Population Genomics of American Mink Using Whole Genome Sequencing Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Whole Genome Sequencing and Variant Calling
2.3. Inbreeding Coefficients and Genetic Distances
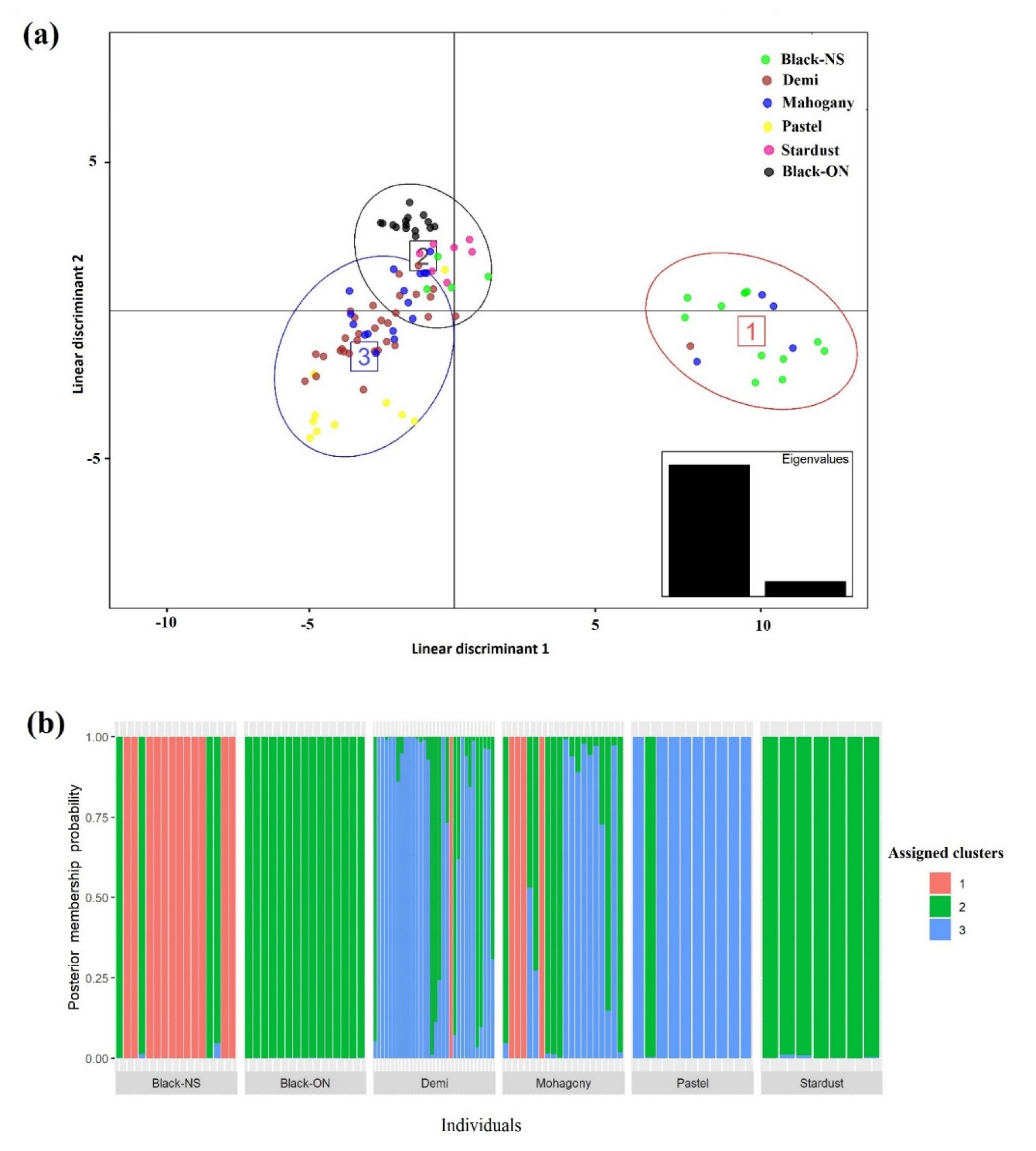
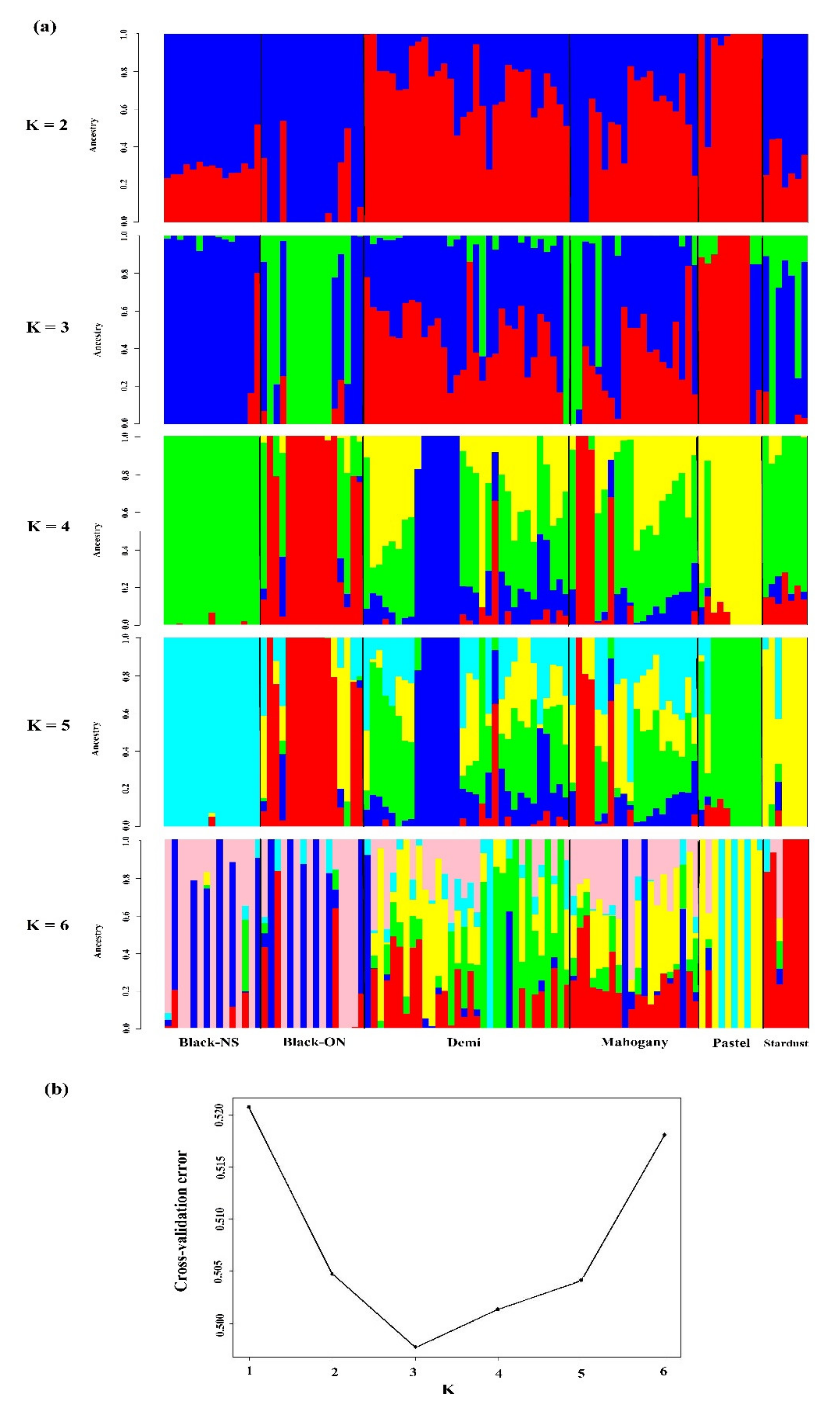
2.4. Genetic Structure and Admixture Patterns
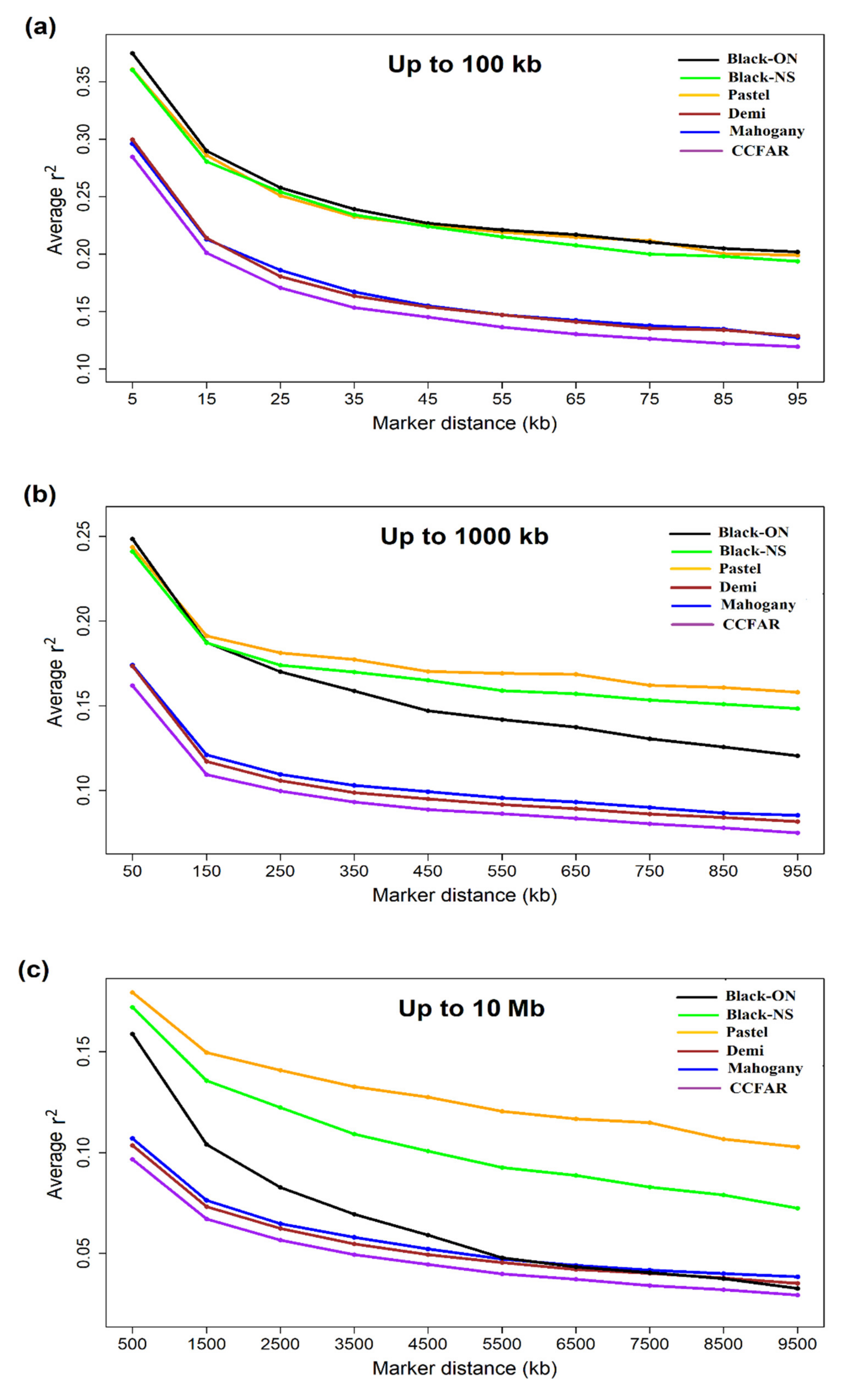
2.5. Linkage Disequilibrium (LD) and Effective Population Size
3. Results
3.1. Data Quality and Population Genetic Parameters
3.2. Genetic Distances
3.3. Genetic Structure and Admixture Patterns
3.4. Linkage Disequilibrium and Effective Population Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Wellmann, R.; Bennewitz, J. Key Genetic Parameters for Population Management. Front. Genet. 2019, 10, 667. [Google Scholar] [CrossRef]
- Karimi, K.; Strucken, E.M.; Moghaddar, N.; Ferdosi, M.H.; Esmailizadeh, A.; Gondro, C. Local and Global Patterns of Admixture and Population Structure in Iranian Native Cattle. BMC Genet. 2016, 17, 108. [Google Scholar] [CrossRef]
- The Bovine HapMap Consortium; Gibbs, A.R.; Taylor, J.F.; Van Tassell, C.; Barendse, W.; Eversole, A.K.; Gill, A.C.; Green, R.D.; Hamernik, D.L.; Kappes, S.M.; et al. Genome-Wide Survey of SNP Variation Uncovers the Genetic Structure of Cattle Breeds. Science 2009, 324, 528–532. [Google Scholar] [CrossRef]
- Kijas, J.W.; Lenstra, J.A.; Hayes, B.; Boitard, S.; Neto, L.R.P.; Cristobal, M.S.; Servin, B.; McCulloch, R.; Whan, V.; Gietzen, K.J.; et al. Genome-Wide Analysis of the World’s Sheep Breeds Reveals High Levels of Historic Mixture and Strong Recent Selection. PLoS Biol. 2012, 10, e1001258. [Google Scholar] [CrossRef]
- Colli, L.; The ADAPTMAP Consortium; Milanesi, M.; Talenti, A.; Bertolini, F.; Chen, M.; Crisà, A.; Daly, K.G.; Del Corvo, M.; Guldbrandtsen, B.; et al. Genome-Wide SNP Profiling of Worldwide Goat Populations Reveals Strong Partitioning of Diversity and Highlights Post-Domestication Migration Routes. Genet. Sel. Evol. 2018, 50, 58. [Google Scholar] [CrossRef]
- Edea, Z.; Kim, S.-W.; Lee, K.-T.; Kim, T.H.; Kim, K.-S. Genetic Structure of and Evidence for Admixture between Western and Korean Native Pig Breeds Revealed by Single Nucleotide Polymorphisms. Asian-Australas. J. Anim. Sci. 2014, 27, 1263–1269. [Google Scholar] [CrossRef]
- Ardlie, K.G.; Kruglyak, L.; Seielstad, M. Patterns of Linkage Disequilibrium in the Human Genome. Nat. Rev. Genet. 2002, 3, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage Disequilibrium—Understanding the Evolutionary Past and Mapping the Medical Future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef]
- Reich, D.E.; Cargill, M.; Bolk, S.; Ireland, J.; Sabeti, P.C.; Richter, D.J.; Lavery, T.; Kouyoumjian, R.; Farhadian, S.F.; Ward, R.; et al. Linkage Disequilibrium in the Human Genome. Nat. Cell Biol. 2001, 411, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Qanbari, S. On the Extent of Linkage Disequilibrium in the Genome of Farm Animals. Front. Genet. 2020, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, C.L.; Parra, E.J.; Bonilla, C.; Hiester, K.; McKeigue, P.M.; Kamboh, M.I.; Hutchinson, R.G.; Ferrell, R.E.; Boerwinkle, E.; Shriver, M.D. Population Structure in Admixed Populations: Effect of Admixture Dynamics on the Pattern of Linkage Disequilibrium. Am. J. Hum. Genet. 2001, 68, 198–207. [Google Scholar] [CrossRef]
- Goddard, M.E.; Hayes, B.J. Mapping Genes for Complex Traits in Domestic Animals and Their Use in Breeding Programmes. Nat. Rev. Genet. 2009, 10, 381–391. [Google Scholar] [CrossRef]
- Hollenbeck, C.M.; Portnoy, D.S.; Gold, J.R. A Method for Detecting Recent Changes in Contemporary Effective Population Size from Linkage Disequilibrium at Linked and Unlinked Loci. Heredity 2016, 117, 207–216. [Google Scholar] [CrossRef]
- Hill, W.G. Estimation of Effective Population Size from Data on Linkage Disequilibrium. Genet. Res. 1981, 38, 209–216. [Google Scholar] [CrossRef]
- Karimi, K.; Koshkoiyeh, A.E.; Asadi-Fozi, M.; Porto-Neto, L.R.; Gondro, C. Prioritization for Conservation of Iranian Native Cattle Breeds Based on Genome-Wide SNP Data. Conserv. Genet. 2015, 17, 77–89. [Google Scholar] [CrossRef]
- Waples, R.K.; Larson, W.A. Estimating Contemporary Effective Population Size in Non-model Species Using Linkage Disequilibrium across Thousands of Loci. Heredity 2016, 117, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, K.; Couvet, D. On the Expected Relationship between Inbreeding, Fitness, and Extinction. Genet. Sel. Evol. 2006, 38, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.T.; Pryce, J.E.; Baes, C.; Maltecca, C. Invited Review: Inbreeding in the Genomics Era: Inbreeding, Inbreeding Depression, and Management of Genomic Variability. J. Dairy Sci. 2017, 100, 6009–6024. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.V. A Taxonomic Review of the Genus Mustela (Mammalia Carnivora). Zoosyst. Ross. 2000, 8, 357–364. [Google Scholar]
- Bowness, E.R. An Historical Perspective on the North American Mink Industry. In Mink Biology, Health and Disease; Hunter, D.B., Lemieux, N., Eds.; University of Guelph: Guelph, ON, Canada, 1996; pp. 1–9. [Google Scholar]
- Joergensen, G. Mink Production; Scientifur: Hillerød, Denmark, 1985; p. 399. [Google Scholar]
- Lagerkvist, G.; Johansson, K.; Lundeheim, N. Selection for Litter Size, Body Weight, and Pelt Quality in Mink (Mustela vison): Correlated Responses. J. Anim. Sci. 1994, 72, 1126–1137. [Google Scholar] [CrossRef]
- Hu, G.; Do, D.N.; Gray, J.; Miar, Y. Selection for Favorable Health Traits: A Potential Approach to Cope with Diseases in Farm Animals. Animals 2020, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Miar, Y. Evaluation of Growth Curve Models for Body Weight in American Mink. Animals 2019, 10, 22. [Google Scholar] [CrossRef]
- Karimi, K.; Sargolzaei, M.; Plastow, G.; Wang, Z.; Miar, Y. Genetic and Phenotypic Parameters for Litter Size, Survival Rate, Gestation Length, and Litter Weight Traits in American Mink1. J. Anim. Sci. 2018, 96, 2596–2606. [Google Scholar] [CrossRef]
- Wiggans, G.; Cole, J.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Knol, E.F.; Nielsen, B.; Knap, P.W. Genomic Selection in Commercial Pig Breeding. Anim. Front. 2016, 6, 15–22. [Google Scholar] [CrossRef]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-Wide Genetic Marker Discovery and Genotyping Using Next-Generation Sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Karimi, K.; Sargolzaei, M.; Plastow, G.S.; Wang, Z.; Miar, Y. Opportunities for Genomic Selection in American Mink: A Simulation Study. PLoS ONE 2019, 14, e0213873. [Google Scholar] [CrossRef]
- Shimatani, Y.; Fukue, Y.; Kishimoto, R.; Masuda, R. Genetic Variation and Population Structure of the Feral American Mink (Neovison vison) in Nagano, Japan, Revealed by Microsatellite Analysis. Mammal Study 2010, 35, 1–7. [Google Scholar] [CrossRef]
- Thirstrup, J.; Ruiz-González, A.; Pujolar, J.M.; Larsen, P.F.; Jensen, J.; Randi, E.; Zalewski, A.; Pertoldi, C. Population Genetic Structure in Farm and Feral American Mink (Neovison Vison) Inferred from RAD Sequencing-Generated Single Nucleotide Polymorphisms1. J. Anim. Sci. 2015, 93, 3773–3782. [Google Scholar] [CrossRef]
- Mora, M.; Medina-Vogel, G.; Sepúlveda, M.A.; Noll, D.; Álvarez-Varas, R.; Vianna, J.A. Genetic Structure of Introduced American Mink (Neovison Vison) in Patagonia: Colonisation Insights and Implications for Control and Management Strategies. Wildl. Res. 2018, 45, 344. [Google Scholar] [CrossRef]
- Lecis, R.; Ferrando, A.; Ruiz-Olmo, J.; Mañas, S.; Roura, D.X. Population Genetic Structure and Distribution of Introduced American Mink (Mustela Vison) in Spain, Based on Microsatellite Variation. Conserv. Genet. 2007, 9, 1149–1161. [Google Scholar] [CrossRef]
- Zalewski, A.; Zalewska, H.; Lunneryd, S.-G.; André, C.; Mikusiński, G. Reduced Genetic Diversity and Increased Structure in American Mink on the Swedish Coast following Invasive Species Control. PLoS ONE 2016, 11, e0157972. [Google Scholar] [CrossRef]
- Karimi, K.; Farid, A.H.; Sargolzaei, M.; Myles, S.; Miar, Y. Linkage Disequilibrium, Effective Population Size and Genomic Inbreeding Rates in American Mink Using Genotyping-by-Sequencing Data. Front. Genet. 2020, 11, 223. [Google Scholar] [CrossRef]
- Turner, P.; Buijs, S.; Rommers, J.M.; Tessier, M. The Code of Practice for the Care and Handling of Farmed Mink; The National Farm Animal Care Council: Rexdale, ON, Canada, 2013; p. 58. [Google Scholar]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yuxin, C.; Li, Z.; et al. SOAPnuke: A Mapreduce Acceleration-Supported Software for Integrated Quality Control and Preprocessing of High-Throughput SE-Quencing Data. GigaScience 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Petersen, B.; Sahana, G.; Madsen, L.B.; Larsen, K.; Thomsen, B.; Bendixen, C.; Lund, M.S.; Guldbrandtsen, B.; Panitz, F. The First Draft Reference Genome of the American Mink (Neovison Vison). Sci. Rep. 2017, 7, 14564. [Google Scholar] [CrossRef]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Broad Institute. Picard Toolkit, Broad Institute. GitHub Repository . Available online: http://broadinstitute.github.io/picard/ (accessed on 3 February 2021).
- Sargolzaei, M. SNP1101 User’s Guide; Higgs Gene Solutions Inc.: Guelph, ON, Canada, 2014; Version 1.0. [Google Scholar]
- McQuillan, R.; Leutenegger, A.-L.; Abdel-Rahman, R.; Franklin, C.S.; Pericic, M.; Barac-Lauc, L.; Smolej-Narancic, N.; Janicijevic, B.; Polasek, O.; Tenesa, A.; et al. Runs of Homozygosity in European Populations. Am. J. Hum. Genet. 2008, 83, 359–372. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Pembleton, L.W.; Cogan, N.O.I.; Forster, J.W. St AMPP: An R Package for Calculation of Genetic Differentiation and Structure of Mixed-Ploidy Level Populations. Mol. Ecol. Resour. 2013, 13, 946–952. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New Tools for the Analysis of Genome-Wide Snp Data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast Model-Based Estimation of Ancestry in Unrelated Individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.G.; Robertson, A. Linkage Disequilibrium in Finite Populations. Theor. Appl. Genet. 1968, 38, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Hui, T.-Y.J.; Burt, A. Estimating Linkage Disequilibrium from Genotypes under Hardy-Weinberg Equilibrium. BMC Genet. 2020, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, H.; Hayes, B.; Berg, P.R.; Roseth, A.; Sundsaasen, K.K.; Nilsen, K.; Lien, S. Construction of a Dense SNP Map for Bovine Chromosome 6 to Assist the Assembly of the Bovine Genome Sequence. Anim. Genet. 2008, 39, 97–104. [Google Scholar] [CrossRef]
- Sved, J. Linkage Disequilibrium and Homozygosity of Chromosome Segments in Finite Populations. Theor. Popul. Biol. 1971, 2, 125–141. [Google Scholar] [CrossRef]
- Hayes, B.J.; Visscher, P.M.; McPartlan, H.C.; Goddard, M.E. Novel Multilocus Measure of Linkage Disequilibrium to Estimate Past Effective Population Size. Genome Res. 2003, 13, 635–643. [Google Scholar] [CrossRef]
- Cai, Z.; Villumsen, T.M.; Asp, T.; Guldbrandtsen, B.; Sahana, G.; Lund, M.S. SNP Markers Associated with Body Size and Pelt Length in American Mink (Neovison Vison). BMC Genet. 2018, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, A.; Miksza-Cybulska, A.; Szmatoła, T.; Jasielczuk, I.; Piestrzyńska-Kajtoch, A.; Fornal, A.; Semik-Gurgul, E.; Bugno-Poniewierska, M. Genotyping-by-Sequencing Performance in Selected Livestock Species. Genomes 2019, 111, 186–195. [Google Scholar] [CrossRef]
- Belliveau, A.M.; Farid, A.; O’Connell, M.; Wright, J.M. Assessment of Genetic Variability in Captive and Wild American Mink (Mustela Vison) Using Microsatellite Markers. Can. J. Anim. Sci. 1999, 79, 7–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Calus, M.P.L.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Estimation of Inbreeding Using Pedigree, 50k SNP Chip Genotypes and Full Sequence Data in Three Cattle Breeds. BMC Genet. 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Bovo, S.; Bertolini, F.; Tinarelli, S.; Dall’Olio, S.; Costa, L.N.; Gallo, M.; Fontanesi, L. Comparative Evaluation of Genomic Inbreeding Parameters in Seven Commercial and Autochthonous Pig Breeds. Animal 2020, 14, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramilo, S.T.; Fernández, J.; Toro, M.Á.; Hernández, D.; Villanueva, B. Genome-Wide Estimates of Coancestry, Inbreeding and Effective Population Size in the Spanish Holstein Population. PLoS ONE 2015, 10, e0124157. [Google Scholar] [CrossRef]
- Pemberton, T.J.; Absher, D.; Feldman, M.W.; Myers, R.M.; Rosenberg, N.A.; Li, J.Z. Genomic Patterns of Homozygosity in Worldwide Human Populations. Am. J. Hum. Genet. 2012, 91, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Howrigan, D.; Simonson, M.A.; Keller, M.C. Detecting Autozygosity through Runs of Homozygosity: A Comparison of Three Autozygosity Detection Algorithms. BMC Genom. 2011, 12, 460. [Google Scholar] [CrossRef]
- Ferenčaković, M.; Hamzić, E.; Gredler, B.; Solberg, T.; Klemetsdal, G.; Curik, I.; Sölkner, J. Estimates of Autozygosity Derived from Runs of Homozygosity: Empirical Evidence from Selected Cattle Populations. J. Anim. Breed. Genet. 2013, 130, 286–293. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Tolone, M.; Di Gerlando, R.; Fontanesi, L.; Sardina, M.T.; Portolano, B. Genomic Inbreeding Estimation in Small Populations: Evaluation of Runs of Homozygosity in Three Local Dairy Cattle Breeds. Animal 2016, 10, 746–754. [Google Scholar] [CrossRef]
- Bush, W.S.; Moore, J.H. Chapter 11: Genome-Wide Association Studies. PLoS Comput. Biol. 2012, 8, e1002822. [Google Scholar] [CrossRef]
- Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar]
- Thomasen, J.R.; Sørensen, A.C.; Su, G.; Madsen, P.; Lund, M.S.; Guldbrandtsen, B. The Admixed Population Structure in Danish Jersey Dairy Cattle Challenges Accurate Genomic Predictions1. J. Anim. Sci. 2013, 91, 3105–3112. [Google Scholar] [CrossRef]
- Brito, L.F.; Jafarikia, M.; Grossi, D.D.A.; Kijas, J.W.; Porto-Neto, L.R.; Ventura, R.V.; Sargolzaei, M.; Schenkel, F.S. Characterization of Linkage Disequilibrium, Consistency of Gametic Phase and Admixture in Australian and Canadian Goats. BMC Genet. 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ødegård, J.; Moen, T.; Santi, N.; Korsvoll, S.A.; Kjøglum, S.; Meuwissen, T.H.E. Genomic Prediction in an Admixed Population of Atlantic Salmon (Salmo Salar). Front. Genet. 2014, 5, 402. [Google Scholar] [CrossRef]
- Toosi, A.; Fernando, R.L.; Dekkers, J.C.M. Genomic Selection in Admixed and Crossbred Populations1. J. Anim. Sci. 2010, 88, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Li, W.-H. Linkage Disequilibrium in Subdivided Populations. Genetics 1973, 75, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.F.; Goldstein, D.B. Consistent Long-Range Linkage Disequilibrium Generated by Admixture in a Bantu-Semitic Hybrid Population. Am. J. Hum. Genet. 2000, 67, 926–935. [Google Scholar] [CrossRef]
- Ohta, T. Linkage Disequilibrium Due to Random Genetic Drift in Finite Subdivided Populations. Proc. Natl. Acad. Sci. USA 1982, 79, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Bashir, A.; Bafna, V. Evidence for Large Inversion Polymorphisms in the Human Genome from Hapmap Data. Genome Res. 2007, 17, 219–230. [Google Scholar] [CrossRef]
- Cáceres, A.; Sindi, S.S.; Raphael, B.J.; Cáceres, M.; González, J.R. Identification of Polymorphic Inversions from Genotypes. BMC Bioinform. 2012, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Lewontin, R.C.; Kojima, K.-I. The Evolutionary Dynamics of Complex Polymorphisms. Evolution 1960, 14, 458. [Google Scholar] [CrossRef]
- Voight, B.F.; Kudaravalli, S.; Wen, X.; Pritchard, J.K. A Map of Recent Positive Selection in the Human Genome. PLoS Biol. 2006, 4, e72. [Google Scholar] [CrossRef]
- Slatkin, M. Linkage Disequilibrium in Growing and Stable Populations. Genetics 1994, 137, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Schmegner, C.; Hoegel, J.; Vogel, W.; Assum, G. Genetic Variability in a Genomic Region with Long-Range Linkage Disequilibrium Reveals Traces of a Bottleneck in the History of the European Population. Qual. Life Res. 2005, 118, 276–286. [Google Scholar] [CrossRef]
- Koch, E.; Ristroph, M.; Kirkpatrick, M. Long Range Linkage Disequilibrium across the Human Genome. PLoS ONE 2013, 8, e80754. [Google Scholar] [CrossRef]
- Franssen, S.U.; Nolte, V.; Tobler, R.; Schlötterer, C. Patterns of Linkage Disequilibrium and Long Range Hitchhiking in Evolving Experimental Drosophila Melanogaster Populations. Mol. Biol. Evol. 2015, 32, 495–509. [Google Scholar] [CrossRef]
- Khatkar, M.S.; Nicholas, F.W.; Collins, A.R.; Zenger, K.R.; Cavanagh, J.A.L.; Barris, W.; Schnabel, R.; Taylor, J.F.; Raadsma, H.W. Extent of Genome-Wide Linkage Disequilibrium in Australian Holstein-Friesian Cattle Based on a High-Density SNP Panel. BMC Genom. 2008, 9, 187. [Google Scholar] [CrossRef] [PubMed]

| Color-Types | Number of Animals | Average MAF | Observed Heterozygosity (%) | FHOM | FPED |
|---|---|---|---|---|---|
| Demi | 32 | 0.214 | 31.12 | −0.189 | 0.006 |
| Pastel | 10 | 0.195 | 29.42 | −0.146 | 0.026 |
| Mahogany | 20 | 0.211 | 30.93 | −0.184 | 0.009 |
| Stardust | 7 | 0.190 | 30.64 | −0.153 | 0.016 |
| Black-NS 1 | 16 | 0.197 | 31.32 | −0.166 | 0.011 |
| CCFAR 2 | 85 | 0.216 | 30.87 | −0.171 | 0.010 |
| Black-ON 3 | 15 | 0.192 | 28.03 | −0.112 | NA 4 |
| Total | 100 | 0.216 | 30.45 | −0.166 | - |
| Color-Types | Minimum Length of ROH | |||||
|---|---|---|---|---|---|---|
| 500 kb | 1 Mb | 2 Mb | ||||
| Number | FROH | Number | FROH | Number | FROH | |
| Demi | 61 ± 17 | 0.072 ± 0.022 | 19 ± 7 | 0.036 ± 0.013 | 3 ± 2 | 0.009 ± 0.006 |
| Pastel | 90 ± 21 | 0.110 ± 0.027 | 30 ± 8 | 0.057 ± 0.016 | 5 ± 2 | 0.016 ± 0.008 |
| Mahogany | 68 ± 24 | 0.078 ± 0.030 | 21 ± 10 | 0.039 ± 0.018 | 3 ± 1 | 0.009 ± 0.005 |
| Stardust | 85 ± 12 | 0.100 ± 0.014 | 29 ± 4 | 0.056 ± 0.009 | 3 ± 2 | 0.011 ± 0.007 |
| Black-NS 1 | 82 ± 27 | 0.097 ± 0.034 | 26 ± 11 | 0.048 ± 0.021 | 3 ± 2 | 0.009 ± 0.007 |
| CCFAR 2 | 72 ± 23 | 0.085 ± 0.029 | 23 ± 10 | 0.043 ± 0.018 | 3 ± 2 | 0.010 ± 0.006 |
| Black-ON 3 | 120 ± 21 | 0.138 ± 0.031 | 39 ± 11 | 0.071 ± 0.019 | 4 ± 2 | 0.012 ± 0.007 |
| Color-Types | Demi | Pastel | Mahogany | Stardust | Black-NS | Black-ON |
|---|---|---|---|---|---|---|
| Demi | 0 | 0.025 | 0.013 | 0.036 | 0.029 | 0.030 |
| Pastel | 0.036 | 0 | 0.036 | 0.065 | 0.055 | 0.058 |
| Mahogany | 0.015 | 0.062 | 0 | 0.034 | 0.021 | 0.028 |
| Stardust | 0.055 | 0.124 | 0.046 | 0 | 0.039 | 0.044 |
| Black-NS 1 | 0.057 | 0.114 | 0.033 | 0.066 | 0 | 0.037 |
| Black-ON 2 | 0.059 | 0.122 | 0.048 | 0.077 | 0.076 | 0 |
| Source of Variation | Degree of Freedom | Sum of Squares | Mean Squared Deviations | Variance Components | Percentage of Variation |
|---|---|---|---|---|---|
| Among farms | 1 | 0.123 | 0.123 | 0.002 | 9 |
| Among color-types | 4 | 0.301 | 0.075 | 0.004 *** | 18 |
| Within color-types | 94 | 1.560 | 0.016 | 0.016 *** | 73 |
| Total | 99 | 1.984 | 0.020 | 0.022 | 100 |
| Number of Scaffolds | Length (Mb) | Number of SNPs | Average r2 ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Demi | Mahogany | Pastel | Black-NS 1 | CCFAR 2 | Black-ON 3 | |||
| 51 | 802.19 | 100,000 | 0.285 ± 0.346 | 0.280 ± 0.361 | 0.344 ± 0.411 | 0.343 ± 0.377 | 0.266 ± 0.334 | 0.366 ± 0.388 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, K.; Ngoc Do, D.; Sargolzaei, M.; Miar, Y. Population Genomics of American Mink Using Whole Genome Sequencing Data. Genes 2021, 12, 258. https://doi.org/10.3390/genes12020258
Karimi K, Ngoc Do D, Sargolzaei M, Miar Y. Population Genomics of American Mink Using Whole Genome Sequencing Data. Genes. 2021; 12(2):258. https://doi.org/10.3390/genes12020258
Chicago/Turabian StyleKarimi, Karim, Duy Ngoc Do, Mehdi Sargolzaei, and Younes Miar. 2021. "Population Genomics of American Mink Using Whole Genome Sequencing Data" Genes 12, no. 2: 258. https://doi.org/10.3390/genes12020258
APA StyleKarimi, K., Ngoc Do, D., Sargolzaei, M., & Miar, Y. (2021). Population Genomics of American Mink Using Whole Genome Sequencing Data. Genes, 12(2), 258. https://doi.org/10.3390/genes12020258







