Abstract
Renal cell carcinoma is a term that represents multiple different disease processes, each driven by different genetic alterations, with distinct histology, and biological potential which necessitates divergent management strategies. This review discusses the genetic alterations seen in several forms of hereditary kidney cancer and how that knowledge can dictate when and how to intervene with a focus on the surgical management of these tumors.
1. Introduction
In the United States, renal cell carcinoma (RCC) diagnoses are expected to reach over 73,000 new cases in 2020 with an estimated 14,800 deaths. RCC is the sixth most common cancer diagnosis in men and eighth most common in women [1]. Whereas RCC was once thought to be a single disease, it is now recognized to be multiple different entities. At least 17 genes have been identified that lead to the development of tumors arising in the kidney, each with different histologies and distinct biologic potential [2,3,4,5,6].
Hereditary kidney cancer is thought to account for 5–8% of all kidney cancer cases [7], though that number is likely an underestimate, with some studies estimating that up to 38% of RCCs have a hereditary component [8]. Numerous hereditary cancer phenotypes have been described that are associated with development of RCC, each with their own specific molecular alteration and typical clinical course. Many of the molecular underpinnings of sporadic RCC have been elucidated by studying hereditary kidney cancer. For instance, alterations of VHL first identified in families with von Hippel-Lindau are present in >90% of cases of sporadic clear cell RCC. Similarly, alterations in MET which were identified in patients with hereditary papillary renal carcinoma (HPRC) are also present in 17% of sporadic type 1 papillary RCCs, and 81% of these cases have altered MET status. Therefore, lessons from the management of hereditary kidney cancer have broad implications for the management of sporadic RCC.
In this review, we discuss the most prevalent hereditary kidney cancer syndromes, their clinical manifestations, genetic alterations, and treatment with a focus on surgical management. Knowledge of the genetic alterations of a kidney tumor, whether germline or somatic, allows for a precision surgical approach that can dictate both when and how to intervene.
2. Von Hippel-Lindau (VHL)
Phenotypic characteristics of von Hippel-Lindau syndrome were first described in the early 1900s relating specifically to angiomas of the eyes, cerebellum, and spine [9,10]. It was not until the late 1980s/early 1990s, via the evaluation of family clusters, that the VHL gene was localized to the short arm of chromosome 3 (3p25–26) [11]. The VHL gene is a tumor suppressor gene, with affected individuals inheriting a single mutated copy of the gene and a normal wild-type allele from the unaffected parent. Tumor formation is initiated when the wild-type allele undergoes an inactivating mutation or deletion in the affected target organs [12]. The VHL gene encodes for a protein that forms a complex with Elongin B, Elongin C, and Cullin-2. Under normoxia, this complex binds with hypoxia inducible factor (HIF) and targets it for ubiquitin-mediated proteasomal destruction. Without a functioning VHL protein and the associated degradation of HIF, the cell acts in a hypoxic state, leading to the upregulation of several angiogenic/cell cycle and proliferation pathways, such as increased VEGF, EPO, TGFaplha, and PDGFBeta [13,14]. Patients with a germline VHL mutation have over 90% disease penetrance by the age of 65. Manifestations include hemangioblastomas of the central nervous system, pheochromocytoma, cystadenomas of the epididymis, cysts and neuroendocrine tumors of the pancreas, endolymphatic sac tumors, and renal cysts/clear cell carcinoma [15]. Specific genotypes are associated with certain phenotypic manifestations, which can help guide screening and management of patients. Deletion and frameshift alterations have a higher propensity for development of clear cell RCC but low risk for pheochromocytoma. In contrast, missense alterations have a higher propensity for development of pheochromocytoma [16,17]. The median age of onset of RCC in patients with VHL is around 35 years of age [18], with approximately 25–60% of patients developing renal cysts and/or RCC in their lifetime [15]. The histologic subtype seen in patients with VHL is clear cell RCC.
3. Birt-Hogg-Dubé (BHD)
Originally described by three Canadian physicians in 1977 examining common skin findings in family members, Birt-Hogg-Dubé is an autosomal dominant condition, with the mutation mapped in 2001 to chromosome 17p11.2 [19] and the novel gene FLCN identified 1 year later [20]. FLCN is a tumor suppressor gene that encodes for the protein folliculin. Whereas its function has not been definitively elucidated, via its interaction with FNIP1, FNIP2 and AMPK, it has been shown to modulate mTOR signaling, TFE3 transcriptional activities, TGFB signaling, and PGC-1a mitochondrial biogenesis [21]. Clinical manifestations of this syndrome include fibrofolliculomas, lung cysts, and RCC. RCC onset is around 50 years of age, with approximately 30% of patients developing renal tumors [22]. Unlike VHL, BHD-associated RCC is associated with several different tumor histologies, with hybrid oncocytic (features of both chromophobe and oncocytoma) being the most prevalent, followed by chromophobe, oncocytoma, clear cell, and papillary subtypes [23].
4. Hereditary Papillary Renal Carcinoma (HPRC)
Hereditary Papillary Renal Carcinoma (HPRC) is an extremely rare autosomal dominant condition that can result in the development of multifocal and bilateral type 1 papillary renal tumors. Unlike the other syndromes described, HPRC is not associated with any clinical manifestations other than renal tumors, making its diagnosis difficult unless a high suspicion exists. Alteration of the MET gene (located on chromosome 7q31) was ultimately identified as the cause of this condition initially based on the study of families with high prevalence of papillary RCC without any identifiable changes on chromosome 3p [24,25]. Missense mutations are specific to the tyrosine kinase domain of the MET gene and act as a proto-oncogene. In normal cells, the interaction of MET kinase and its ligand, hepatocyte gross factor (HGF), results in phosphorylation of several tyrosines that are vital to cell proliferation and differentiation. In affected individuals, the activating mutation allows inappropriate upregulation of the numerous downstream regulators by the MET kinase without its interaction with HGF [26]. Penetrance is over 90% by age 60 and the median age of diagnosis of renal tumors is 57 years (range 46–63) [27].
5. Hereditary Leiomyomatosis Renal Cell Cancer (HLRCC)
Contrary to the indolent nature of HPRC, Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) is associated with a more aggressive type 2 papillary RCC. HLRCC was first suggested as a hereditary syndrome in 1958, when dermatologists noted families with numerous cutaneous leiomyomas [28]. Its association with uterine leiomyomas (named Reed’s syndrome) was described in 1973 [29], and its association with the development of aggressive RCC was described in 2001. This coincided with the discovery of the associated germline mutation of the FH gene on chromosome 1q42.3–q43 [30,31]. FH encodes a Krebs cycle enzyme, fumarate hydratase, which is involved in the conversion of fumarate into malate. As tumor suppressor gene, HLRCC-affected cells are characterized by increased aerobic glycolysis and impaired oxidative phosphorylation. This alteration in glucose metabolism is an example of the Warburg Effect [32]. Additionally, a pseudohypoxic state develops with the inhibition of prolyl hydroxylase leading to increased HIF1a and its associated downstream effects [33]. The most common clinical manifestations in affected individuals include cutaneous leiomyomas in all genders and uterine leiomyomas in women, with high rates of early hysterectomy seen. Approximately 15% of those affected develop RCC over the course of their lifetime, and tumors have been seen in patients as young as 10 years old. These are aggressive tumors, and many patients present with locally advanced or distant disease even in the setting of small tumor sizes [34].
6. Succinate Dehydrogenase (SDH)-Deficient RCC
Just recently added to the World Health Organization classification of RCC [35], Succinate dehydrogenase (SDH)-deficient RCC is associated with a germline mutation in the genes encoding any of the SDH subunits (SDHA, SDHB, SDHC, SDHD). Initially described as a cause of hereditary paraganglioma/pheochromocytoma syndrome [36], SDH is another Krebs cycle enzyme that catalyzes the change of succinate to fumarate. Without a functioning SDH complex, succinate accumulates which leads to stabilization of HIF1a [37], creating a pseudohypoxic state and an increase in aerobic glycolysis similar to what is seen in HLRCC-associated tumors. Originally described in 2004, mutations in the SDHB subunit are the most frequently seen in SDH-deficient RCC [38]. ln addition to pheochromocytoma, paraganglioma, and RCC, patients are at risk for gastrointestinal stromal tumors. SDH-deficient RCC tends to present early (median age of 30 years in NCI series) and represent an aggressive disease with high potential for metastatic spread even at small sizes [39].
7. BAP1-Tumor Predisposition Syndrome (BAP1-TPS)
First implicated in the development of malignant mesothelioma and uveal melanoma, germline BRCA1-associated protein-1 (BAP1) mutations are a relatively recently discovered tumor predisposition syndrome [40]. The gene that encodes for BAP1 is located on chromosome 3p21.1 and functions as a de-ubiquinating enzyme, with functions related to cellular integrity via cell cycle control and DNA damage repair. Recently, its role in apoptosis was described via stabilizing the type 3 inositol-1,4-5, triphosphate receptor, which modulates calcium release into the cytosol. Decreased levels of BAP1 lead to decreased fidelity of DNA damage repair in the nucleus along with decreased triggering of cell death when DNA damage has accumulated [41,42]. In addition to mesothelioma and uveal melanoma, patients are predisposed to develop cutaneous melanomas and RCC [43]. Examination of families with a BAP1 mutation and RCC showed the development of early onset tumors, many of which were fast growing and with higher Fuhrman grade on pathologic analysis [44]. Whereas less than 100 families with this syndrome have been described, the frequency of RCC is reported to be 3–10% [45,46]. Somatic BAP1 loss in sporadic RCC has been linked to poor prognoses [47,48]. This fact, along with early onset and higher grade tumors in patients with germline mutation, suggest that the renal cancers seen in this syndrome are potentially more aggressive.
8. Tuberous Sclerosis Complex (TSC)
TSC is a multisystem disorder first described in the late 19th century [49]. It is inherited in an autosomal dominant fashion with mutations mapped in the 1990s to the TSC1 and TSC2 genes located on chromosome 9q34 and 16p13, respectively [50,51]. TSC1 and TSC2 are tumor suppressor genes that follows Knudson’s two-hit model with development of the manifestations occurring with loss of the 2nd hit mutation/inactivation. TSC1 encodes the protein hamartin and TSC2 encodes the protein tuberin, which together form heterodimers to interact with several proteins [52]. Its primary downstream effect appears to inhibit the mammalian target of rapamycin (mTOR) pathway, which plays a role in cell proliferation and growth. Clinical manifestations primarily develop in the skin, central nervous system, eyes, lungs, and kidneys, with some patients developing seizures or mental retardation. The most noticeable changes are skin changes, including the development of hypopigmented macules, shagreen patches, facial angiofibroma, and ungual fibroma. Renal manifestations are primarily the development of bilateral multifocal cysts and angiomyolipoma [53]. Whereas the risk of RCC appears to only be slightly higher than the general population with a rate of about 3–5%, patients with TSC appear to develop malignant tumors at an earlier age. Series of TSC-related RCCs have shown that mean ages are between 30–42 years, and there is a slight female predominance [54,55]. Kidney cancers seen represent a wide range of histologies, with clear cell, papillary, chromophobe, hybrid tumors, and oncocytomas all being reported [53,55].
9. Clinical Management
The first step in precision management of these cancers remains a high clinical suspicion for a hereditary cancer syndrome to trigger appropriate genetic counseling and potential germline testing prior to planned surgery. Besides the more obvious clinical symptoms of the syndromes with visually apparent clinical manifestations, age at diagnosis and family history are important clues to prompt a genetic evaluation. Age at diagnosis of less than 46 is proposed as an age cutoff to prompt germline testing [18]. Additionally, the presence of bilateral/multifocal disease and/or a family history of RCC should prompt the need for genetic evaluation. At minimum, a detailed history focused on manifestations of hereditary syndromes, a family pedigree, and a physical examination focused on cutaneous manifestations of these syndromes should strongly be considered. Whereas some urologists may feel comfortable evaluating for these syndromes, there should be a low threshold for referral to a genetic counselor. This early suspicion can allow for genetic evaluation and diagnosis prior to any planned surgery so that germline mutations discovered can guide the surgical approach and planning.
The mainstay of treatment of hereditary RCC is nephron sparing surgery for localized disease and systemic therapy for metastatic disease. The goals of treatment are to minimize the risk of metastasis, to minimize the number of repeat ipsilateral renal surgeries over a patient’s life, and to maximize renal preservation when possible. For localized disease, decades of experience managing these patients have made it possible to tailor the approach based on the specific germline mutation as a direct result of the biological nature of the renal tumors. Biologic aggressiveness is associated with each specific germline mutation. Similarly, growth rates are providing additional data to help guide management. In a study of 292 genetically defined patients, growth rates were analyzed from 435 tumors. Bap1-deficient tumors had the fastest growth rate (median 0.6 cm/year), while tumors with MET-activated tumors had the slowest growth rate (0.015 cm/year) [56].
10. Precision Surgery: When to Intervene
Several hereditary syndromes are managed with an initial period of active surveillance until the largest solid tumor reaches 3 cm. Longitudinal follow-up has shown this to be a safe strategy in patients with VHL, HPRC, and BHD, and this strategy has been extrapolated to TSC [57]. Growth rates help guide the frequency of active surveillance of renal lesions, especially in VHL patients who undergo imaging every 12–36 months based on their established tumor growth and current size. In general, patients with no current renal tumors are surveilled less frequently, up to every 36 months, while those with larger tumors are reimaged more frequently. BAP1-altered tumors often exhibit faster growth and have been associated with high grade, high stage, and low survival. Whereas active surveillance can be utilized, it is likely prudent to image these tumors more frequently. This active surveillance approach is not recommended in patients with HLRCC or SHD-deficient tumors, and immediate surgery is the preferred strategy. Surgery is the primary recommended management of localized kidney cancer, with nephron sparing surgery preferred for small renal masses and patients with hereditary syndromes given the lifelong risk of tumor development and potential need for numerous surgical resections [58]. Table 1 illustrates the differences in our surveillance approaches for several hereditary kidney cancer syndromes.

Table 1.
Examples of lesions seen in several hereditary kidney cancer syndromes, with difference in initial active surveillance and surgical approach noted.
11. Precision Surgery: How to Intervene
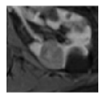
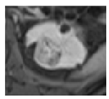
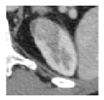
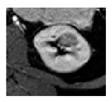
Surgical management of hereditary syndromes can vary in several respects. The most notable are the type of surgical approach (open or minimally invasive), and the extent of resection (tumor enucleation or wide excision). Figure 1 shows an example of the difference between a minimally invasive tumor enucleation for VHL and an open wide excision partial nephrectomy for HLRCC.
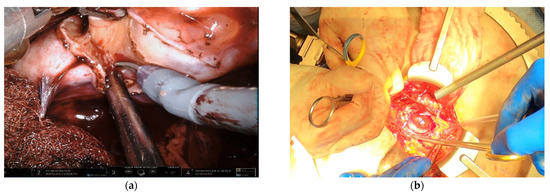
Figure 1.
(a) Intraoperative view of a robotic tumor enucleation performed on a patient with von Hippel-Lindau (VHL) syndrome. Note: The plane of dissection is between the normal renal parenchyma and tumor pseudocapsule. (b) Intraoperative view of an open partial nephrectomy with wide excision performed on a patient with Hereditary Leiomyomatosis Renal Cell Cancer (HLRCC). Note that the plane of dissection is approximately 1 cm wider than the base of the tumor.
For less biologically aggressive tumors, such as those seen VHL, HPRC, TSC, and BHD, our default approach is minimally invasive partial nephrectomy with enucleation of the renal tumors. This approach maximizes normal renal tissue preservation. Enucleation is performed by incising the renal capsule circumferentially around the tumor and gently separating the normal parenchyma from the tumor capsule. Whereas most of the dissection is performed bluntly, if perforating blood vessels are encountered, they can be controlled with cautery and cut or cut sharply and suture ligated later. If the true enucleation plane is entered and followed, bleeding tends to be minimal, especially in the setting of pneumoperitoneum, and many of these enucleations can be performed without hilar clamping. One important point regarding enucleation is that sometimes patients lack a robust pseudocapsule. In general, VHL tumors have a robust pseudocapsule, while those in HPRC and BHD are sometimes more fragile. In this setting, a small normal margin can be taken if appropriate to help prevent tumor spillage [59].
HLRCC, SDH, and BAP1 are associated with more aggressive tumors. HLRCC specifically can have infiltrating tumor cells into normal parenchyma that cannot be appreciated with imaging or on intraoperative examination. Partial nephrectomy, if performed, requires a margin of normal parenchyma, and intraoperative frozen sections should be considered to ensure adequate resection has been performed. Open approaches should be strongly considered given the increased tactile feedback to help minimize risk of tumor spillage [59]. If a wide margin cannot be obtained because of the size or depth of a tumor, one should consider radical nephrectomy, particularly for HLRCC.
Removal of locoregional lymph nodes at time of surgery for RCC continues to be controversial. Results of the only randomized trial to date (EORTC 30881) did not show a difference in either progression free survival or overall survival between patients undergoing lymph node dissection at time of radical nephrectomy [60]. Critics of this trial discuss the higher rates of low-risk patients included and overall low lymph node yields. The histopathologic diagnoses for the patients in the trial were not included, but a reasonable assumption is that the majority were sporadic clear cell RCC patients. The role of lymph node dissection in a patient with a hereditary germline mutation is even less clear. Given the aggressiveness of the tumors seen in certain germline mutations (specifically HLRCC and SDH-deficient tumors), we routinely perform a local regional lymph node dissection at time of surgery in these settings for larger or more complex solid tumors, even in the setting of preoperatively normal lymph nodes.
12. Conclusions
Knowledge of a patient’s germline genetic alterations have multiple implications. Not only can it dictate screening for the patient and their family, but it is imperative in delivering a precision surgical approach that can maximize oncologic benefit while minimizing surgical morbidity. Knowledge of when to employ active surveillance and when to resect, when a minimally invasive approach is preferred and when an open operation is required, and the extent of a margin to take is directly inferred from a patient’s germline or tumor’s somatic alteration.
Author Contributions
Writing—original draft preparation, P.T.G.; writing—review and editing, M.W.B., W.M.L.; supervision, M.W.B., W.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has been supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miller, K.D.; Siegel, R.L.; Khan, R.; Jemal, A. Cancer Statistics. Cancer Rehabil. 2018, 70, 7–30. [Google Scholar] [CrossRef]
- Ball, M.W.; Shuch, B.M. Inherited kidney cancer syndromes. Curr. Opin. Urol. 2019, 29, 334–343. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Vocke, C.D.; Lang, M.; Chen, X.; Zhao, Y.; Tran, B.; Tandon, M.; Schmidt, L.S.; Ball, M.W.; Linehan, W.M. A germline 1;3 translocation disrupting the VHL gene: A novel genetic cause for von Hippel-Lindau. J. Med. Genet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Vocke, C.D.; Ricketts, C.J.; Metwalli, A.R.; Ball, M.W.; Schmidt, L.S.; Linehan, W.M. Clinical and Molecular Characterization of Microphthalmia-Associated Transcription Factor (MITF)-Related Renal Cell Carcinoma. Urology 2020. [Google Scholar] [CrossRef] [PubMed]
- Vocke, C.D.; Ricketts, C.J.; Ball, M.W.; Schmidt, L.S.; Metwalli, A.R.; Middelton, L.A.; Killian, J.K.; Khan, J.; Meltzer, P.S.; Simonds, W.F.; et al. CDC73 Germline Mutation in a Family with Mixed Epithelial and Stromal Tumors. Urology 2019, 124, 91–97. [Google Scholar] [CrossRef]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef]
- Haas, N.B.; Nathanson, K.L. Hereditary Kidney Cancer Syndromes. Adv. Chronic Kidney Dis. 2014, 21, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.H.; Graff, R.E.; Holst, K.K.; Moeller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Von Hippel, E. Die anatomische Grundlage der von mir beschriebenen „sehr seltenen Erkrankung der Netzhaut. Albrecht Graefes Arch. Ophthalmol. 1911, 79, 350–377. [Google Scholar] [CrossRef]
- Lindau, A. Zur frage der angiomatosis retinae und ihrer hirnkomplikationen. Acta Ophthalmol. 2009, 4, 193–226. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Genetics of human cancer. Ann. Rev. Genet. 1986, 20, 231–251. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFα Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Kaelin, W.G. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. von Hippel-Lindau disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef]
- Hes, F.; Zewald, R.; Peeters, T.; Sijmons, R.; Links, T.; Verheij, J.; Matthijs, G.; Legius, E.; Mortier, G.; Van Der Torren, K.; et al. Genotype-phenotype correlations in families with deletions in the von Hippel-Lindau (VHL) gene. Qual. Life Res. 2000, 106, 425–431. [Google Scholar] [CrossRef]
- Maher, E.R.; Webster, A.R.; Richards, F.M.; Green, J.S.; A Crossey, P.; Payne, S.J.; Moore, A.T. Phenotypic expression in von Hippel-Lindau disease: Correlations with germline VHL gene mutations. J. Med. Genet. 1996, 33, 328–332. [Google Scholar] [CrossRef][Green Version]
- Shuch, B.; Vourganti, S.; Ricketts, C.J.; Middleton, L.; Peterson, J.; Merino, M.J.; Metwalli, A.R.; Srinivasan, R.; Linehan, W.M. Defining Early-Onset Kidney Cancer: Implications for Germline and Somatic Mutation Testing and Clinical Management. J. Clin. Oncol. 2014, 32, 431–437. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Warren, M.B.; Nickerson, M.L.; Weirich, G.; Matrosova, V.; Toro, J.R.; Turner, M.L.; Duray, P.; Merino, M.; Hewitt, S.; et al. Birt-Hogg-Dubé Syndrome, a Genodermatosis Associated with Spontaneous Pneumothorax and Kidney Neoplasia, Maps to Chromosome 17p11.2. Am. J. Hum. Genet. 2001, 69, 876–882. [Google Scholar] [CrossRef]
- Nickerson, M.L.; Warren, M.B.; Toro, J.R.; Matrosova, V.; Glenn, G.; Turner, M.L.; Duray, P.; Merino, M.; Choyke, P.; Pavlovich, C.P.; et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2002, 2, 157–164. [Google Scholar] [CrossRef]
- Schmidt, L.S. Birt-Hogg-Dubé syndrome: From gene discovery to molecularly targeted therapies. Fam. Cancer 2012, 12, 357–364. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Grubb, R.L., 3rd; Hurley, K.; Glenn, G.M.; Toro, J.; Schmidt, L.S.; Torres-Cabala, C.; Merino, M.J.; Zbar, B.; Choyke, P.; et al. Evaluation and management of renal tumors in the Birt-Hogg-Dubé syndrome. J. Urol. 2005, 173, 1482–1486. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Walther, M.M.; Eyler, R.A.; Hewitt, S.M.; Zbar, B.; Linehan, W.M.; Merino, M.J. Renal tumors in the Birt-Hogg-Dubé syndrome. Am. J. Surg. Pathol. 2002, 26, 1542–1552. [Google Scholar] [CrossRef]
- Zbar, B.; Glenn, G.; Lubensky, I.; Choyke, P.; Walther, M.M.; Magnusson, G.; Bergerheim, U.S.; Pettersson, S.; Amin, M.; Hurley, K.; et al. Original Articles: Kidney Cancer: Hereditary Papillary Renal Cell Carcinoma: Clinical Studies in 10 Families. J. Urol. 1995, 153, 907–912. [Google Scholar] [CrossRef]
- Schmidt, L.; Duh, F.-M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Pathirage, G.D.; Alessio, G.; Donald, P.B. Hereditary Papillary Renal Carcinoma Type, I. Curr. Mol. Med. 2004, 4, 855–868. [Google Scholar]
- Schmidt, L.S.; Nickerson, M.L.; Angeloni, D.; Glenn, G.M.; Walther, M.M.; Albert, P.S.; Warren, M.B.; Choyke, P.L.; Torres-Cabala, C.A.; Merino, M.J.; et al. Early onset hereditary papillary renal carcinoma: Germline missense mutations in the tyrosine kinase domain of the met proto-oncogene. J. Urol. 2004, 172, 1256–1261. [Google Scholar] [CrossRef]
- Kloepfer, H.W.; Krafchuk, J.; Derbes, V.; Burks, J. Hereditary Multiple Leiomyoma of the Skin. Am. J. Hum. Genet. 1958, 10, 48–52. [Google Scholar]
- Reed, W.B.; Walker, R.; Horowitz, R. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm. Venereol. 1973, 53, 409–416. [Google Scholar]
- Launonen, V.; Vierimaa, O.; Kiuru, M.; Isola, J.; Roth, S.; Pukkala, E.; Sistonen, P.; Herva, R.; Aaltonen, L.A. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.M.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.M.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Jung, Y.J.; Mole, D.R.; Lee, S.; Torres-Cabala, C.; Chung, Y.-L.; Merino, M.; Trepel, J.; Zbar, B.; Toro, J.; et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Grubb, R.L., 3rd; Franks, M.E.; Toro, J.; Middelton, L.; Choyke, L.; Fowler, S.; Torres-Cabala, C.; Glenn, G.M.; Choyke, P.; Merino, M.J.; et al. Hereditary leiomyomatosis and renal cell cancer: A syndrome associated with an aggressive form of inherited renal cancer. J. Urol. 2007, 177, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Amin, M.; Smith, S.; Trpkov, K. Chapter 1: Succinate dehydrogenase (SDH) deficient renal carcinoma. In WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; Moch, H., Humphrey, P.A., Ulbright, T.M., Reuter, V.E., Eds.; IARC: Lyon, France, 2016; pp. 35–36. [Google Scholar]
- Bardella, C.; Pollard, P.J.; Tomlinson, I. SDH mutations in cancer. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1432–1443. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Vanharanta, S.; Buchta, M.; McWhinney, S.R.; Virta, S.K.; Peçzkowska, M.; Morrison, C.D.; Lehtonen, R.; Januszewicz, A.; Järvinen, H.; Juhola, M.; et al. Early-Onset Renal Cell Carcinoma as a Novel Extraparaganglial Component of SDHB-Associated Heritable Paraganglioma. Am. J. Hum. Genet. 2004, 74, 153–159. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Shuch, B.; Vocke, C.D.; Metwalli, A.R.; Bratslavsky, G.; Middelton, L.; Yang, Y.; Wei, M.-H.; Pautler, S.E.; Peterson, J.; et al. Succinate Dehydrogenase Kidney Cancer: An Aggressive Example of the Warburg Effect in Cancer. J. Urol. 2012, 188, 2063–2071. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef]
- Bononi, A.; Giorgi, C.; Patergnani, S.; Larson, D.; Verbruggen, K.; Tanji, M.; Pellegrini, L.; Signorato, V.; Olivetto, F.; Pastorino, S.; et al. BAP1 regulates IP3R3-mediated Ca (2+) flux to mitochondria suppressing cell trans-formation. Nature 2017, 546, 549–553. [Google Scholar] [CrossRef]
- Yu, H.; Mashtalir, N.; Daou, S.; Hammond-Martel, I.; Ross, J.; Sui, G.; Hart, G.W.; Rauscher, F.J.; Drobetsky, E.; Milot, E.; et al. The Ubiquitin Carboxyl Hydrolase BAP1 Forms a Ternary Complex with YY1 and HCF-1 and Is a Critical Regulator of Gene Expression. Mol. Cell. Biol. 2010, 30, 5071–5085. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Hebert, L.; Jacquemin, V.; Gad, S.; Caux-Moncoutier, V.; Dubois-D’Enghien, C.; Richaudeau, B.; Renaudin, X.; Sellers, J.; Nicolas, A.; et al. Germline BAP1 Mutations Predispose to Renal Cell Carcinomas. Am. J. Hum. Genet. 2013, 92, 974–980. [Google Scholar] [CrossRef]
- Farley, M.N.; Schmidt, L.S.; Mester, J.L.; Pena-Llopis, S.; Pavia-Jimenez, A.; Christie, A.; Vocke, C.D.; Ricketts, C.J.; Peterson, J.; Middeltone, L.; et al. A Novel Germline Mutation in BAP1 Predisposes to Familial Clear-Cell Renal Cell Carcinoma. Mol. Cancer Res. 2013, 11, 1061–1071. [Google Scholar] [CrossRef]
- Rai, K.R.; Pilarski, R.; Cebulla, C.M.; Abdelrahman, M.H. Comprehensive review ofBAP1tumor predisposition syndrome with report of two new cases. Clin. Genet. 2016, 89, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Flores, E.G.; Emi, M.; Johnson, T.A.; Tsunoda, T.; Behner, D.; Hoffman, H.; Hesdorffer, M.; Nasu, M.; Napolitano, A.; et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015, 11, e1005633. [Google Scholar] [CrossRef]
- Joseph, R.W.; Kapur, P.; Serie, D.J.; Parasramka, M.; Ho, T.H.; Cheville, J.C.; Frenkel, E.; Parker, A.S.; Brugarolas, J. Clear Cell Renal Cell Carcinoma Subtypes Identified by BAP1 and PBRM1 Expression. J. Urol. 2016, 195, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Bourneville, D.J.A.N. Sclerose tubereuse der circonvolutions cerebrales: Idiotie et epilepsie hemiplegique. Arch de Neurologie. 1880, 1, 81–91. [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993, 75, 1305–1315. [Google Scholar] [CrossRef]
- Van Slegtenhorst, M.; De Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; Ouweland, A.V.D.; Halley, D.; Young, J.; et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef]
- Van Slegtenhorst, M.; Nellist, M.; Nagelkerken, B.; Cheadle, J.; Snell, R.; van den Ouweland, A.; Reuser, A.; Sampson, J.; Halley, D.; van der Sluijs, P. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum. Mol. Genet. 1998, 7, 1053–1057. [Google Scholar] [CrossRef]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The Tuberous Sclerosis Complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tretiakova, M.S.; Troxell, M.L.; Osunkoya, A.O.; Fadare, O.; Sangoi, A.R.; Shen, S.S.; Lopez-Beltran, A.; Mehra, R.; Heider, A.; et al. Tuberous sclerosis-associated renal cell carcinoma: A clinicopathologic study of 57 separate carcinomas in 18 patients. Am. J. Surg. Pathol. 2014, 38, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cornejo, K.M.; Sadow, P.M.; Cheng, L.; Wang, M.; Xiao, Y.; Jiang, Z.; Oliva, E.; Jozwiak, S.; Nussbaum, R.L.; et al. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Am. J. Surg. Pathol. 2014, 38, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.W.; An, J.Y.; Gomella, P.T.; Gautam, R.; Ricketts, C.J.; Vocke, C.D.; Schmidt, L.S.; Merino, M.J.; Srinivasan, R.; Malayeri, A.A.; et al. Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention. J. Clin. Oncol. 2020, 38, 1146–1153. [Google Scholar] [CrossRef]
- Herring, J.C.; Enquist, E.G.; Chernoff, A.; Linehan, W.M.; Choyke, P.L.; Walther, M.M. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J. Urol. 2001, 165, 777–781. [Google Scholar] [CrossRef]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef]
- Hemal, A.K.; Menon, M. Robotics in Genitourinary Surgery; Springer: Berlin, Germany, 2018. [Google Scholar]
- Blom, J.H.; Van Poppel, H.; Maréchal, J.M.; Jacqmin, D.; Schröder, F.H.; De Prijck, L.; Sylvester, R. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 30881. Eur. Urol. 2009, 55, 28–34. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).