Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Blood Transcriptomic Data
2.2. Analysis of Transcriptomic Data
2.3. Functional Insights into the Significant Genes
2.4. Network Analysis
3. Results
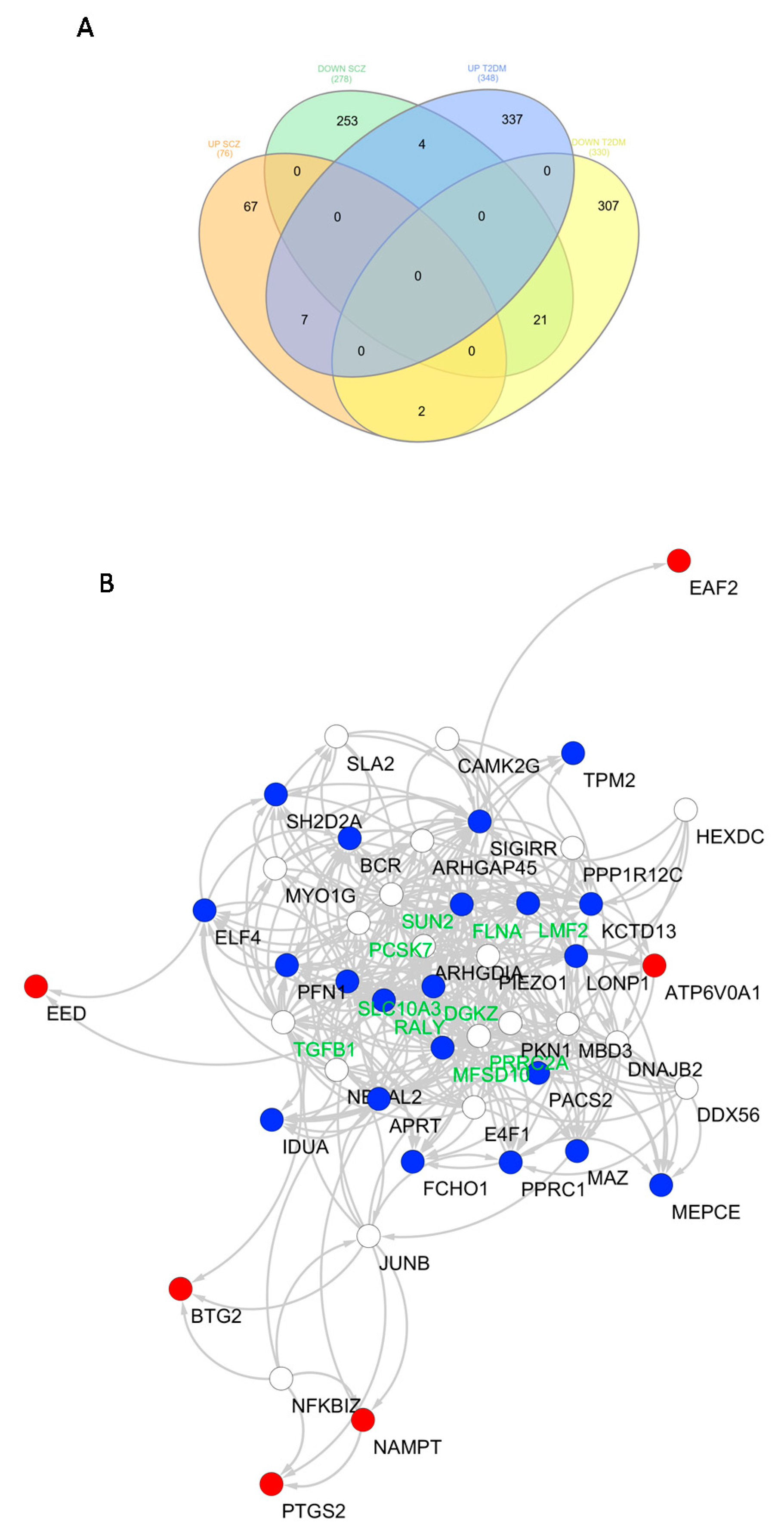
3.1. Identification of Common Transcriptional Signatures between SCZ and T2DM PBMCs
3.2. Identification of Common Functional Gene Ontology Terms in SCZ and T2DM PBMCs
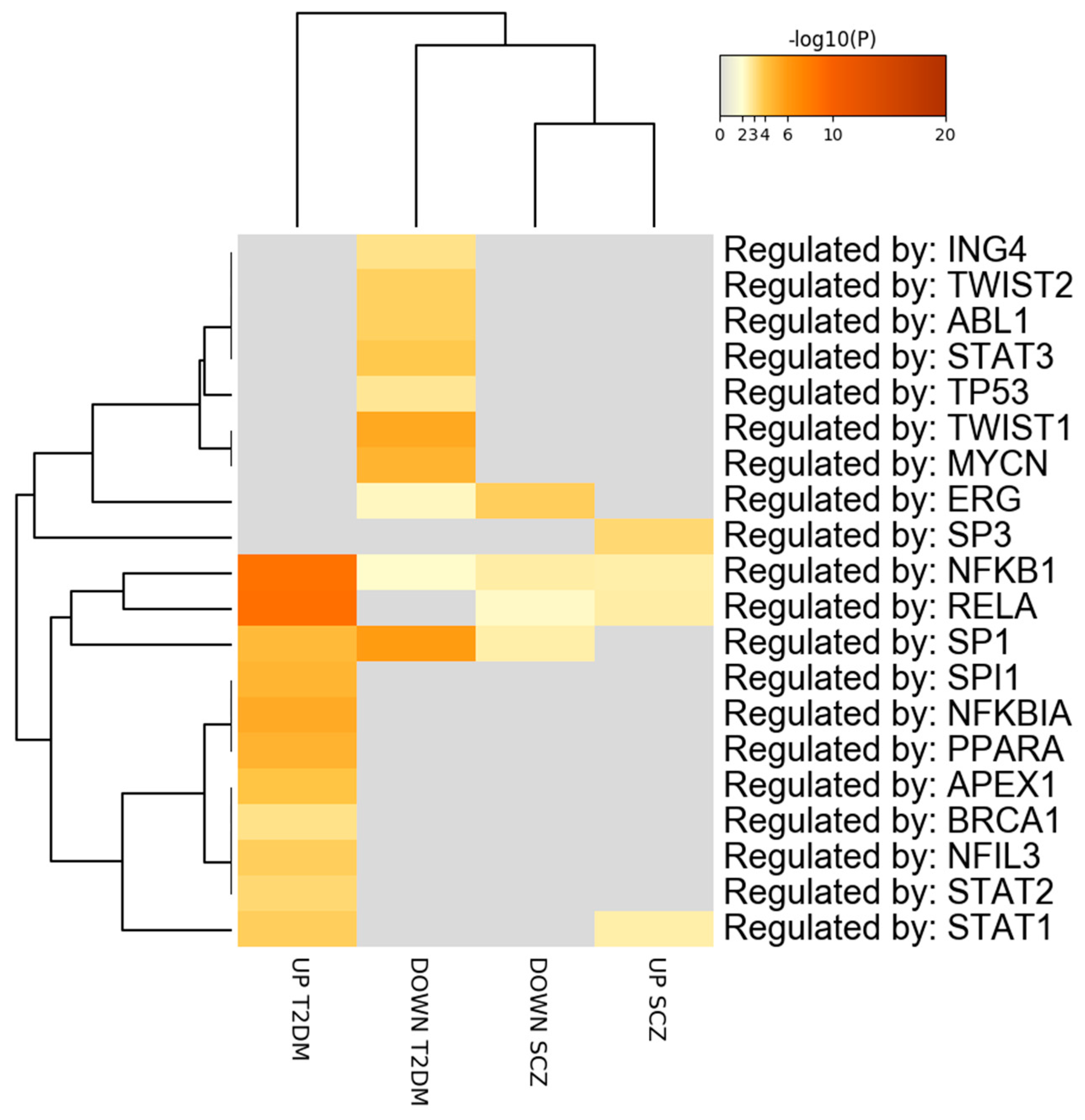
3.3. Prediction of Transcription Factor Overlapping between SCZ and T2DM PBMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rouillon, F.; Sorbara, F. Schizophrenia and diabetes: Epidemiological data. Eur. Psychiatry 2005, 20, S345–S348. [Google Scholar] [CrossRef]
- Lin, P.I.; Shuldiner, A.R. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr. Res. 2010, 123, 234–243. [Google Scholar] [CrossRef]
- Suvisaari, J.; Keinänen, J.; Eskelinen, S.; Mantere, O. Diabetes and schizophrenia. Curr. Diabetes Rep. 2016, 16, 16. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Mitchell, A.J. Diabetes mellitus and severe mental illness: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 79–89. [Google Scholar] [CrossRef]
- Young, S.L.; Taylor, M.; Lawrie, S.M. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J. Psychopharmacol. 2015, 29, 353–362. [Google Scholar] [CrossRef]
- Vancampfort, D.; Correll, C.U.; Galling, B.; Probst, M.; De Hert, M.; Ward, P.B.; Rosenbaum, S.; Gaughran, F.; Lally, J.; Stubbs, B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: A systematic review and large scale meta-analysis. World Psychiatry 2016, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Hopkins, D.; Peveler, R.C.; Holt, R.I.G.; Woodward, M.; Ismail, K. First-v. second-generation antipsychotics and risk for diabetes in schizophrenia: Systematic review and meta-analysis. Br. J. Psychiatry 2008, 192, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Hackinger, S.; Prins, B.; Mamakou, V.; Zengini, E.; Marouli, E.; Brčić, L.; Serafetinidis, I.; Lamnissou, K.; Kontaxakis, V.; Dedoussis, G. Evidence for genetic contribution to the increased risk of type 2 diabetes in schizophrenia. Transl. Psychiatry 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Purcell, S.M.; Wray, N.R.; Stone, J.L.; Visscher, P.M. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009, 460, 748–752. [Google Scholar]
- Stringer, S.; Kahn, R.S.; de Witte, L.D.; Ophoff, R.A.; Derks, E.M. Genetic liability for schizophrenia predicts risk of immune disorders. Schizophr. Res. 2014, 159, 347–352. [Google Scholar] [CrossRef]
- Padmanabhan, J.L.; Nanda, P.; Tandon, N.; Mothi, S.S.; Bolo, N.; McCarroll, S.; Clementz, B.A.; Gershon, E.S.; Pearlson, G.D.; Sweeney, J.A.; et al. Polygenic risk for type 2 diabetes mellitus among individuals with psychosis and their relatives. J. Psychiatr. Res. 2016, 77, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Islam, T.; Turanli, B.; Zaman, T.; Faruquee, H.M.; Rahman, M.M.; Mollah, M.N.H.; Nanda, R.K.; Arga, K.Y.; Gov, E.; et al. Network-based approach to identify molecular signatures and therapeutic agents in Alzheimer’s disease. Comput. Biol. Chem. 2019, 78, 431–439. [Google Scholar] [CrossRef]
- Rahman, M.R.; Islam, T.; Zaman, T.; Shahjaman, M.; Karim, M.R.; Huq, F.; Quinn, J.M.W.; Holsinger, R.M.D.; Gov, E.; Moni, M.A. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer’s disease: Insights from a systems biomedicine perspective. Genomics 2020, 112, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gu, C.; Huo, Y.; Li, X.; Luo, X.-J. The integrated landscape of causal genes and pathways in schizophrenia. Transl. Psychiatry 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Wu, T.; Zhu, S.; Deng, L.; Cui, G. Axon guidance pathway genes are associated with schizophrenia risk. Exp. Ther. Med. 2018, 16, 4519–4526. [Google Scholar] [CrossRef]
- Petralia, M.C.; Ciurleo, R.; Saraceno, A.; Pennisi, M.; Basile, M.S.; Fagone, P.; Bramanti, P.; Nicoletti, F.; Cavalli, E. Meta-Analysis of Transcriptomic Data of Dorsolateral Prefrontal Cortex and of Peripheral Blood Mononuclear Cells Identifies Altered Pathways in Schizophrenia. Genes 2020, 11, 390. [Google Scholar] [CrossRef]
- Ding, L.; Fan, L.; Xu, X.; Fu, J.; Xue, Y. Identification of core genes and pathways in type 2 diabetes mellitus by bioinformatics analysis. Mol. Med. Rep. 2019, 20, 2597–2608. [Google Scholar] [CrossRef]
- Zhong, M.; Wu, Y.; Ou, W.; Huang, L.; Yang, L. Identification of key genes involved in type 2 diabetic islet dysfunction: A bioinformatics study. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef]
- Kaizer, E.C.; Glaser, C.L.; Chaussabel, D.; Banchereau, J.; Pascual, V.; White, P.C. Gene expression in peripheral blood mononuclear cells from children with diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 3705–3711. [Google Scholar] [CrossRef]
- Gardiner, E.J.; Cairns, M.J.; Liu, B.; Beveridge, N.J.; Carr, V.; Kelly, B.; Scott, R.J.; Tooney, P.A. Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J. Psychiatr. Res. 2013, 47, 425–437. [Google Scholar] [CrossRef]
- Bousman, C.A.; Chana, G.; Glatt, S.J.; Chandler, S.D.; May, T.; Lohr, J.; Kremen, W.S.; Tsuang, M.T.; Everall, I.P. Positive symptoms of psychosis correlate with expression of ubiquitin proteasome genes in peripheral blood. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 1336–1341. [Google Scholar] [CrossRef]
- Bousman, C.A.; Chana, G.; Glatt, S.J.; Chandler, S.D.; Lucero, G.R.; Tatro, E.; May, T.; Lohr, J.B.; Kremen, W.S.; Tsuang, M.T.; et al. Preliminary evidence of ubiquitin proteasome system dysregulation in schizophrenia and bipolar disorder: Convergent pathway analysis findings from two independent samples. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153B, 494–502. [Google Scholar] [CrossRef] [PubMed]
- van Beveren, N.J.M.; Buitendijk, G.H.S.; Swagemakers, S.; Krab, L.C.; Röder, C.; de Haan, L.; van der Spek, P.; Elgersma, Y. Marked reduction of AKT1 expression and deregulation of AKT1-associated pathways in peripheral blood mononuclear cells of schizophrenia patients. PLoS ONE 2012, 7, e32618. [Google Scholar] [CrossRef] [PubMed]
- Toro-Domínguez, D.; Martorell-Marugán, J.; López-Domínguez, R.; García-Moreno, A.; González-Rumayor, V.; Alarcón-Riquelme, M.E.; Carmona-Sáez, P. ImaGEO: Integrative gene expression meta-analysis from GEO database. Bioinformatics 2019, 35, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.; Gallopin, M.; Celeux, G.; Jaffrézic, F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 2013, 29, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. Network Analyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Konishi, S. Normalizing and variance stabilizing transformations for intraclass correlations. Ann. Inst. Stat. Math. 1985, 37, 87–94. [Google Scholar] [CrossRef]
- Hansen, K.D.; Irizarry, R.A.; Wu, Z. Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Kalinka, A.T. The probability of drawing intersections: Extending the hypergeometric distribution. arXiv 2013, arXiv:1305.0717. [Google Scholar]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, K.; Franz, M.; Rodriguez, H.; Montojo, J.; Lopes, C.T.; Bader, G.D.; Morris, Q. GeneMANIA Prediction Server 2013 Update. Nucleic Acids Res. 2013, 41, W115–W122. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mazzon, E.; Cavalli, E.; Bramanti, A.; Petralia, M.C.; Mangano, K.; Al-Abed, Y.; Bramati, P.; Nicoletti, F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J. Neuroimmunol. 2018, 322, 46–56. [Google Scholar] [CrossRef]
- Fagone, P.; Patti, F.; Mangano, K.; Mammana, S.; Coco, M.; Touil-Boukoffa, C.; Chikovani, T.; Di Marco, R.; Nicoletti, F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neuroimmunol. 2013, 261, 82–86. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Mammana, S.; Basile, M.S.; Lombardo, S.D.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Overexpression of Macrophage Migration Inhibitory Factor and Its Homologue D-Dopachrome Tautomerase as Negative Prognostic Factor in Neuroblastoma. Brain Sci. 2019, 9, 284. [Google Scholar] [CrossRef]
- Rahman, M.R.; Petralia, M.C.; Ciurleo, R.; Bramanti, A.; Fagone, P.; Shahjaman, M.; Wu, L.; Sun, Y.; Turanli, B.; Arga, K.Y.; et al. Comprehensive analysis of RNA-seq gene expression profiling of brain transcriptomes reveals novel genes, regulators, and pathways in autism spectrum disorder. Brain Sci. 2020, 10, 747. [Google Scholar] [CrossRef]
- Fagone, P.; Mangano, K.; Pesce, A.; Portale, T.R.; Puleo, S.; Nicoletti, F. Emerging therapeutic targets for the treatment of hepatic fibrosis. Drug Discov. Today 2016, 21, 369–375. [Google Scholar] [CrossRef]
- Günther, S.; Fagone, P.; Jalce, G.; Atanasov, A.G.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today 2019, 24, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant expression of MHC class II in melanoma attracts inflammatory tumor-specific CD4+T-cells, which dampen CD8+T-cell antitumor reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Rothweiler, F.; Michaelis, M.; Brauer, P.; Otte, J.; Weber, K.; Fehse, B.; Doerr, H.W.; Wiese, M.; Kreuter, J.; Al-Abed, Y.; et al. Anticancer effects of the nitric oxide-modified saquinavir derivative saquinavir-NO against multidrug-resistant cancer cells. Neoplasia 2010, 12, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The therapeutic potential of mTOR inhibitors in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic-Ivanic, D.; Fagone, P.; McCubrey, J.; Bendtzen, K.; Mijatovic, S.; Nicoletti, F. HIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int. J. Cancer 2017, 140, 1713–1726. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Schwartsburd, P.M. Catabolic and anabolic faces of insulin resistance and their disorders: A new insight into circadian control of metabolic disorders leading to diabetes. Future Sci. OA 2017, 3, FSO201. [Google Scholar] [CrossRef]
- Yarwood, R.; Hellicar, J.; Woodman, P.G.; Lowe, M. Membrane trafficking in health and disease. Dis. Model. Mech. 2020, 13, dmm043448. [Google Scholar] [CrossRef]
- Schubert, K.O.; Föcking, M.; Prehn, J.H.M.; Cotter, D.R. Hypothesis review: Are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol. Psychiatry 2012, 17, 669–681. [Google Scholar] [CrossRef]
- Wang, X.; Christian, K.M.; Song, H.; Ming, G. Synaptic dysfunction in complex psychiatric disorders: From genetics to mechanisms. Genome Med. 2018, 10, 9. [Google Scholar] [CrossRef]
- Van Kesteren, C.; Gremmels, H.; De Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E.C. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J. Diabetes Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Chase, K.A.; Cone, J.J.; Rosen, C.; Sharma, R.P. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Pouget, J.G.; Han, B.; Wu, Y.; Mignot, E.; Ollila, H.M.; Barker, J.; Spain, S.; Dand, N.; Trembath, R.; Martin, J.; et al. Cross-disorder analysis of schizophrenia and 19 immune-mediated diseases identifies shared genetic risk. Hum. Mol. Genet. 2019, 28, 3498–3513. [Google Scholar] [CrossRef]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Joosten, L.A.B.; Koenen, T.; Van Tits, B.; Van Diepen, J.A.; Van Den Berg, S.A.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Masedu, F.; Alvaro, S.; Airò, P.; Battafarano, N.; Cantarini, L.; Cantatore, F.P.; Carlino, G.; D’Abrosca, V.; Frassi, M.; et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 2019, 16, e1002901. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, C.; Sun, L.; Li, J.; Qing, Y.; Hu, X.; Yang, X.; Li, Y.; Xu, C.; Zhang, J.; et al. Leukocyte Proteomic Profiling in First-Episode Schizophrenia Patients: Does Oxidative Stress Play Central Roles in the Pathophysiology Network of Schizophrenia? Antioxid. Redox Signal. 2019, 31, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.K.; Pokharia, D.; Tripathi, K. Relationship between oxidative stress and apoptotic markers in lymphocytes of diabetic patients with chronic non healing wound. Diabetes Res. Clin. Pract. 2011, 94, 377–384. [Google Scholar] [CrossRef]
- Hashimoto, R.; Ohi, K.; Yasuda, Y.; Fukumoto, M.; Yamamori, H.; Takahashi, H.; Iwase, M.; Okochi, T.; Kazui, H.; Saitoh, O. Variants of the RELA gene are associated with schizophrenia and their startle responses. Neuropsychopharmacology 2011, 36, 1921–1931. [Google Scholar] [CrossRef]
- Song, X.-Q.; Lv, L.-X.; Li, W.-Q.; Hao, Y.-H.; Zhao, J.-P. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol. Psychiatry 2009, 65, 481–488. [Google Scholar] [CrossRef]
- Volk, D.W.; Moroco, A.E.; Roman, K.M.; Edelson, J.R.; Lewis, D.A. The role of the nuclear factor-κB transcriptional complex in cortical immune activation in schizophrenia. Biol. Psychiatry 2019, 85, 25–34. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Rezaeepoor, M.; Hoseini-Aghdam, M.; Sheikh, V.; Eftekharian, M.M.; Behzad, M. Evaluation of interleukin-23 and JAKs/STATs/SOCSs/ROR-ct expression in type 2 diabetes mellitus patients treated with or without sitagliptin. J. Interf. Cytokine Res. 2020, 40, 515–523. [Google Scholar] [CrossRef]
- Sharma, R.P.; Rosen, C.; Melbourne, J.K.; Feiner, B.; Chase, K.A. Activated phosphorylated STAT1 levels as a biologically relevant immune signal in schizophrenia. Neuroimmunomodulation 2017, 23, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Papas, T.S.; Reddy, E.S.P. Erg, a human ets-related gene on chromosome 21:Aternative splicing, polyadenylation, and translation. Science 1987, 237, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Loughran, S.J.; Kruse, E.A.; Hacking, D.F.; de Graaf, C.A.; Hyland, C.D.; Willson, T.A.; Henley, K.J.; Ellis, S.; Voss, A.K.; Metcalf, D.; et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat. Immunol. 2008, 9, 810–819. [Google Scholar] [CrossRef]
- Yi, H.K.; Fujimura, Y.; Ouchida, M.; Prasad, D.D.K.; Rao, V.N.; Reddy, E.S.P. Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias. Oncogene 1997, 14, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Schwarz, M.J.; Müller, N. Inflammatory processes in schizophrenia: A promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol. Ther. 2011, 132, 96–110. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.C.; Chen, Y.L.; Pan, Y.H.; Ling, W.; Tian, F.; Zhang, X.X.; Zhao, H.L. Changes of transforming growth factor beta 1 in patients with type 2 diabetes and diabetic nephropathy. Medicine (Baltimore) 2017, 96, e6583. [Google Scholar] [CrossRef]
- Abbasi, F.; Amiri, P.; Sayahpour, F.A.; Pirmoradi, S.; Abolhalaj, M.; Larijani, B.; Bazzaz, J.T.; Amoli, M.M. TGF-β and IL-23 gene expression in unstimulated PBMCs of patients with diabetes. Endocrine 2012, 41, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Finardi, A.; Brambilla, P.; Furlan, R. Immune signature in PBMCs of patients with bipolar disorder and schizophrenia. Neurol. Psychiatry Brain Res. 2016, 22, 11. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.J.; Lee, H.-J.; Seki, T.; Hong, K.-H.; Park, J.; Beppu, H.; Lim, I.K.; Yoon, J.-W.; Li, E.; et al. B-Cell Translocation Gene 2 (Btg2) Regulates Vertebral Patterning by Modulating Bone Morphogenetic Protein/Smad Signaling. Mol. Cell. Biol. 2004, 24, 10256–10262. [Google Scholar] [CrossRef]

| Accession | Source/Tissue | Sample | Patients Characteristics | Healthy Controls Characteristics | Platform |
|---|---|---|---|---|---|
| Schizophrenia | |||||
| GSE18312 | PBMCs | 13 SCZ patients and 8 healthy controls | Age (years): 43.6 ± 8.6 | Age (years): 44.6 ± 6.5 | Affymetrix Human Exon 1.0 ST Array |
| % female: 30.7 | % female: 37.5 | ||||
| Race: European-American 38.4% Hispanic 15.3% African-American 46.2% | Race: European-American 65.5% Hispanic 12.5% Asian 12.5% African-American 12.5% | ||||
| GSE27383 | PBMCs | 43 SCZ subjects and 29 controls | Age (years): 23 ± 4 | Age (years): 23.9 ± 4.1 | Affymetrix Human Genome U133 Plus 2.0 Array |
| Race: European 48.8% Surinamese/African 14.6% Cape Verdean 2.4% Surinamese/Hindustani 14.6% Moroccan/North African 4.9% Asian 2.4% Mixed 7.3% Unknown 4.9% | Race: European 82.6% Surinamese/African 3.4% Surinamese/Hindustani 3.4% Asian 3.4% Mixed 6.9% | ||||
| Type 2 diabetes mellitus | |||||
| GSE9006 | PBMCs | 12 T2DM patients and 24 healthy controls | Age (years): 14 ± 2.3 | Age (years): 11.3 ± 4.6 | Affymetrix Human Genome U133A Array |
| % female: 58 | % female: 58 | ||||
| Race: Caucasian 16.6% Hispanic 16.6% African-American 58.3% Asian 8.3% | Race: Caucasian 45.8% Hispanic 29.1% Mixed or unknown 25% | ||||
| Genes Symbol | Description | Regulation |
|---|---|---|
| BTG2 | BTG anti-proliferation factor 2 | Upregulated |
| EED | embryonic ectoderm development | Upregulated |
| HBP1 | HMG-box transcription factor 1 | Upregulated |
| PTGS2 | prostaglandin-endoperoxide synthase 2 | Upregulated |
| NAMPT | nicotinamide phosphoribosyltransferase | Upregulated |
| ATP6V0A1 | ATPase H+ transporting V0 subunit a1 | Upregulated |
| EAF2 | ELL associated factor 2 | Upregulated |
| LONP1 | lon peptidase 1, mitochondrial | Downregulated |
| RALY | RALY heterogeneous nuclear ribonucleoprotein | Downregulated |
| PACS2 | phosphofurin acidic cluster sorting protein 2 | Downregulated |
| SH2D2A | SH2 domain containing 2A | Downregulated |
| DGKZ | diacylglycerol kinase zeta | Downregulated |
| MEPCE | methylphosphate capping enzyme | Downregulated |
| KCTD13 | potassium channel tetramerization domain containing 13 | Downregulated |
| ELF4 | E74 like ETS transcription factor 4 | Downregulated |
| MFSD10 | major facilitator superfamily domain containing 10 | Downregulated |
| MAZ | MYC associated zinc finger protein | Downregulated |
| SIGIRR | single Ig and TIR domain containing | Downregulated |
| FCHO1 | FCH domain only 1 | Downregulated |
| BCR | BCR, RhoGEF and GTPase activating protein | Downregulated |
| PPRC1 | peroxisome proliferator-activated receptor γ, coactivator-related 1 | Downregulated |
| TPM2 | tropomyosin 2 | Downregulated |
| IDUA | iduronidase, α-L- | Downregulated |
| PFN1 | profilin 1 | Downregulated |
| LMF2 | lipase maturation factor 2 | Downregulated |
| FLNA | filamin A | Downregulated |
| APRT | adenine phosphoribosyltransferase | Downregulated |
| SLC10A3 | solute carrier family 10 member 3 | Downregulated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.R.; Islam, T.; Nicoletti, F.; Petralia, M.C.; Ciurleo, R.; Fisicaro, F.; Pennisi, M.; Bramanti, A.; Demirtas, T.Y.; Gov, E.; et al. Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis. Genes 2021, 12, 237. https://doi.org/10.3390/genes12020237
Rahman MR, Islam T, Nicoletti F, Petralia MC, Ciurleo R, Fisicaro F, Pennisi M, Bramanti A, Demirtas TY, Gov E, et al. Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis. Genes. 2021; 12(2):237. https://doi.org/10.3390/genes12020237
Chicago/Turabian StyleRahman, Md Rezanur, Tania Islam, Ferdinando Nicoletti, Maria Cristina Petralia, Rosella Ciurleo, Francesco Fisicaro, Manuela Pennisi, Alessia Bramanti, Talip Yasir Demirtas, Esra Gov, and et al. 2021. "Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis" Genes 12, no. 2: 237. https://doi.org/10.3390/genes12020237
APA StyleRahman, M. R., Islam, T., Nicoletti, F., Petralia, M. C., Ciurleo, R., Fisicaro, F., Pennisi, M., Bramanti, A., Demirtas, T. Y., Gov, E., Islam, M. R., Mussa, B. M., Moni, M. A., & Fagone, P. (2021). Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis. Genes, 12(2), 237. https://doi.org/10.3390/genes12020237









