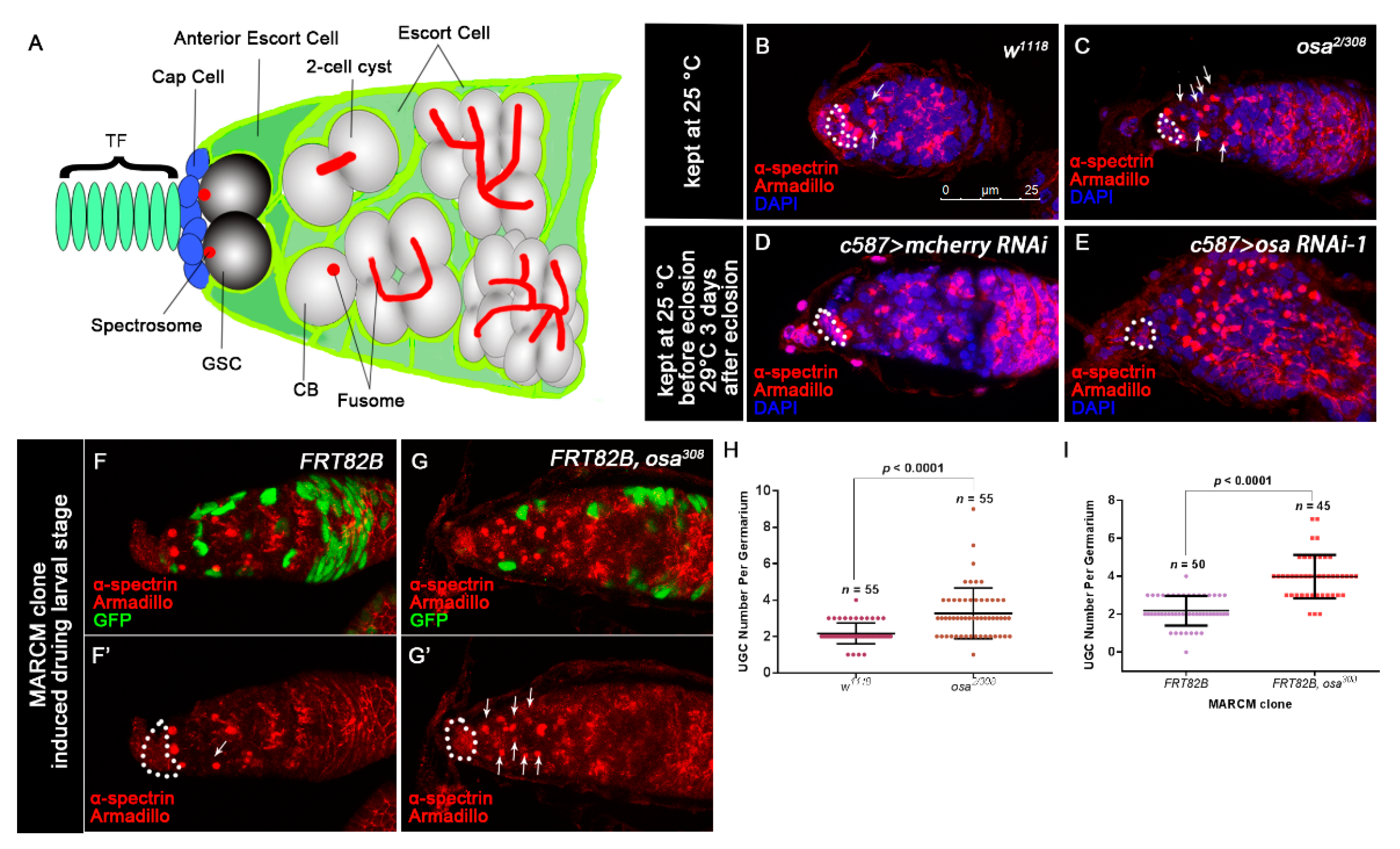
3.1. Osa Is Required in Escort Cells for GSC Progeny Differentiation
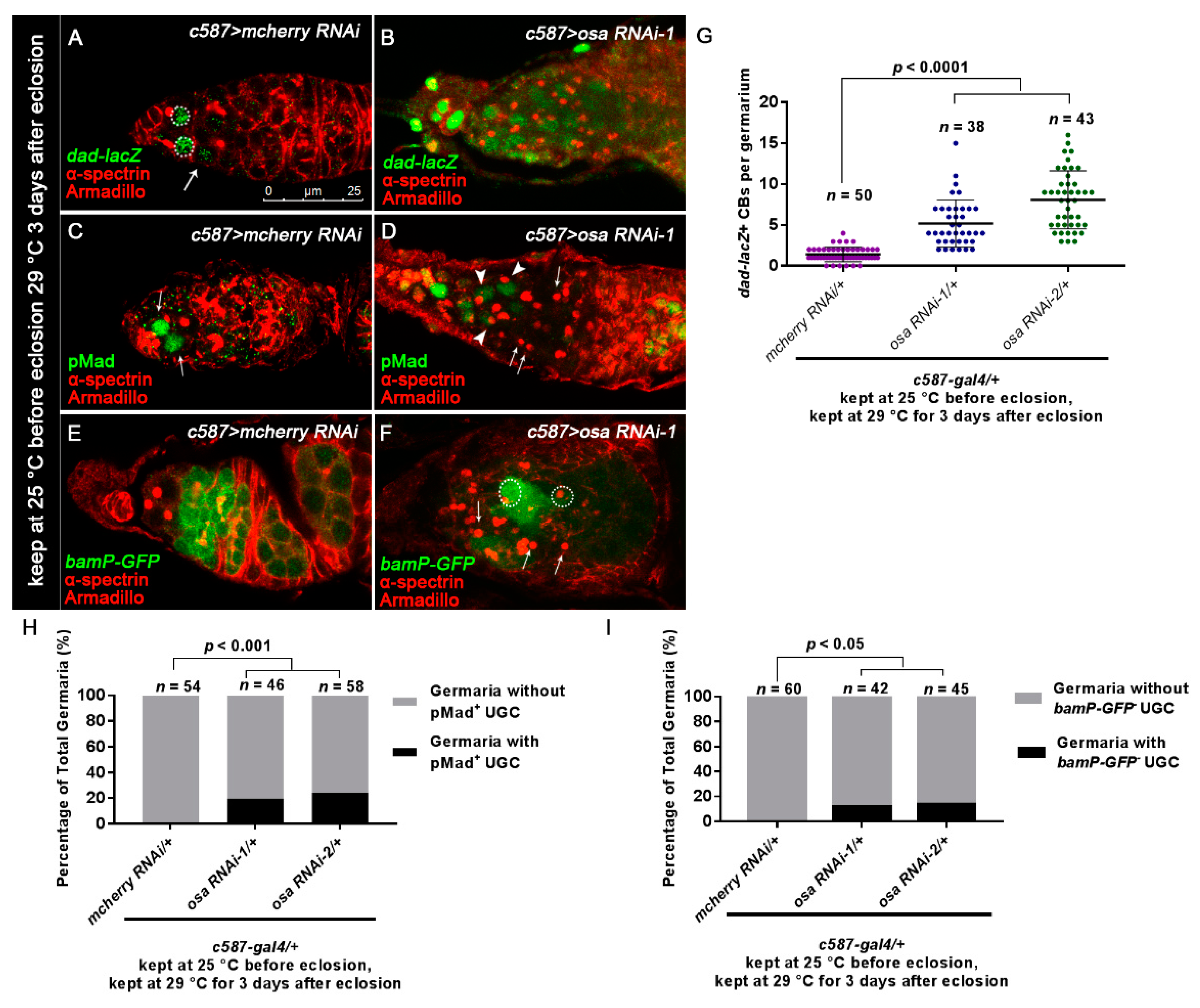
3.2. Osa Is Required for the Restriction of BMP Signaling Outside the GSC Niche
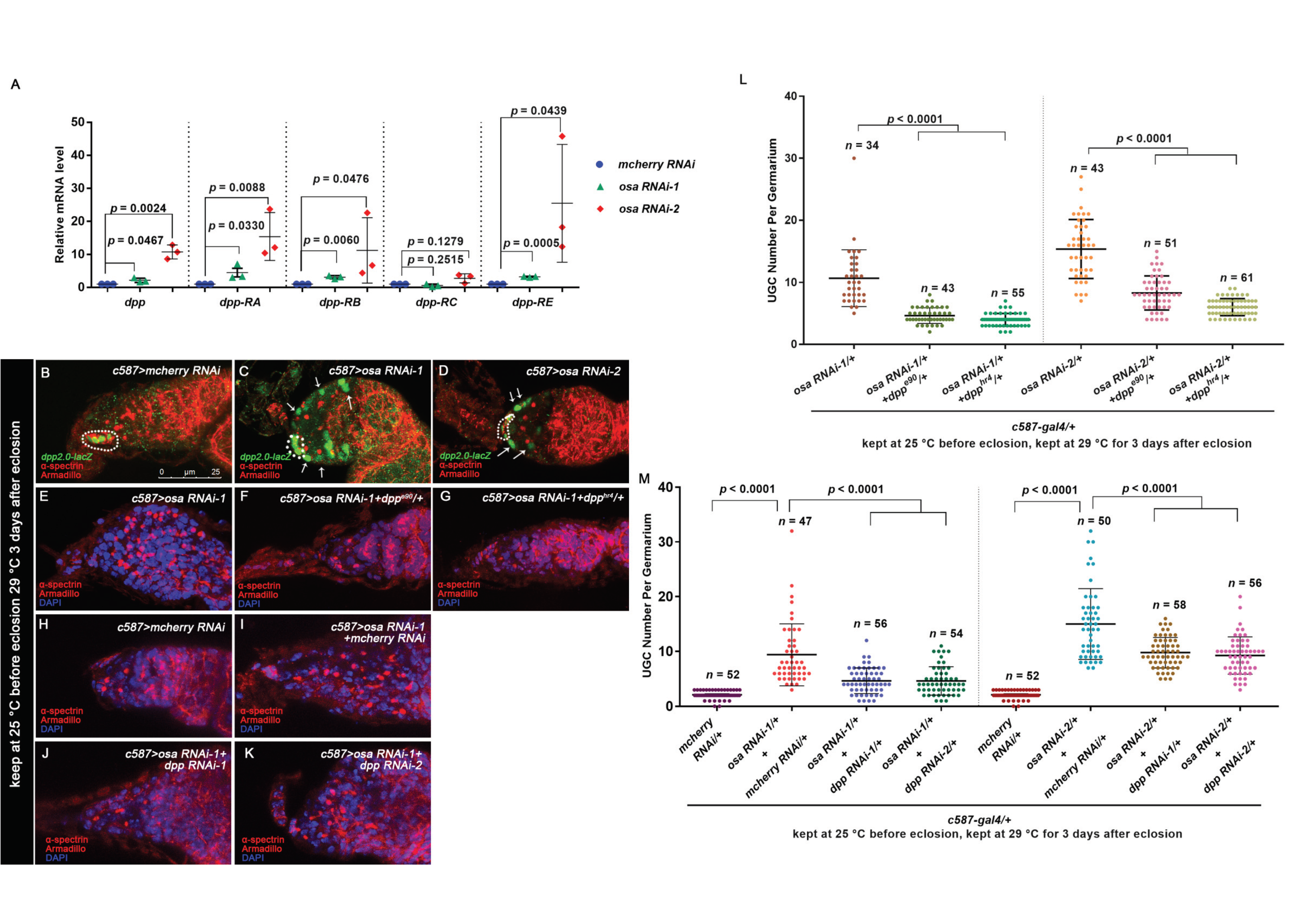
3.3. Osa Restricts Dpp Transcription in Escort Cells
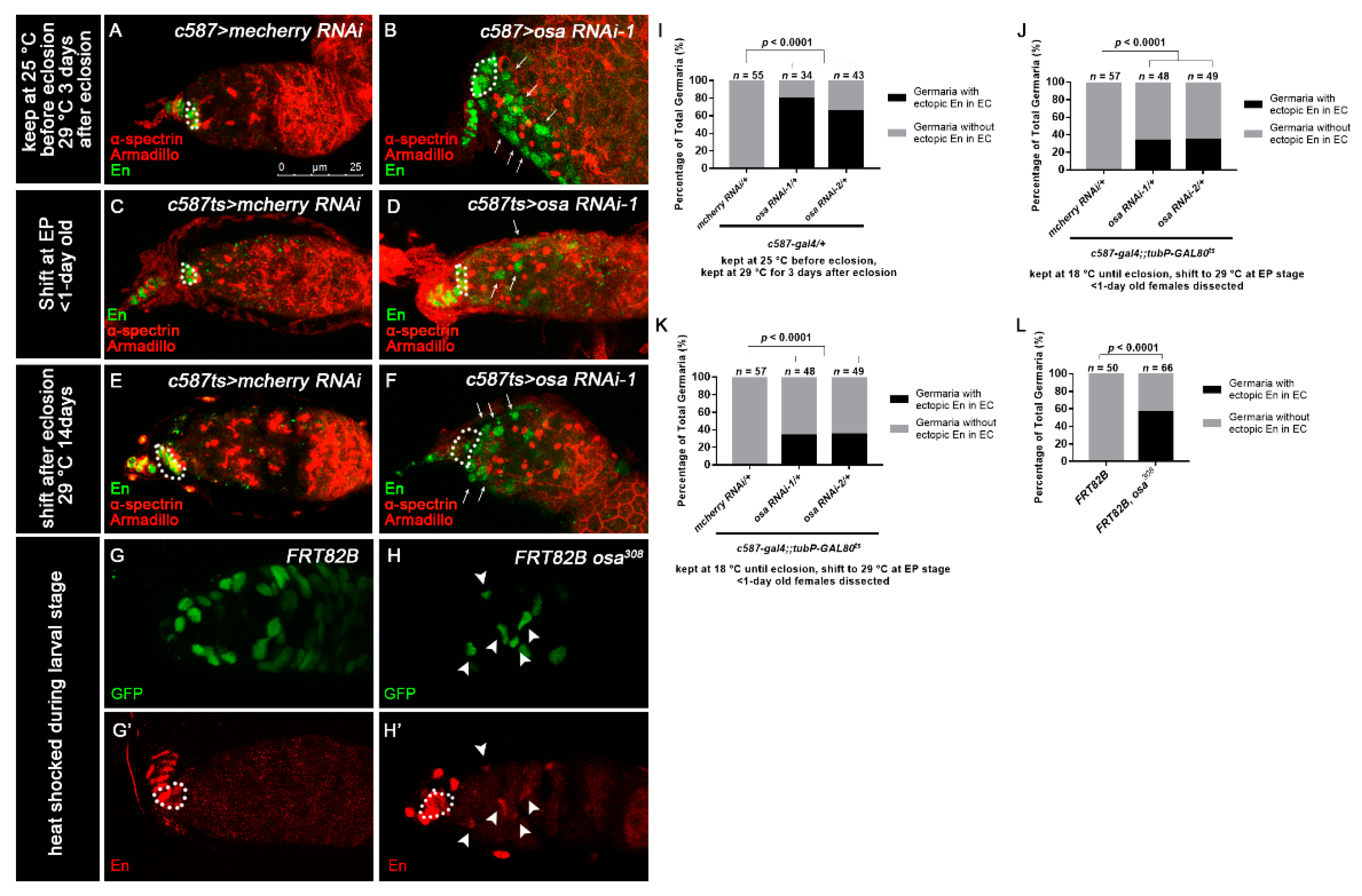
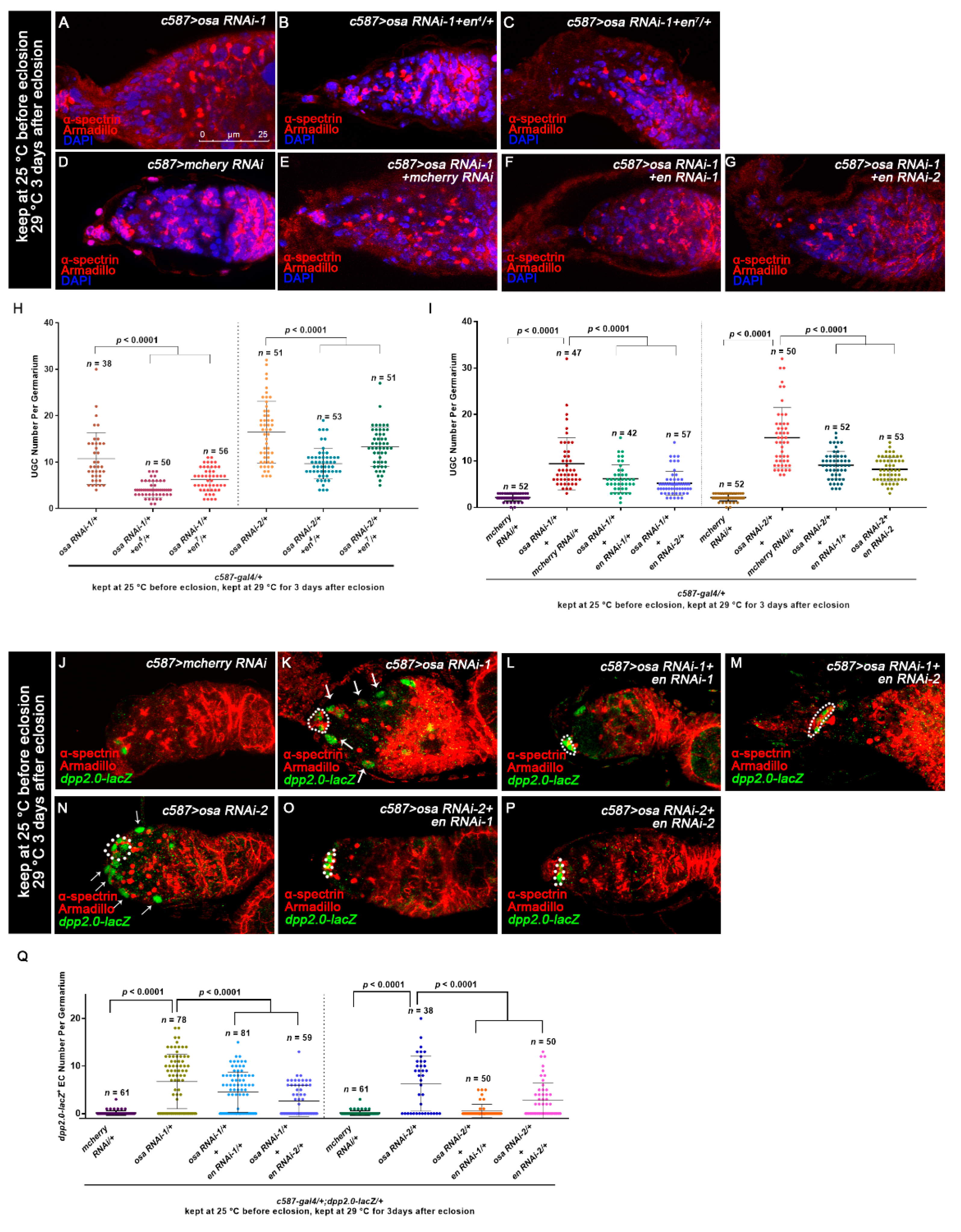
3.4. Osa Limits Engrailed Expression in Escort Cells
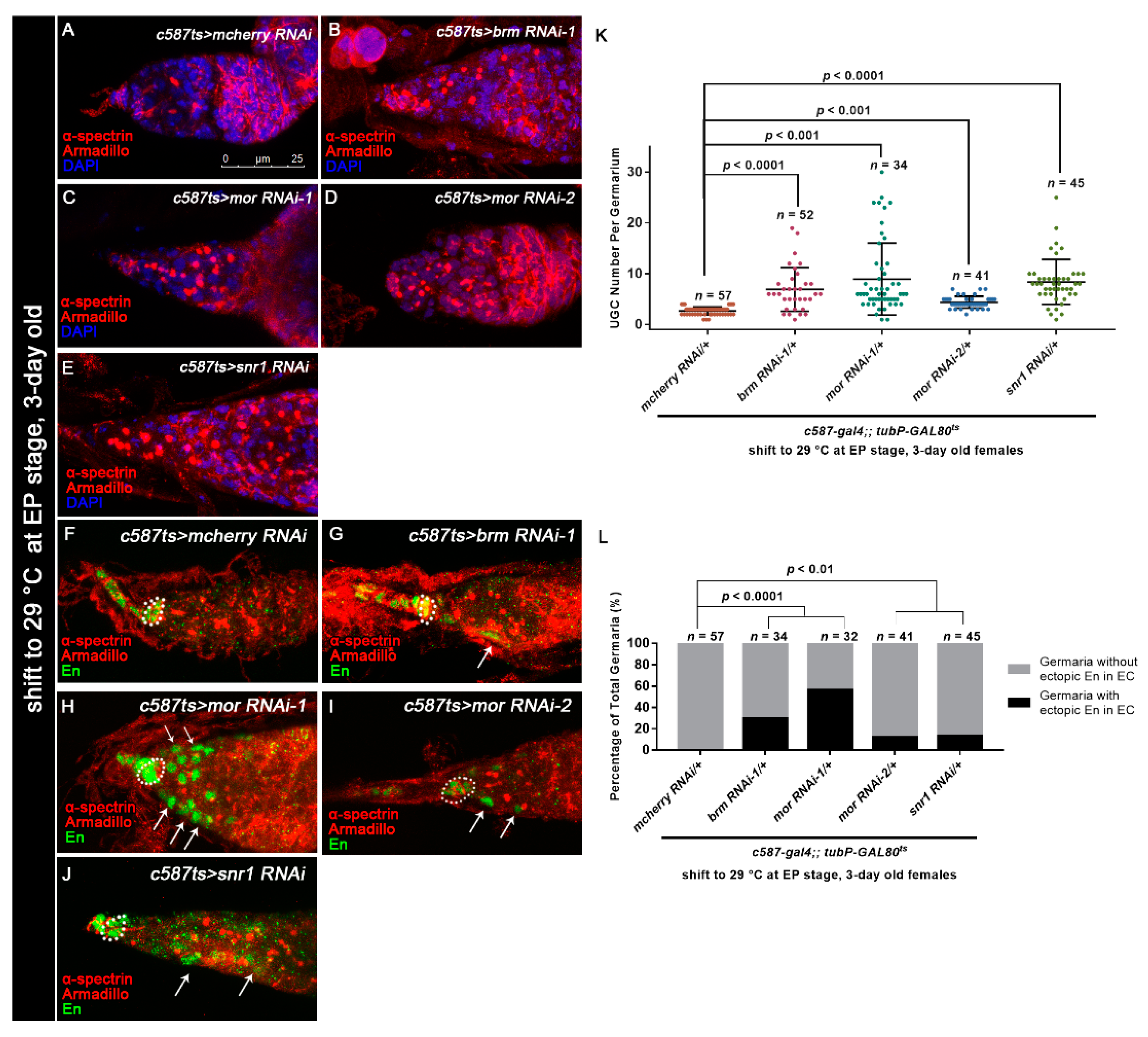
3.5. Osa Regulates Germline Differentiation through the BAP Complex
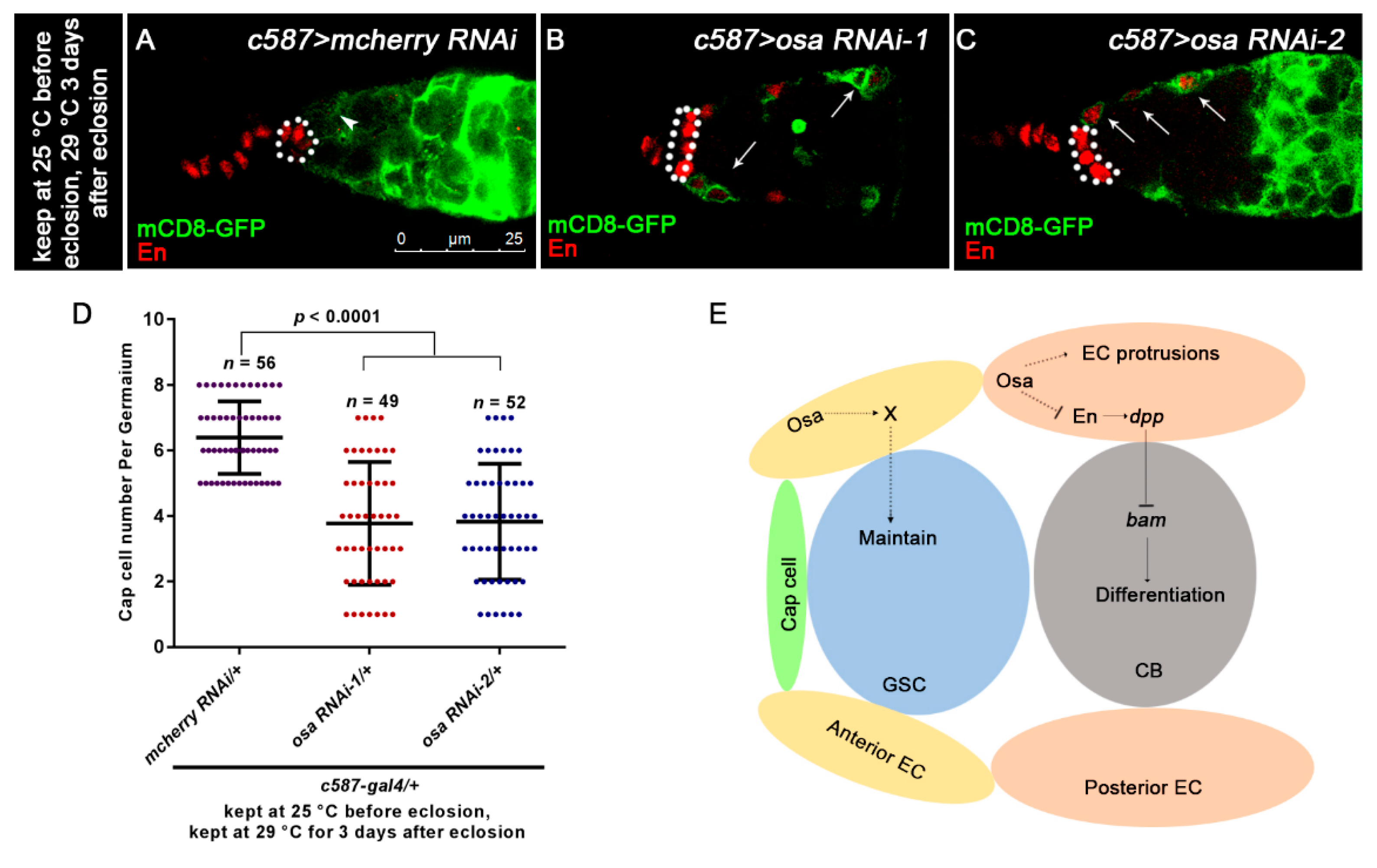
3.6. Osa Maintains Escort Cell Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Xie, T. STEM CELL NICHE: Structure and Function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Lin, H. The stem-cell niche theory: Lessons from flies. Nat. Rev. Genet 2002, 3, 931–940. [Google Scholar] [CrossRef]
- Chen, D.; McKearin, D. Gene Circuitry Controlling a Stem Cell Niche. Curr. Biol. 2005, 15, 179–184. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, G.; Chai, P.C.; Luo, L.; Liu, S.; Yang, Y.; Baeg, G.-H.; Cai, Y. Coordinated niche-associated signals promote germline homeostasis in the Drosophila ovary. J. Cell Biol. 2015, 211, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Page-McCaw, A. Wnt6 maintains anterior escort cells as an integral component of the germline stem cell niche. Development 2018, 145, dev158527. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Spradling, A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000, 290, 328–330. [Google Scholar] [CrossRef]
- Kirilly, D.; Wang, S.; Xie, T. Self-maintained escort cells form a germline stem cell differentiation niche. Developement 2011, 138, 5087–5097. [Google Scholar] [CrossRef]
- Morris, L.X.; Spradling, A.C. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 2011, 138, 2207–2215. [Google Scholar] [CrossRef]
- Xie, T.; Spradling, A.C. Decapentaplegic is Essential for the Maintenance and Division of Germline Stem Cells in the Drosophila Ovary. Cell 1998, 94, 251–260. [Google Scholar] [CrossRef]
- Chen, D.; McKearin, D. Dpp Signaling Silences bam Transcription Directly to Establish Asymmetric Divisions of Germline Stem Cells. Curr. Biol. 2003, 13, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- McKearin, D.M.; Spradling, A.C. bag-of-marbles: A Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990, 4, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, B.; McKearin, D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 1997, 124, 3651–3662. [Google Scholar]
- Song, X. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 2004, 131, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Konig, A. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 2011, 30, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.; Cortez, Y.M.; Wong-Deyrup, S.; Tavares, L.; Schowalter, S.; Flora, P.; Hill, C.; Nasrallah, M.A.; Chittur, S.; Rangan, P. Transposon Dysregulation Modulates dWnt4 Signaling to Control Germline Stem Cell Differentiation in Drosophila. PLoS Genet. 2016, 12, e1005918. [Google Scholar] [CrossRef]
- Banisch, T.U.; Maimon, I.; Dadosh, T.; Gilboa, L. Escort cells generate a dynamic compartment for germline stem cell differentiation via combined Stat and Erk signalling. Development 2017, 144, 1937–1947. [Google Scholar] [CrossRef]
- Luo, L.; Wang, H.; Fan, C.; Liu, S.; Cai, Y. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J. Cell Biol. 2015, 209, 595–608. [Google Scholar] [CrossRef]
- Liu, M.; Lim, T.M.; Cai, Y. The Drosophila Female Germline Stem Cell Lineage Acts to Spatially Restrict DPP Function Within the Niche. Sci. Signal. 2010, 3, ra57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, L.; Wang, S.; Zhou, J.; McDowell, W.; Park, J.; Haug, J.; Staehling, K.; Tang, H.; Xie, T. Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation. PLoS Genet. 2011, 7, e1002426. [Google Scholar] [CrossRef]
- Eliazer, S.; Shalaby, N.A.; Buszczak, M. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 2011, 108, 7064–7069. [Google Scholar] [CrossRef] [PubMed]
- Eliazer, S.; Palacios, V.; Wang, Z.; Kollipara, R.K.; Kittler, R.; Buszczak, M. Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells. PLoS Genet. 2014, 10, e1004200. [Google Scholar] [CrossRef]
- Jin, Z.; Flynt, A.S.; Lai, E.C. Drosophila piwi mutants exhibit germline stem cell tumors that are sustained by elevated Dpp signaling. Curr. Biol. 2013, 23, 1442–1448. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Do, T.; Song, X.; Inaba, M.; Nishimoto, Y.; Liu, L.-P.; Gao, Y.; Mao, Y.; Li, H.; et al. Piwi Is Required in Multiple Cell Types to Control Germline Stem Cell Lineage Development in the Drosophila Ovary. PLoS ONE 2014, 9, e90267. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.; Xin, T.; He, J.; Tan, J.; Gao, Y.; Feng, S.; He, L.; Zhao, G.; Li, M. dBre1/dSet1-dependent pathway for histone H3K4 trimethylation has essential roles in controlling germline stem cell maintenance and germ cell differentiation in the Drosophila ovary. Dev. Biol. 2013, 379, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Control of germline stem cell differentiation by Polycomb and Trithorax group genes in the niche microenvironment. Development 2016, 143, 3449–3458. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, J.; Hu, Y.; Wang, F.; Wang, X.; Qiao, H.-H.; Xu, J.; Mao, D.; Ren, X.; Pan, L.-X.; et al. Histone H1 defect in escort cells triggers germline tumor in Drosophila ovary. Dev. Biol. 2017, 424, 40–49. [Google Scholar] [CrossRef]
- Mohrmann, L.; Langenberg, K.; Krijgsveld, J.; Kal, A.J.; Heck, A.J.R.; Verrijzer, C.P. Differential Targeting of Two Distinct SWI/SNF-Related Drosophila Chromatin-Remodeling Complexes. Mol. Cell. Biol. 2004, 24, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.T.; Furukawa, T.; Tanese, N.; Treisman, J.E. Osa associates with the Brahma chromatin remodeling complex and romotes the activation of some target genes. EMBO J. 1999, 18, 7029–7040. [Google Scholar] [CrossRef]
- Mohrmann, L.; Verrijzer, C.P. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2005, 1681, 59–73. [Google Scholar] [CrossRef]
- Reisman, D.; Glaros, S.; Thompson, E. The SWI/SNF complex and cancer. Oncogene 2009, 28, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Tamkun, J.W.; Deuring, R.; Scott, M.P.; Kissinger, M.; Pattatucci, A.M.; Kaufman, T.C.; Kennison, J.A. brahma: A regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2SWI2. Cell 1992, 68, 561–572. [Google Scholar] [CrossRef]
- Crosby, M.A. The trithorax group gene moira encodes a brahma-associated putative chromatin-remodeling factor in Drosophila melanogaster. Mol. Cell. Biol. 1999, 19, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Dingwall, A.K.; Beek, S.J.; McCallum, C.M.; Tamkun, J.W.; Kalpana, G.V.; Goff, S.P.; Scott, M.P. The Drosophila snr1 and brm proteins are related to yeast SWI/SNF proteins and are components of a large protein complex. Mol. Biol. Cell 1995, 6, 777–791. [Google Scholar] [CrossRef]
- Moshkin, Y.M.; Mohrmann, L.; van Ijcken, W.F.; Verrijzer, C.P. Functional Differentiation of SWI/SNF Remodelers in Transcription and Cell Cycle Control. Mol. Cell. Biol. 2006, 27, 651–661. [Google Scholar] [CrossRef]
- Vázquez, M.; Moore, L.; Kennison, J.A. The trithorax group gene osa encodes an ARID-domain protein that genetically interacts with the brahma chromatin-remodeling factor to regulate transcription. Development 1999, 126, 733–742. [Google Scholar] [PubMed]
- Zeng, X.; Lin, X.; Hou, S.X. The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development 2013, 140, 3532–3540. [Google Scholar] [CrossRef]
- Eroglu, E.; Burkard, T.R.; Jiang, Y.; Saini, N.; Homem, C.C.F.; Reichert, H.; Knoblich, J.A. SWI/SNF Complex Prevents Lineage Reversion and Induces Temporal Patterning in Neural Stem Cells. Cell 2014, 156, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Baig, J.; Chanut, F.; Kornberg, T.B.; Klebes, A. The Chromatin-Remodeling Protein Osa Interacts With CyclinE in Drosophila Eye Imaginal Discs. Genetics 2010, 184, 731–744. [Google Scholar] [CrossRef]
- Terriente-Felix, A.; de Celis, J.F. A subunit of the BAP chromatin-remodelling complex, participates in the regulation of gene expression in response to EGFR signalling in the Drosophila wing. Dev. Biol. 2009, 329, 350–361. [Google Scholar] [CrossRef]
- Collins, R.T.; Treisman, J.E. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 2000, 14, 3140–3152. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.A.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Pollack, J.R. The Spectrum of SWI/SNF Mutations, Ubiquitous in Human Cancers. PLoS ONE 2013, 8, e55119. [Google Scholar] [CrossRef] [PubMed]
- Ronan, J.L.; Wu, W.; Crabtree, G.R. From neural development to cognition: Unexpected roles for chromatin. Nat. Rev. Genet. 2013, 14, 347–359. [Google Scholar] [CrossRef]
- Mathur, R. ARID1A loss in cancer: Towards a mechanistic understanding. Pharmacol. Ther. 2018, 190, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Gilboa, L. Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila. Development 2013, 140, 4145–4154. [Google Scholar] [CrossRef]
- Gancz, D.; Lengil, T.; Gilboa, L. Coordinated Regulation of Niche and Stem Cell Precursors by Hormonal Signaling. PLoS Biol. 2011, 9, e1001202. [Google Scholar] [CrossRef]
- Li, Q.; Xin, T.; Chen, W.; Zhu, M.; Li, M. Lethal(2)giant larvae is required in the follicle cells for formation of the initial AP asymmetry and the oocyte polarity during Drosophila oogenesis. Cell Res. 2008, 18, 372–384. [Google Scholar] [CrossRef]
- Kai, T.; Williams, D.; Spradling, A.C. The expression profile of purified Drosophila germline stem cells. Dev. Biol. 2005, 283, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yue, L.; Spradling, A.C. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 1994, 120, 947–956. [Google Scholar] [PubMed]
- Kai, T.; Spradling, A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4633–4638. [Google Scholar] [CrossRef]
- Decotto, E.; Spradling, A.C. The Drosophila Ovarian and Testis Stem Cell Niches: Similar Somatic Stem Cells and Signals. Dev. Cell 2005, 9, 501–510. [Google Scholar] [CrossRef]
- Lai, C.-M.; Lin, K.-Y.; Kao, S.-H.; Chen, Y.-N.; Huang, F.; Hsu, H.-J. Hedgehog signaling establishes precursors for germline stem cell niches by regulating cell adhesion. J. Cell Biol. 2017, 216, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-H.; Xie, T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development 2003, 130, 2579–2588. [Google Scholar] [CrossRef]
- McGuire, S.E.; Mao, Z.; Davis, R.L. Spatiotemporal Gene Expression Targeting with the TARGET and Gene-Switch Systems in Drosophila. Sci. Signal. 2004, 2004, pl6. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Winter, C.; Marticke, S.S.; Lee, A.; Luo, L. Essential Roles of Drosophila RhoA in the Regulation of Neuroblast Proliferation and Dendritic but Not Axonal Morphogenesis. Neuron 2000, 25, 307–316. [Google Scholar] [CrossRef]
- He, J.; Xuan, T.; Xin, T.; An, H.; Wang, J.; Zhao, G.; Li, M. Evidence for Chromatin-Remodeling Complex PBAP-Controlled Maintenance of the Drosophila Ovarian Germline Stem Cells. PLoS ONE 2014, 9, e103473. [Google Scholar] [CrossRef] [PubMed]
- Tsuneizumi, K.; Nakayama, T.; Kamoshida, Y.; Kornberg, T.B.; Christian, J.L.; Tabata, T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nat. Cell Biol. 1997, 389, 627–631. [Google Scholar] [CrossRef]
- Zhao, R.; Xuan, Y.; Li, X.; Xi, R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell 2008, 7, 344–354. [Google Scholar] [CrossRef]
- Chen, D.; McKearin, D.M. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 2003, 130, 1159–1170. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Song, X.; Ma, X.; Zhu, X.; Mao, Y.; Yang, Z.; Ni, J.; Li, H.; Malanowski, K.E.; et al. Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. eLife 2015, 4, e08174. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Siah, C.K.; Cai, Y. Engrailed acts with Nejire to control decapentaplegic expression in the Drosophila ovarian stem cell niche. Development 2017, 144, 3224–3231. [Google Scholar] [CrossRef]
- Treisman, J.E.; Luk, A.; Rubin, G.M.; Heberlein, U. eyelid antagonizes wingless signaling during Drosophila development and has homology to the Bright family of DNA-binding proteins. Genes Dev. 1997, 11, 1949–1962. [Google Scholar] [CrossRef]
- Song, X.; Call, G.B.; Kirilly, D.; Xie, T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 2007, 134, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Drummond-Barbosa, D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev. Biol. 2011, 350, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, A.S.; Shcherbata, H.R. Stereotypical architecture of the stem cell niche is spatiotemporally established by miR-125-dependent coordination of Notch and steroid signaling. Development 2018, 145, dev159178. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′-3′) |
|---|---|
| dpp | Forward primer: AGCCGATGAAGAAGCTCTACG |
| Reverse primer: ATGTCGTAGACAAGCACCTGGTA | |
| dpp-RA | Forward primer: TTGGAGCGTAACTGAGCGG |
| Reverse primer: CGTTTGAAAAGTCGCCAGCA | |
| dpp-RB | Forward primer: GTTTCGTACTTGGCTCATTGCG |
| Reverse primer: CGTTTGAAAAGTCGCCAGCA | |
| dpp-RC | Forward primer: GGGCGATCCATCCATCAAAC |
| Reverse primer: CGTTTGAAAAGTCGCCAGCA | |
| dpp-RE | Forward primer: TGCCAGATACGAAGAGTTGGG |
| Reverse primer: CGTTTGAAAAGTCGCCAGCA | |
| rp49 | Forward primer: TCCTACCAGCTTCAAGATGAC |
| Reverse primer: CACGTTGTGCACCAGGAACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Li, M.; Hao, X.; Lu, Y.; Zhang, L.; Wu, G. The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation. Genes 2021, 12, 363. https://doi.org/10.3390/genes12030363
Hu X, Li M, Hao X, Lu Y, Zhang L, Wu G. The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation. Genes. 2021; 12(3):363. https://doi.org/10.3390/genes12030363
Chicago/Turabian StyleHu, Xiaolong, Mengjie Li, Xue Hao, Yi Lu, Lei Zhang, and Geng Wu. 2021. "The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation" Genes 12, no. 3: 363. https://doi.org/10.3390/genes12030363
APA StyleHu, X., Li, M., Hao, X., Lu, Y., Zhang, L., & Wu, G. (2021). The Osa-Containing SWI/SNF Chromatin-Remodeling Complex Is Required in the Germline Differentiation Niche for Germline Stem Cell Progeny Differentiation. Genes, 12(3), 363. https://doi.org/10.3390/genes12030363






