Paracellular and Transcellular Leukocytes Diapedesis Are Divergent but Interconnected Evolutionary Events
Abstract
1. Introduction
The Origin and the Nature of These Systems Is Not Yet Known
2. Methods
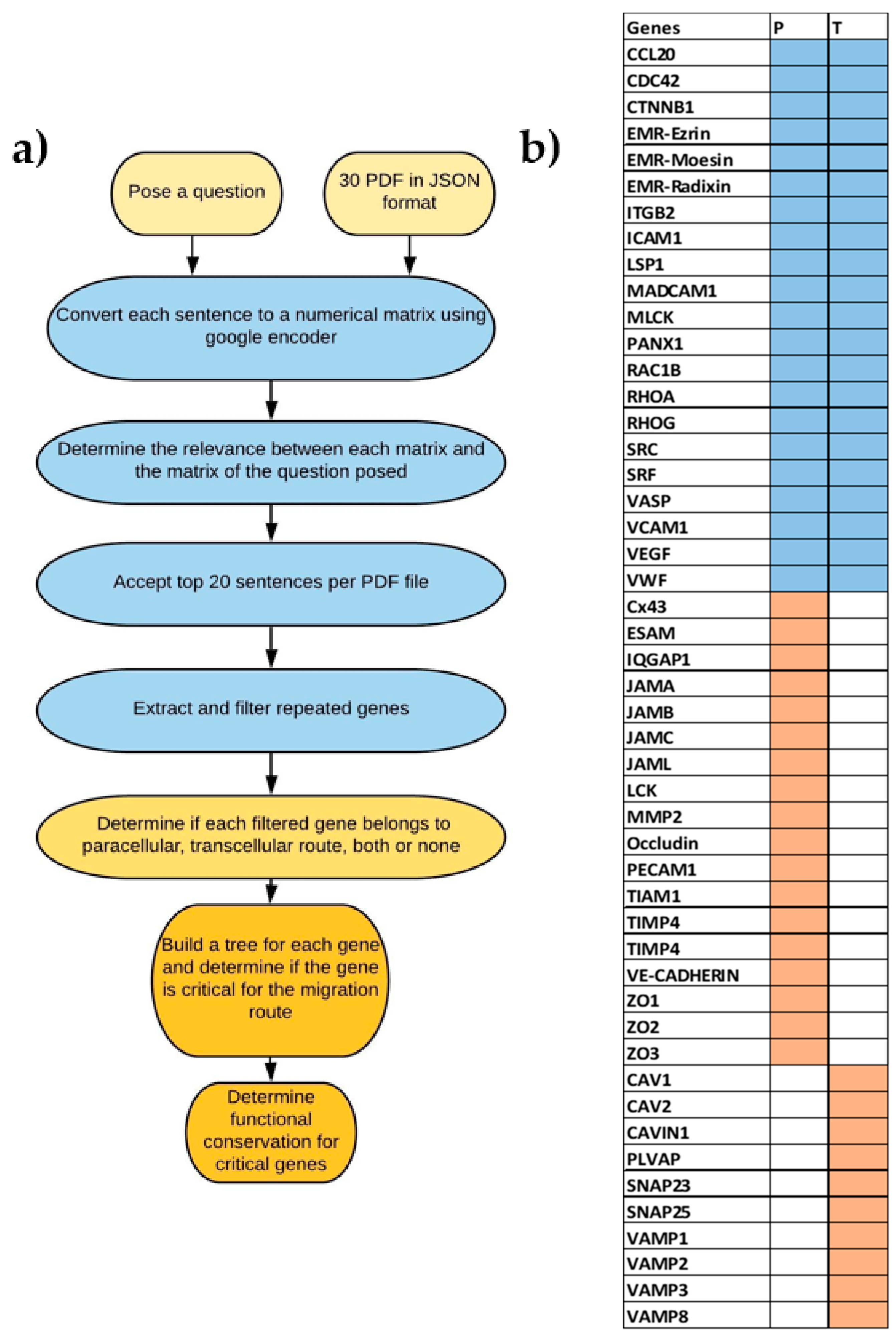
2.1. Text Mining Using AI
2.2. Data Used and Phylogenetic Tree
2.3. Ancestral Sequence Reconstruction (ASR)
2.4. HHsearch
3. Results
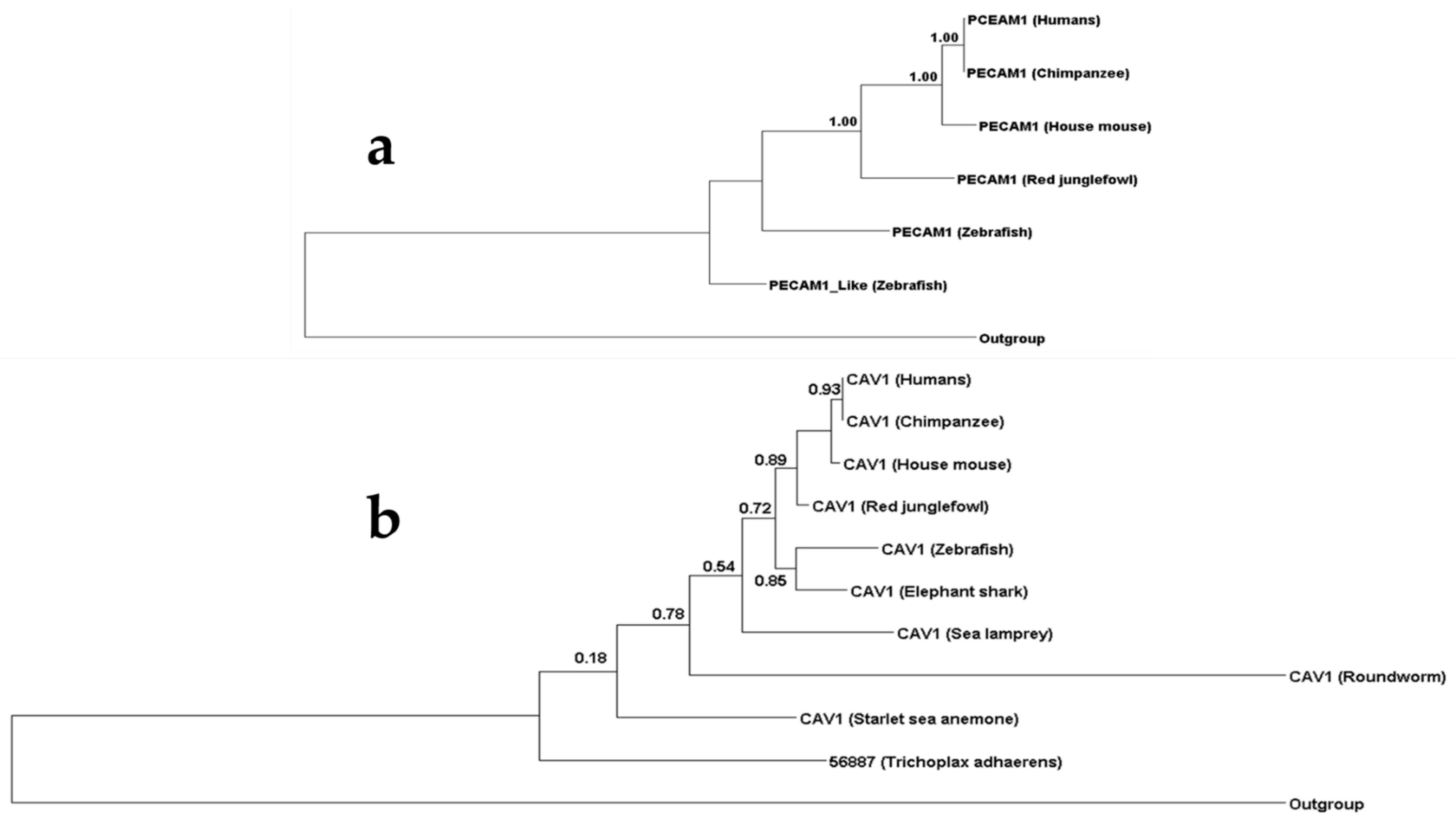
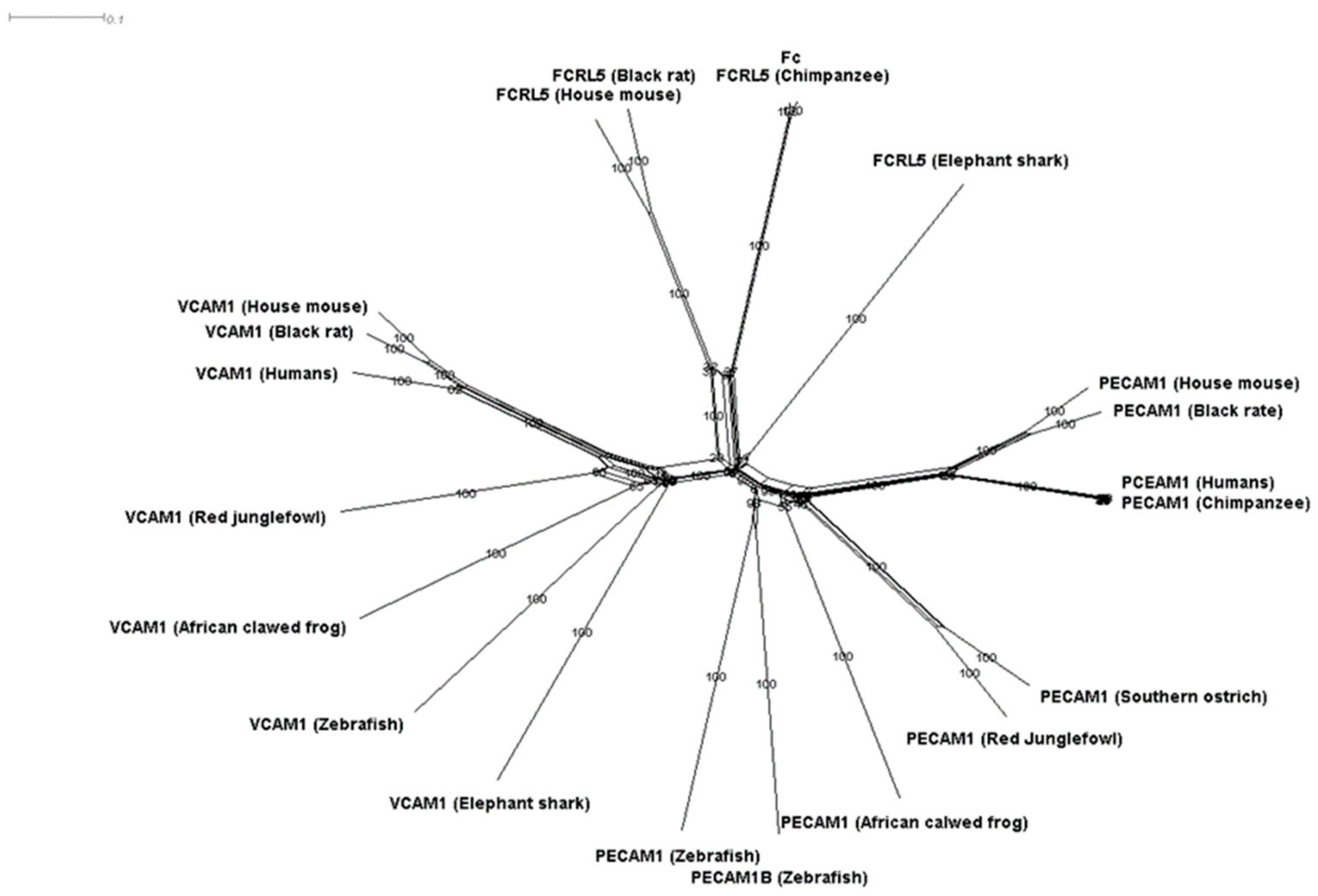
3.1. Phylogenetic Analysis of Components of the Diapedesis Mechanism
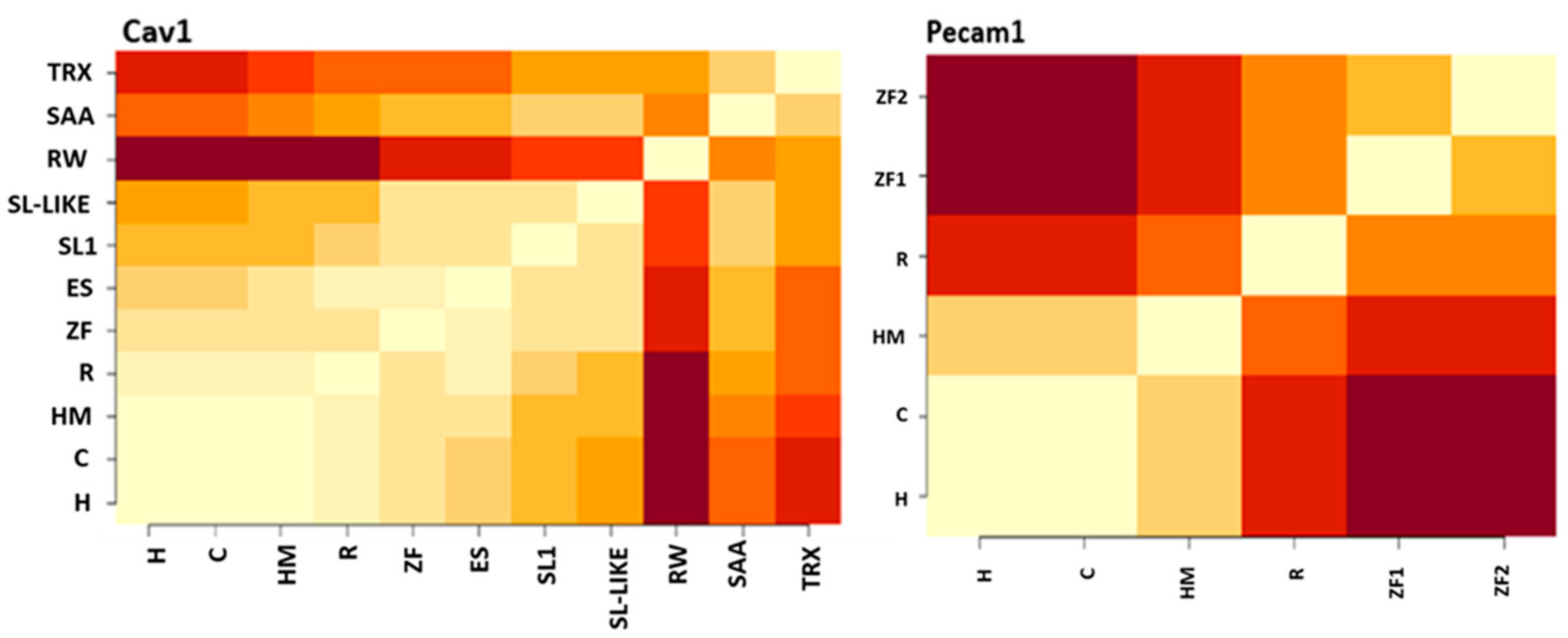
3.2. PECAM1 Evolved to Function as a Mechanical Sensor
4. Discussion
4.1. Origin of Transmigration Routes
4.2. Transmigration in Lower Invertebrates
4.3. Transmigration in Insects
4.4. Transmigration in Lampreys
4.5. Functional Aspects of the Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Kubick, N.; Flournoy, P.C.H.; Enciu, A.-M.; Manda, G.; Mickael, M.-E. Drugs Modulating CD4+ T Cells Blood–Brain Barrier Interaction in Alzheimer’s Disease. Pharmaceutics 2020, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Targeting blood–brain barrier changes during inflammatory pain: An opportunity for optimizing CNS drug delivery. Ther. Deliv. 2011, 2, 1015–1041. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Reglero-Real, N.; Colom, B.; Bodkin, J.V.; Nourshargh, S. Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2048–2057. [Google Scholar] [CrossRef]
- Abadier, M.; Boscacci, R.; Vestweber, D.; Barnum, S.; Deutsch, U.; Engelhardt, B.; Lyck, R.; Cardoso-Alves, L.; Jahromi, N.H. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur. J. Immunol. 2015, 45, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by ‘invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef]
- Monsonego, A.; Nemirovsky, A.; Harpaz, I. CD4 T cells in immunity and immunotherapy of Alzheimer’s disease. Immunology 2013, 139, 438–446. [Google Scholar] [CrossRef]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2008, 119, 182–192. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Rijvers, L.; Smolders, J.; Van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of Leukocyte Transendothelial Migration. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.P. Regulation of Endothelial Adherens Junctions by Tyrosine Phosphorylation. Mediat. Inflamm. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. The regulation of transendothelial migration: New knowledge and new questions. Cardiovasc. Res. 2015, 107, 310–320. [Google Scholar] [CrossRef]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Carman, C.V.; Sage, P.T.; Sciuto, T.E.; De La Fuente, M.A.; Geha, R.S.; Ochs, H.D.; Dvorak, H.F.; Dvorak, A.M.; Springer, T.A. Transcellular Diapedesis Is Initiated by Invasive Podosomes. Immunity 2007, 26, 784–797. [Google Scholar] [CrossRef]
- Wu, H.; Deng, R.; Chen, X.; Wong, W.C.; Chen, H.; Gao, L.; Nie, Y.; Wu, W.; Shen, J. Caveolin-1 Is Critical for Lymphocyte Trafficking into Central Nervous System during Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2016, 36, 5193–5199. [Google Scholar] [CrossRef]
- Carman, C.V.; Springer, T.A. Trans-cellular migration: Cell–cell contacts get intimate. Curr. Opin. Cell Biol. 2008, 20, 533–540. [Google Scholar] [CrossRef]
- Lutz, S.E.; Smith, J.R.; Kim, D.H.; Olson, C.V.L.; Ellefsen, K.; Bates, J.M.; Gandhi, S.P.; Agalliu, D. Caveolin1 Is Required for Th1 Cell Infiltration, but Not Tight Junction Remodeling, at the Blood-Brain Barrier in Autoimmune Neuroinflammation. Cell Rep. 2017, 21, 2104–2117. [Google Scholar] [CrossRef]
- Kunwar, P.S.; Starz-Gaiano, M.; Bainton, R.J.; Heberlein, U.; Lehmann, R. Tre1, a G Protein-Coupled Receptor, Directs Transepithelial Migration of Drosophila Germ Cells. PLoS Biol. 2003, 1, e80. [Google Scholar] [CrossRef] [PubMed]
- Kubick, N.; Pajares, M.; Enache, I.; Manda, G.; Mickael, M.-E. Repurposing Zileuton as a Depression Drug Using an AI and In Vitro Approach. Molecules 2020, 25, 2155. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2009, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Kubick, N.; Brösamle, D.; Mickael, M.-E. Molecular Evolution and Functional Divergence of the IgLON Family. Evol. Bioinform. 2018, 14, 1176934318775081. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R.; Posada, D. ProtTest: Selection of best-fit models of protein evolution. Bioinformatics 2005, 21, 2104–2105. [Google Scholar] [CrossRef] [PubMed]
- Mickael, M.E.; Rajput, A.; Steyn, J.; Wiemerslage, L.; Bürglin, T. An optimised phylogenetic method sheds more light on the main branching events of rhodopsin-like superfamily. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2016, 20, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Lethiec, F.; Duroux, P.; Gascuel, O. PHYML Online--a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005, 33, W557–W559. [Google Scholar] [CrossRef]
- Kalinina, O.V.; Mironov, A.A.; Gelfand, M.S.; Rakhmaninova, A.B. Automated selection of positions determining functional specificity of proteins by comparative analysis of orthologous groups in protein families. Protein Sci. 2004, 13, 443–456. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F. Modeling blood–brain barrier pathology in cerebrovascular disease in vitro: Current and future paradigms. Fluids Barriers CNS 2020, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tanos, B.E.; Bay, A.E.P.; Salvarezza, S.; Vivanco, I.; Mellinghoff, I.; Osman, M.; Sacks, D.B.; Rodriguez-Boulan, E. IQGAP1 controls tight junction formation through differential regulation of claudin recruitment. J. Cell Sci. 2015, 128, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.Y.; Huber, M.; Beauchemin, N.; Famiglietti, J.; Albelda, S.M.; Veillette, A. Regulation of Mouse PECAM-1 Tyrosine Phosphorylation by the Src and Csk Families of Protein-tyrosine Kinases. J. Biol. Chem. 1998, 273, 15765–15772. [Google Scholar] [CrossRef] [PubMed]
- Rojas, H.; Ritter, C.; Dal-Pizzol, F. Mechanisms of dysfunction of the blood-brain barrier in critically ill patients: Emphasis on the role of matrix metalloproteinases. Rev. Bras. Ter. Intensiv. 2011, 23, 222–227. [Google Scholar] [CrossRef]
- Shen, Q.; Lee, E.S.; Pitts, R.L.; Wu, M.H.; Yuan, S.Y. Tissue Inhibitor of Metalloproteinase-2 Regulates Matrix Metalloproteinase-2–Mediated Endothelial Barrier Dysfunction and Breast Cancer Cell Transmigration through Lung Microvascular Endothelial Cells. Mol. Cancer Res. 2010, 8, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Virgintino, D.; Robertson, D.; Errede, M.; Benagiano, V.; Tauer, U.; Roncali, L.; Bertossi, M. Expression of caveolin-1 in human brain microvessels. Neuroscience 2002, 115, 145–152. [Google Scholar] [CrossRef]
- Bastiani, M.; Parton, R.G. Caveolae at a glance. J. Cell Sci. 2010, 123, 3831–3836. [Google Scholar] [CrossRef]
- Bosma, E.K.; Van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood–brain and blood–retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 2018, 15, 1–17. [Google Scholar] [CrossRef]
- Hasan, N.; Corbin, D.; Hu, C. Fusogenic Pairings of Vesicle-Associated Membrane Proteins (VAMPs) and Plasma Membrane t-SNAREs—VAMP5 as the Exception. PLoS ONE 2010, 5, e14238. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Hughes, J.M.; Vogels, I.M.; Schalkwijk, C.G.; Van Noorden, C.J.; Schlingemann, R.O. Altered expression of genes related to blood–retina barrier disruption in streptozotocin-induced diabetes. Exp. Eye Res. 2009, 89, 4–15. [Google Scholar] [CrossRef]
- Steiner, O.; Coisne, C.; Cecchelli, R.; Boscacci, R.; Deutsch, U.; Engelhardt, B.; Lyck, R. Differential Roles for Endothelial ICAM-1, ICAM-2, and VCAM-1 in Shear-Resistant T Cell Arrest, Polarization, and Directed Crawling on Blood–Brain Barrier Endothelium. J. Immunol. 2010, 185, 4846–4855. [Google Scholar] [CrossRef] [PubMed]
- Doring, A.; Pfeiffer, F.; Meier, M.; Dehouck, B.; Tauber, S.; Deutsch, U.; Engelhardt, B. TET inducible expression of the ?4?7-integrin ligand MAdCAM-1 on the blood-brain barrier does not influence the immunopathogenesis of experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2011, 41, 813–821. [Google Scholar] [CrossRef]
- Heemskerk, N.; Van Rijssel, J.; Van Buul, J.D. Rho-GTPase signaling in leukocyte extravasation. Cell Adhes. Migr. 2014, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kishi, H.; Ye, L.-H.; Nakamura, A.; Okagaki, T.; Iwata, A.; Tanaka, T.; Kohama, K. Structure and function of smooth muscle myosin light chain kinase. In Taurine 6; Springer Nature: Berlin/Heidelberg, Germany, 1998; Volume 453, pp. 229–234. [Google Scholar]
- Busche, S.; Descot, A.; Julien, S.; Genth, H.; Posern, G. Epithelial cell-cell contacts regulate SRF-mediated transcription via Rac-actin-MAL signalling. J. Cell Sci. 2008, 121, 1025–1035. [Google Scholar] [CrossRef]
- Wittchen, E.S. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front. Biosci. 2009, 14, 2522–2545. [Google Scholar] [CrossRef]
- Bond, S.R.; Naus, C.C. The pannexins: Past and present. Front. Physiol. 2014, 5, 58. [Google Scholar] [CrossRef]
- Lutz, S.E.; González-Fernández, E.; Ventura, J.C.C.; Pérez-Samartín, A.; Tarassishin, L.; Negoro, H.; Patel, N.K.; Suadicani, S.O.; Lee, S.C.; Matute, C.; et al. Contribution of Pannexin1 to Experimental Autoimmune Encephalomyelitis. PLoS ONE 2013, 8, e66657. [Google Scholar] [CrossRef]
- Petri, B.; Kaur, J.; Long, E.M.; Li, H.; Parsons, S.A.; Butz, S.; Phillipson, M.; Vestweber, D.; Patel, K.D.; Robbins, S.M.; et al. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood 2011, 117, 942–952. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Yang, Z.-H.; Liu, Y.-H.; Ying, H.-Q.; Zhang, H.; Xue, Y.-X. Vascular Endothelial Growth Factor Increases Permeability of the Blood–Tumor Barrier via Caveolae-Mediated Transcellular Pathway. J. Mol. Neurosci. 2011, 44, 122–129. [Google Scholar] [CrossRef]
- Broermann, A.; Winderlich, M.; Block, H.; Frye, M.; Rossaint, J.; Zarbock, A.; Cagna, G.; Linnepe, R.; Schulte, D.; Nottebaum, A.F.; et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J. Exp. Med. 2011, 208, 2393–2401. [Google Scholar] [CrossRef]
- Suidan, G.L.; Brill, A.; De Meyer, S.F.; Voorhees, J.R.; Cifuni, S.M.; Cabral, J.E.; Wagner, D.D. Endothelial Von Willebrand Factor Promotes Blood–Brain Barrier Flexibility and Provides Protection from Hypoxia and Seizures in Mice. Arter. Thromb. Vasc. Biol. 2013, 33, 2112–2120. [Google Scholar] [CrossRef]
- Minshall, R.D.; Tiruppathi, C.; Vogel, S.M.; Niles, W.D.; Gilchrist, A.; Hamm, H.E.; Malik, A.B. Endothelial Cell-Surface Gp60 Activates Vesicle Formation and Trafficking via Gi-Coupled Src Kinase Signaling Pathway. J. Cell Biol. 2000, 150, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Muller, W.A. Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration. Eur. J. Immunol. 2008, 38, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Natkanski, E.; Lee, W.-Y.; Mistry, B.; Casal, A.; Molloy, J.E.; Tolar, P. B Cells Use Mechanical Energy to Discriminate Antigen Affinities. Science 2013, 340, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D.; Wild, M.K. A new player in lymphocyte homing. Nat. Immunol. 2008, 9, 347–348. [Google Scholar] [CrossRef]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho Family of Ras-Like GTPases in Eukaryotes. Mol. Biol. Evol. 2006, 24, 203–216. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of Transendothelial Migration of Leukocytes. Circ. Res. 2009, 105, 223–230. [Google Scholar] [CrossRef]
- Collins, C.; Guilluy, C.; Welch, C.; O’Brien, E.T.; Hahn, K.; Superfine, R.; Burridge, K.; Tzima, E. Localized Tensional Forces on PECAM-1 Elicit a Global Mechanotransduction Response via the Integrin-RhoA Pathway. Curr. Biol. 2012, 22, 2087–2094. [Google Scholar] [CrossRef]
- Marmon, S.; Hinchey, J.; Oh, P.; Cammer, M.; De Almeida, C.J.; Gunther, L.; Raine, C.S.; Lisanti, M.P. Caveolin-1 Expression Determines the Route of Neutrophil Extravasation through Skin Microvasculature. Am. J. Pathol. 2009, 174, 684–692. [Google Scholar] [CrossRef]
- Mamdouh, Z.; Chen, X.; Pierini, L.M.; Maxfield, F.R.; Muller, W.A. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nat. Cell Biol. 2003, 421, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.K.; Wang, N.; Leckband, D. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J. Cell Sci. 2015, 128, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.; Van Der Kammen, R.A.; Janssen, H.; Ellenbroek, S.I.; Collard, J.G. The Rac activator Tiam1 controls efficient T-cell trafficking and route of transendothelial migration. Blood 2009, 113, 6138–6147. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, A. Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model. Appl. Sci. 2020, 10, 6644. [Google Scholar] [CrossRef]
- Mickael, M. Modelling Baroreceptors Function. Ph.D. Thesis, Durham University, Durham, UK, 2012. [Google Scholar]
- Dusi, S.; Angiari, S.; Pietronigro, E.C.; Lopez, N.; Angelini, G.; Zenaro, E.; Della Bianca, V.; Tosadori, G.; Paris, F.; Amoruso, A.; et al. LFA-1 Controls Th1 and Th17 Motility Behavior in the Inflamed Central Nervous System. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Kubick, N.; Flournoy, P.C.H.; Klimovich, P.; Manda, G.; Mickael, M. What has single-cell RNA sequencing revealed about microglial neuroimmunology? Immunity Inflamm. Dis. 2020, 8, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Kamm, K.; Schierwater, B.; DeSalle, R. Innate immunity in the simplest animals—Placozoans. BMC Genom. 2019, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Hemmrich, G.; Ball, E.; Hayward, D.C.; Khalturin, K.; Funayama, N.; Agata, K.; Bosch, T.C. The innate immune repertoire in Cnidaria—Ancestral complexity and stochastic gene loss. Genome Biol. 2007, 8, 1–13. [Google Scholar] [CrossRef]
- Engelmann, I.; Pujol, N. Innate Immunity in C. elegans. Adv. Exp. Med. Biol. 2010, 708, 105–121. [Google Scholar]
- Schrödel, T.; Prevedel, R.; Aumayr, K.; Zimmer, M.; Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 2013, 10, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Shaham, S. Glial Development and Function in the Nervous System ofCaenorhabditis elegans. Cold Spring Harb. Perspect. Biol. 2015, 7, a020578. [Google Scholar] [CrossRef]
- Parton, R.G.; Tillu, V.A.; Collins, B.M. Caveolae. Curr. Biol. 2018, 28, R402–R405. [Google Scholar] [CrossRef]
- Walser, P.J.; Ariotti, N.; Howes, M.; Ferguson, C.; Webb, R.; Schwudke, D.; Leneva, N.; Cho, K.-J.; Cooper, L.; Rae, J.; et al. Constitutive Formation of Caveolae in a Bacterium. Cell 2012, 150, 752–763. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Bohórquez, D.V. The now and then of gut-brain signaling. Brain Res. 2018, 1693, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.; Nixon, S.J.; Howes, M.T.; Abi-Rached, L.; Wakeham, D.E.; Hanzal-Bayer, M.; Ferguson, C.; Hill, M.M.; Fernandez-Rojo, M.; Brown, D.A.; et al. Evolutionary analysis and molecular dissection of caveola biogenesis. J. Cell Sci. 2008, 121, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Dighe, S.N.; De La Mora, E.; Chan, S.; Kantham, S.; McColl, G.; Miles, J.A.; Veliyath, S.K.; Sreenivas, B.Y.; Nassar, Z.D.; Silman, I.; et al. Rivastigmine and metabolite analogues with putative Alzheimer’s disease-modifying properties in a Caenorhabditis elegans model. Commun. Chem. 2019, 2, 35. [Google Scholar] [CrossRef]
- Parker, S.; Peterkin, H.S.; Baylis, H.A. Muscular dystrophy associated mutations in caveolin-1 induce neurotransmission and locomotion defects in Caenorhabditis elegans. Invertebr. Neurosci. 2007, 7, 157–164. [Google Scholar] [CrossRef]
- Behr, M.; Riedel, D.; Schuh, R. The Claudin-like Megatrachea Is Essential in Septate Junctions for the Epithelial Barrier Function in Drosophila. Dev. Cell 2003, 5, 611–620. [Google Scholar] [CrossRef]
- Thuma, L.; Carter, D.; Weavers, H.; Martin, P. Drosophila immune cells extravasate from vessels to wounds using Tre1 GPCR and Rho signaling. J. Cell Biol. 2018, 217, 3045–3056. [Google Scholar] [CrossRef] [PubMed]
- Pombal, M.A.; Alvarez-Otero, R.; Pérez-Fernández, J.; Solveira, C.; Megías, M. Development and Organization of the Lamprey Telencephalon with Special Reference to the GABAergic System. Front. Neuroanat. 2011, 5, 20. [Google Scholar] [CrossRef]
- Di Rocco, R.T.; Imre, I.; Johnson, N.; Brown, G.E. Behavioural response of adult sea lamprey (Petromyzon marinus) to predator and conspecific alarm cues: Evidence of additive effects. Hydrobiologia 2015, 767, 279–287. [Google Scholar] [CrossRef]
- Bundgaard, M. Brain barrier systems in the lamprey. I. Ultrastructure and permeability of cerebral blood vessels. Brain Res. 1982, 240, 55–64. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Mirshafiey, A. Th17 Cell, the New Player of Neuroinflammatory Process in Multiple Sclerosis. Scand. J. Immunol. 2011, 74, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kiapour, N.; Kapoor, S.; Merrill, J.R.; Xia, Y.; Ban, W.; Cohen, S.M.; Midkiff, B.R.; Jewells, V.; Shih, Y.-Y.I.; et al. IL-11 antagonist suppresses Th17 cell-mediated neuroinflammation and demyelination in a mouse model of relapsing-remitting multiple sclerosis. Clin. Immunol. 2018, 197, 45–53. [Google Scholar] [CrossRef]
- Hoepner, R.; Faissner, S.; Salmen, A.; Gold, R.; Chan, A. Efficacy and Side Effects of Natalizumab Therapy in Patients with Multiple Sclerosis. J. Cent. Nerv. Syst. Dis. 2014, 6, 41–49. [Google Scholar] [CrossRef] [PubMed]

| Species | Brain Structure | Immune Cells | BBB | Critical Component | Known Diapedesis Mechanism | |
|---|---|---|---|---|---|---|
| Cav1 | Pecam1 | |||||
| T. adhaerens | No brain detected | No known immune cells | No | Yes | no | NA |
| N. vectensis | Two brain pathways observed | Ameboyctes | unknown | Yes | no | NA |
| C. elegans | Brain-like structure | No adaptive immune system or mobile immune cells identified | glia | Yes | No | NA |
| D. melanogaster | Brain-like structure | Hemocytes | septal | no | no | Likely Paracellular |
| C. intestinalis | 177 neurons identified | Hemocytes | unknown | Yes | No | Likely transcellular |
| P. marinus | Brian structure exits | VLAR, VLARB and VLARC cells | Yes | Yes | No | Likely transcellular |
| Marine vertebrates | Brian structure exists | Adaptive and innate system developed | yes | Yes | Yes | Transcellular and paracellular |
| Terrestrial vertebrates | Brian structure exists | Adaptive and innate system developed | yes | Yes | Yes | Transcellular and paracellular |
| Species | In Both Migration Routes | Paracellular Only | Transcellular Only |
|---|---|---|---|
| T. adhaerens | CDC42, CTNNB1, MLCK, RAC1B, RHOA, RHOG, SRC, SRF, VASP and VWF | IQGAP1, LCK, TIAM1, TIMP1 and TIMP4, ZO orthologs | CAV orthologs, VAMP orthologs, and SNAP orthologs |
| N. vectensis | CDC42, CTNNB1, MLCK, RAC1B, RHOA, RHOG, SRC, SRF, VASP and VWF | IQGAP1, TIAM1, ZO orthologs | CAV orthologs, VAMP orthologs, and SNAP orthologs |
| C. elegans | CDC42, RAC1B, RHOA RHOG, SRC, SRF, VASP, MLCK and VWF | IQGAP1, TIAM1 | CAV orthologs, VAMP orthologs, and SNAP orthologs |
| D. melanogaster | CDC42, CTNNB1, MLCK, RAC1B, RHOA, RHOG, SRC, SRF, VASP, VEGFA, and VWF | LCK, MMP2, TIAM1 and TIMP1 | VAMP orthologs and SNAP orthologs |
| C. intestinalis | CDC42, CTNNB1, MLCK RAC1B, RHOA, SRC, SRF, VASP, VEGFA, VWF and CLDN5 | IQGAP1, LCK, TIMP1 and ZO2 | CAV orthologs, VAMP orthologs, and SNAP orthologs |
| P. marinus | CDC42, CTNNB1, MLCK, RAC1B, RHOA, RHOG, SRC, SRF, VASP, VEGFA and VWF | CLDN5, Cx43, IQGAP1, JAMC, JAML, LCK, MMP2, Occludin, TIAM1, ZO1, ZO2 | CAV orthologs, VAMP orthologs, and SNAP orthologs |
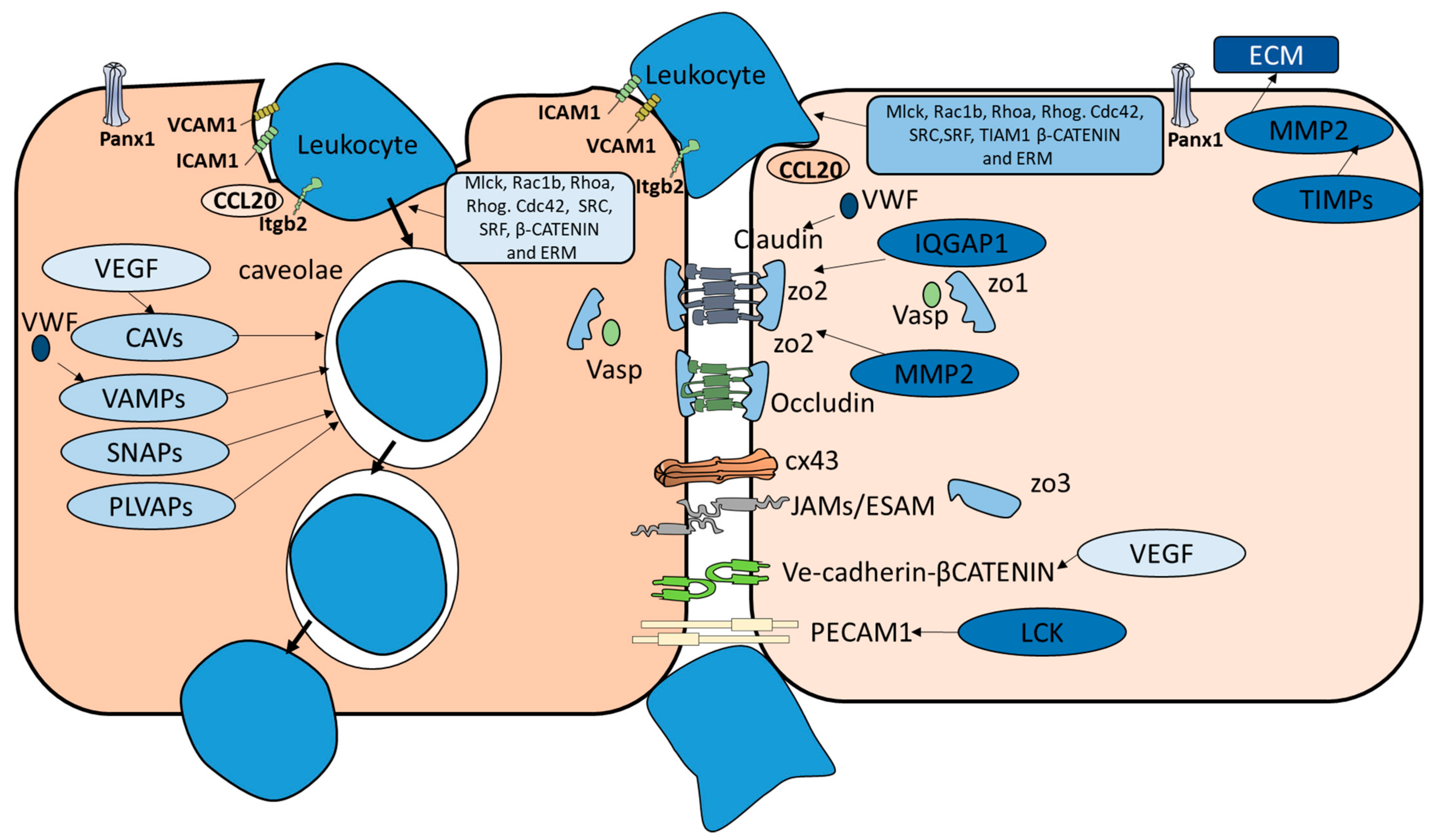
| Marine vertebrates | CCL20, CDC42, CTNNB1, ICAM1, LSP1, MLCK, PANX1, RAC1B, RHOA, RHOG, SRC, SRF, VASP, VCAM1, VEGFA, and VWF | CLDN5, Cx43, ESAM, JAMA, JAMB, JAMC, LCK, MMP2, Occludin, TIAM1, VE-CADHERIN, ZO1, ZO2 | CAV1, CAV2, SNAP23, SNAP25, VAMP2, VAMP3, VAMP8 |
| Terrestrial vertebrates | CCL20, CDC42, CTNNB1, ICAM1, LSP1, MADCAM1, MLCK, PANX1, RAC1B, RHOA, RHOG, SRC, SRF, VASP, VCAM1, VEGFA and VWF | CLDN5, Cx43, ESAM, IQGAP1, JAMA, JAMB, JAMC, JAML, LCK, MMP2, Occludin, PECAM1, TIAM1, TIMP1, TIMP4, VE-CADHERIN, ZO1, ZO2, ZO3 | CAV1, CAV2, CAVIN1, PLVAP, SNAP23, SNAP25, VAMP1, VAMP2, VAMP3, VAMP8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickael, M.-E.; Kubick, N.; Klimovich, P.; Flournoy, P.H.; Bieńkowska, I.; Sacharczuk, M. Paracellular and Transcellular Leukocytes Diapedesis Are Divergent but Interconnected Evolutionary Events. Genes 2021, 12, 254. https://doi.org/10.3390/genes12020254
Mickael M-E, Kubick N, Klimovich P, Flournoy PH, Bieńkowska I, Sacharczuk M. Paracellular and Transcellular Leukocytes Diapedesis Are Divergent but Interconnected Evolutionary Events. Genes. 2021; 12(2):254. https://doi.org/10.3390/genes12020254
Chicago/Turabian StyleMickael, Michel-Edwar, Norwin Kubick, Pavel Klimovich, Patrick Henckell Flournoy, Irmina Bieńkowska, and Mariusz Sacharczuk. 2021. "Paracellular and Transcellular Leukocytes Diapedesis Are Divergent but Interconnected Evolutionary Events" Genes 12, no. 2: 254. https://doi.org/10.3390/genes12020254
APA StyleMickael, M.-E., Kubick, N., Klimovich, P., Flournoy, P. H., Bieńkowska, I., & Sacharczuk, M. (2021). Paracellular and Transcellular Leukocytes Diapedesis Are Divergent but Interconnected Evolutionary Events. Genes, 12(2), 254. https://doi.org/10.3390/genes12020254






