DRD4 Exon 3 Gene Polymorphisms in Patients Diagnosed with Polysubstance Use Disorder and Co-Occurrence of a Depressive Episode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measures
2.3. Genotyping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCabe, S.E.; West, B.T.; Jutkiewicz, E.M.; Boyd, C.J. Multiple DSM-5 substance use disorders: A national study of US adults. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2625. [Google Scholar] [CrossRef]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef]
- Bahorik, A.L.; Leibowitz, A.; Sterling, S.A.; Travis, A.; Weisner, C.; Satre, D.D. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J. Affect. Disord. 2017, 213, 168–171. [Google Scholar] [PubMed]
- Bahji, A.; Mazhar, M.N.; Hudson, C.C.; Nadkarni, P.; MacNeil, B.A.; Hawken, E. Prevalence of substance use disorder comorbidity among individuals with eating disorders: A systematic review and meta-analysis. Psychiatry Res. 2019, 273, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Zhu, Y.S.; Huo, Z.H.; Sun, R.F.; Yu, B.; Wang, Y.P.; Li, S.B. Association study of polymorphisms in the promoter region of DRD4 with schizophrenia, depression, and heroin addiction. Brain Res. 2010, 1359, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Masiak, J.; Chmielowiec, J.; Chmielowiec, K.; Grzywacz, A. DRD4, DRD2, DAT1, and ANKK1 Genes Polymorphisms in Patients with Dual Diagnosis of Polysubstance Addictions. J. Clin. Med. 2020, 9, 3593. [Google Scholar] [CrossRef]
- Van Tol, H.H.; Wu, C.M.; Guan, H.C.; Ohara, K.; Bunzow, J.R.; Civelli, O.; Kennedy, J.; Seeman, P.; Niznik, H.B.; Jovanovic, V. Multiple dopamine D4 receptor variants in the human population. Nature 1992, 358, 149–152. [Google Scholar]
- Asghari, V.; Sanyal, S.; Buchwaldt, S.; Paterson, A.; Jovanovic, V.; Van Tol, H.H. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 1995, 65, 1157–1165. [Google Scholar] [CrossRef]
- Vandenbergh, D.J.; Rodriguez, L.A.; Hivert, E.; Schiller, J.H.; Villareal, G.; Pugh, E.W.; Uhl, G.R. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: No interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am. J. Med. Genet. 2000, 96, 678–683. [Google Scholar] [CrossRef]
- Munafò, M.R.; Yalcin, B.; Willis-Owen, S.A.; Flint, J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: Meta-analysis and new data. Biol. Psychiatry 2008, 63, 197–206. [Google Scholar] [CrossRef]
- Eisenberg, D.T.; MacKillop, J.; Modi, M.; Beauchemin, J.; Dang, D.; Lisman, S.A.; Wilson, D.S. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav. Brain Funct. 2007, 3, 1–14. [Google Scholar] [CrossRef]
- Benjamin, J.; Li, L.; Patterson, C.; Greenberg, B.D.; Murphy, D.L.; Hamer, D.H. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat. Genet. 1996, 12, 81–84. [Google Scholar] [CrossRef]
- Ebstein, R.P.; Novick, O.; Umansky, R.; Priel, B.; Osher, Y.; Blaine, D.; Belmaker, R.H. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat. Genet. 1996, 12, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Schinka, J.A.; Letsch, E.A.; Crawford, F.C. DRD4 and novelty seeking: Results of meta-analyses. Am. J. Med. Genet. 2002, 114, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.; Lesch, K.P.; Jatzke, S.; Paetzold, F.; Brocke, B. Further evidence for a modulation of Novelty Seeking by DRD4 exon III, 5-HTTLPR, and COMT val/met variants. Mol. Psychiatry 2003, 8, 371–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penke, L.; Denissen, J.J.; Miller, G.F. The evolutionary genetics of personality. Eur. J. Personal. 2007, 21, 549–587. [Google Scholar] [CrossRef]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768. [Google Scholar] [CrossRef]
- Costa, P.; McCrae, R.R. The Revised NEO Personality Inventory (NEO-PI-R); Sage Publications Inc.: Thousand Oaks, CA, USA, 2008; Volume 2, pp. 179–198. [Google Scholar]
- Shao, C.; Li, Y.; Jiang, K.; Zhang, D.; Xu, Y.; Lin, L.; Wang, Q.; Zhao, M.; Jin, L. Dopamine D4 receptor polymorphism modulates cue-elicited heroin craving. Chin. Psychopharmacol. 2006, 186, 185–190. [Google Scholar] [CrossRef]
- Ray, L.A.; Bryan, A.; Mackillop, J.; McGeary, J.; Hesterberg, K.; Hutchison, K.E. The dopamine D receptor (DRD4) gene exon III polymorphism, problematic alcohol use and novelty seeking: Direct and mediated genetic effects. Addict. Biol 2009, 14, 238–244. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Alshudaifat, K.M.; Anani, J.Y. Association of the DRD4 exon III and 5-HTTLPR VNTR polymorphisms with substance abuse in Jordanian Arab population. Gene 2020, 733, 144267. [Google Scholar] [CrossRef]
- López, L.S.; Sayed-Tabatabaei, F.A.; Croes, E.A. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: A meta-analysis. Biol. Psychiatry 2005, 57, 999–1003. [Google Scholar] [CrossRef]
- Gafarov, V.; Gromova, E.; Maximov, V.; Bakulin, I.; Gafarova, A. Association of Personal Anxiety with Dopamine Receptor D4 (DRD4), DAT Genes Polymorphism. Anxiety Disord.-New Achiev. 2020. [Google Scholar] [CrossRef]
- Bobadilla, L.; Vaske, J.; Asberg, K. Dopamine receptor (D4) polymorphism is related to comorbidity between marijuana abuse and depression. Addict. Behav. 2013, 38, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Gescher, D.M.; Kahl, K.G.; Hillemacher, T.; Frieling, H.; Kuhn, J.; Frodl, T. Epigenetics in personality disorders: Today’s insights. Front. Psychiatry 2018, 9, 579. [Google Scholar] [CrossRef]
- Carter, J.A.; Herbst, J.H.; Stoller, K.B.; King, V.; Kidorf, M.S.; Costa, P.T.; Brooner, R.K. Short-term stability of NEO-PI-R personality trait scores in opioid-dependent outpatients. Psychol Addict Behav. 2001, 15, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Brooner, R.K.; Schmidt, C.W.; Herbst, J.H. Personality trait charcteristics of opioid abusers with and without comorbid personality disorders. In Personality Disorders: And the Five-Factor Model of Personality; Costa, P.T., Widiger, T.A., Eds.; American Psychological Association: Washington, DC, USA, 2002. [Google Scholar]
- Piedmont, R.L.; Ciarrocchi, J.W. The utility of the revised NEO personality inventory in an outpatient, drug rehabilitation context. Psychol. Addict Behav. 1999, 13, 213–226. [Google Scholar] [CrossRef]
- Kornør, H.; Nordvik, H. Five-factor model personality traits in opioid dependence. BMC Psychiatry 2007, 7, 37. [Google Scholar] [CrossRef]
- Strobel, A.; Wehr, A.; Michel, A. Association between the dopamine D4 receptor (DRD4) exon III polymorphism and measures of Novelty Seeking in a German population. Mol. Psychiatryn 1999, 4, 378–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekelund, J.; Suhonen, J.; Järvelin, M.R.; Peltonen, L.; Lichtermann, D. No association of the –521 C/T polymorphism in the promoter of DRD4 with novelty seeking. Mol. Psychiatry 2001, 6, 618–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strobel, A.; Lesch, K.P.; Hohenberger, K.; Jatzke, S.; Gutzeit, H.O.; Anacker, K.; Brocke, B. No association between dopamine D4 receptor gene exon III and –521C/T polymorphism and novelty seeking. Mol. Psychiatry 2002, 7, 537–538. [Google Scholar] [CrossRef][Green Version]
- Jönsson, E.G.; Ivo, R.; Gustavsson, J.P.; Geijer, T.; Forslund, K.; Mattila-Evenden, M.; Rylander, G.; Cichon, S.; Propping, P.; Bergman, H.; et al. No association between dopamine D4 receptor variants and Novelty Seeking. Mol. Psychiatry 2002, 7, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.; Spinath, F.M.; Angleitner, A.; Riemann, R.; Lesch, K.P. Lack of Association between Polymorphisms of the Dopamine D4 Receptor Gene and Personality. Neuropsychobiology 2003, 47, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chew, S.H.; Ebstein, R.P. The role of D4 receptor gene exon III polymorphisms in shaping human altruism and prosocial behavior. Front. Hum. Neurosci. 2013, 7, 195. [Google Scholar] [CrossRef] [PubMed]
| Type of Substance/Addiction | All Patients Diagnosed with PUD MDD (n = 95) | |
|---|---|---|
| n | % | |
| Behavioral addiction | 43 | 45.3 |
| Designer drugs | 21 | 22.1 |
| F10.2—alcohol | 56 | 58.9 |
| F11.2—opiates | 21 | 22.1 |
| F12.2—cannabinols | 69 | 72.6 |
| F13.2—sedatives and hypnotics | 14 | 14.7 |
| F14.2—cocaine | 8 | 8.4 |
| F15.2—stimulants | 78 | 82.1 |
| F16.2—hallucinogenic | 13 | 13.7 |
| F19.2—mixed addictions | 60 | 63.2 |
| Hardy–Weinberg Equilibrium Calculator Including Analysis for Ascertainment Bias | Observed (Expected) | Test χ2 | |||
|---|---|---|---|---|---|
| χ2 | p | ||||
| DRD4 Ex3 PUD MDD | s/s | 63 (64) | l allele freq = 0.18 s allele freq = 0.82 | 0.529 | >0.05 |
| s/l | 30 (27.9) | ||||
| l/l | 2 (3) | ||||
| DRD4 Ex3 PUD | s/s | 127 (128.2) | l allele freq = 0.21 s allele freq = 0.79 | 0.245 | >0.05 |
| s/l | 71 (68.6) | ||||
| l/l | 8 (9.2) | ||||
| DRD4 Ex3 control subjects | s/s | 177 (169.7) | l allele freq = 0.25 s allele freq = 0.75 | 5.075 | <0.05 |
| s/l | 98 (112.6) | ||||
| l/l | 26 (18.7) | ||||
| Group | DRD4 Ex3 | ||||
|---|---|---|---|---|---|
| Genotypes | Alleles | ||||
| s/s n (%) | s/l n (%) | l/l n (%) | s n (%) | l n (%) | |
| A: PUD MDD n = 95 | 63 (66) | 30 (32) | 2 (2) | 156 (82) | 34 (18) |
| B: PUD n = 206 | 127 (62) | 71 (34) | 8 (4) | 325 (79) | 87 (21) |
| C: Control n = 301 | 177 (59) | 98 (33) | 26 (9) | 452 (75) | 150 (25) |
| χ2 (p value) | A/B: 1.01 (0.605) A/C: 5.05 (0.079) B/C: 4.42 (0.110) | A/B: 0.84 (0.359) A/C: 3.99 (0.046 *) B/C: 1.970 (0.160) | |||
| STAI/NEO Five Factor Inventory/ | A: PUD MDD (n = 95) | B: PUD (n = 206) | C: Control (n = 301) | A/C: Z (p-Value) | B/C: Z (p-Value) | A/B: Z (p-Value) |
|---|---|---|---|---|---|---|
| STAI trait/scale | 7.62 ± 2.25 | 6.87 ± 2.25 | 5.16 ± 2.17 | 7.062 (0.0000 *) | 7.768 (0.0000 *) | 3.665 (0.0002 *) |
| STAI state/scale | 6.65 ± 2.21 | 5.54 ± 2.43 | 4.68 ± 2.14 | 8.207 (0.0000 *) | 4.162 (0.0000 *) | 2.754 (0.0059 *) |
| Neuroticism/scale | 7.34 ± 2.00 | 6.45 ± 2.20 | 4.67 ± 2.01 | 9.264 (0.0000 *) | 8.683 (0.0000 *) | 3.094 (0.0020 *) |
| Extraversion/scale | 5.44 ± 2.36 | 5.90 ± 2.01 | 6.37 ± 1.97 | −3.640 (0.0003 *) | −2.412 (0.0147 *) | −1.837 (0.0602) |
| Openness/scale | 5.46 ± 2.01 | 4.80 ± 2.00 | 4.53 ± 1.61 | 4.136 (0.0000 *) | 1.401 (0.1545) | 2.557 (0.0106 *) |
| Agreeability/scale | 4.05 ± 1.99 | 4.41 ± 1.90 | 5.60 ± 2.09 | −6.286 (0.0000 *) | −6.165 (0.0000 *) | −1.959 (0.0501 *) |
| Conscientiousness/scale | 5.01 ± 2.14 | 5.85 ± 2.29 | 6.07 ± 2.15 | −4.102 (0.0000 *) | −1.014 (0.3059) | −3.006 (0.0027 *) |
| STAI/NEO Five Factor Inventory | DRD4 Ex3 | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PUD MDD (n = 95) | Control (n = 301) | s/s (n = 240) | s/l (n = 127) | l/l (n = 28) | Factor | F (p-Value) | ɳ2 | Power (Alfa = 0.05) | |
| STAI trait/scale | 7.62 ± 2.25 | 5.16 ± 2.17 | 5.72 ± 2.54 | 5.87 ± 2.21 | 5.32 ± 2.34 | intercept | F1,389 = 499.54 (p < 0.0001) * | 0.563 | 1.000 |
| PUD MDD/control | F1,389 = 17.06 (p < 0.000) * | 0.042 | 0.985 | ||||||
| DRD4 Ex3 | F2,389 = 0.02 (p = 0.976) | 0.0001 | 0.054 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 0.64 (p = 0.527) | 0.003 | 0.157 | ||||||
| STAI state/scale | 6.65 ± 2.21 | 4.68 ± 2.14 | 5.10 ± 2,33 | 5.39 ± 2.24 | 4.61 ± 2.39 | intercept | F1,389 = 386.18 (p < 0.0001) * | 0.498 | 1.000 |
| PUD MDD/control | F1,389 = 8.40 (p = 0.004) * | 0.021 | 0.824 | ||||||
| DRD4 Ex3 | F2,389 = 0.88 (p = 0.415) | 0.004 | 0.201 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 0.32 (p = 0.729) | 0.002 | 0.100 | ||||||
| Neuroticism/scale | 7.34 ± 2.00 | 4.67 ± 2.01 | 5.31 ± 2.27 | 5.45 ± 2.32 | 4.50 ± 2.51 | intercept | F1,389 = 515.96 (p < 0.0001) * | 0.571 | 1.000 |
| PUD MDD/control | F1,389 = 26.51 (p < 0.0001) * | 0.064 | 0.999 | ||||||
| DRD4 Ex3 | F2,389 = 0.73 (p = 0.480) | 0.004 | 0.174 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 0.04 (p = 0.954) | 0.0002 | 0.057 | ||||||
| Extraversion/scale | 5.44 ± 2.36 | 6.37 ± 1.97 | 6.20 ± 2.04 | 5.90 ± 2.19 | 6.89 ± 2.11 | intercept | F1,389 = 588.12 (p < 0.0001) * | 0.602 | 1.000 |
| PUD MDD/control | F1,389 = 0.003 (p = 0.953) | 0.00001 | 0.050 | ||||||
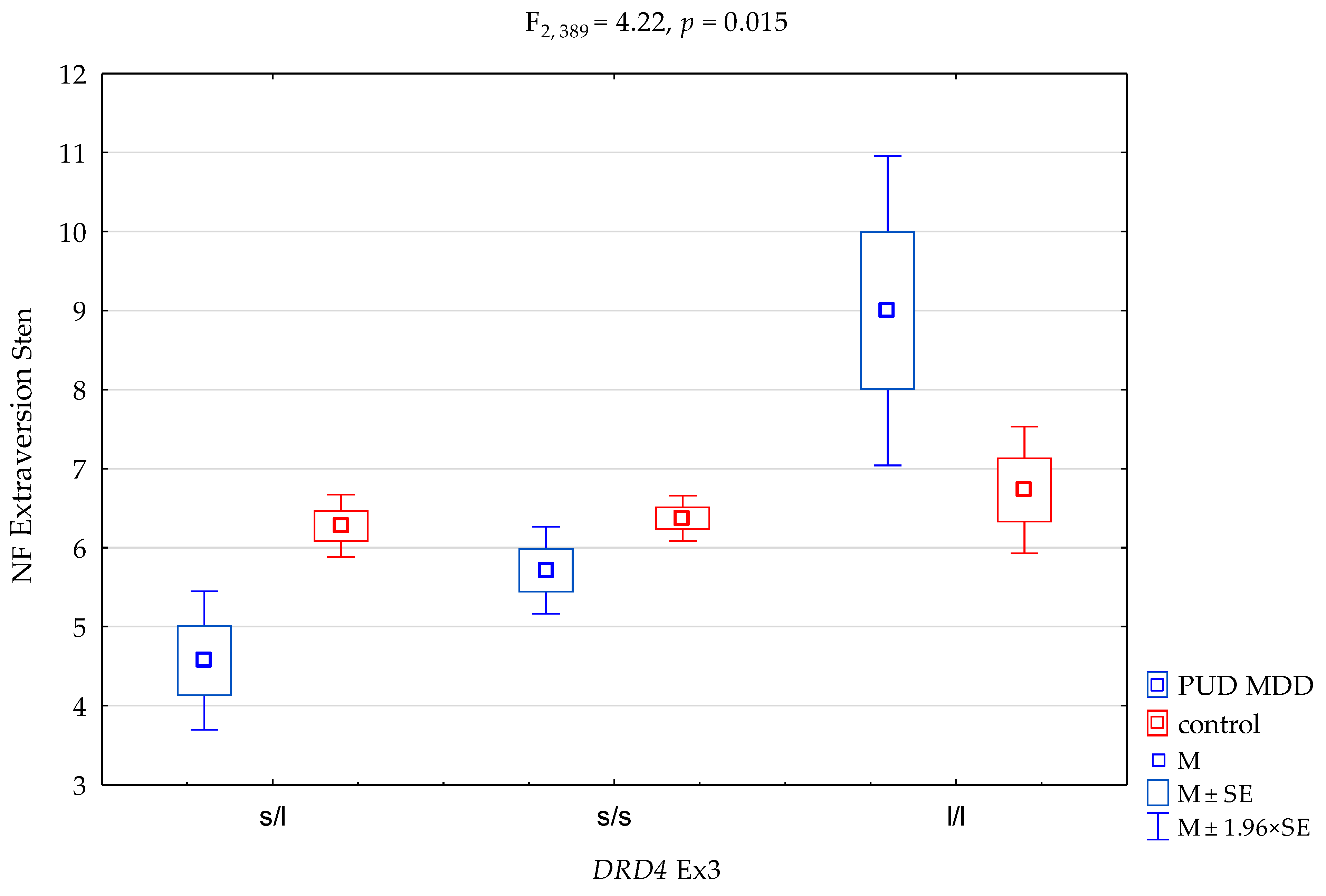
| DRD4 Ex3 | F2,389 = 6.23 (p = 0.002) * | 0.031 | 0.893 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 4.22 (p = 0.015) * | 0.021 | 0.738 | ||||||
| Openness/scale | 5.46 ± 2.01 | 4.53 ± 1.61 | 4.64 ± 1.77 | 4.96 ± 1.74 | 4.75 ± 1.67 | intercept | F1,389 = 568.45 (p < 0.0001) * | 0.594 | 1.000 |
| PUD MDD/control | F1,389 = 9.83 (p = 0.002) * | 0.024 | 0.879 | ||||||
| DRD4 Ex3 | F2,389 = 1.66 (p = 0.190) | 0.008 | 0.350 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 0.88 (p = 0.416) | 0.004 | 0.201 | ||||||
| Agreeability/scale | 4.05 ± 1.99 | 5.60 ± 2.09 | 5.12 ± 2.21 | 5.41 ± 2.12 | 5.36 ± 1.97 | intercept | F1,389 = 313.18 (p < 0.0001) * | 0.447 | 1.000 |
| PUD MDD/control | F1,389 = 9.69 (p = 0.002) | 0.024 | 0.874 | ||||||
| DRD4 Ex3 | F2,389 = 2.37 (p = 0.554) | 0.003 | 0.142 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 0.30 (p = 0.071) | 0.0004 | 0.061 | ||||||
| Conscientiousness/scale | 5.01 ± 2.14 | 6.07 ± 2.15 | 5.80 ± 2.21 | 5.75 ± 2.18 | 6.39 ± 2.10 | intercept | F1,389 = 510.13 (p < 0.0001) * | 0.568 | 1.000 |
| PUD MDD/control | F1,389 = 0.18 (p = 0.668) | 0.0005 | 0.071 | ||||||
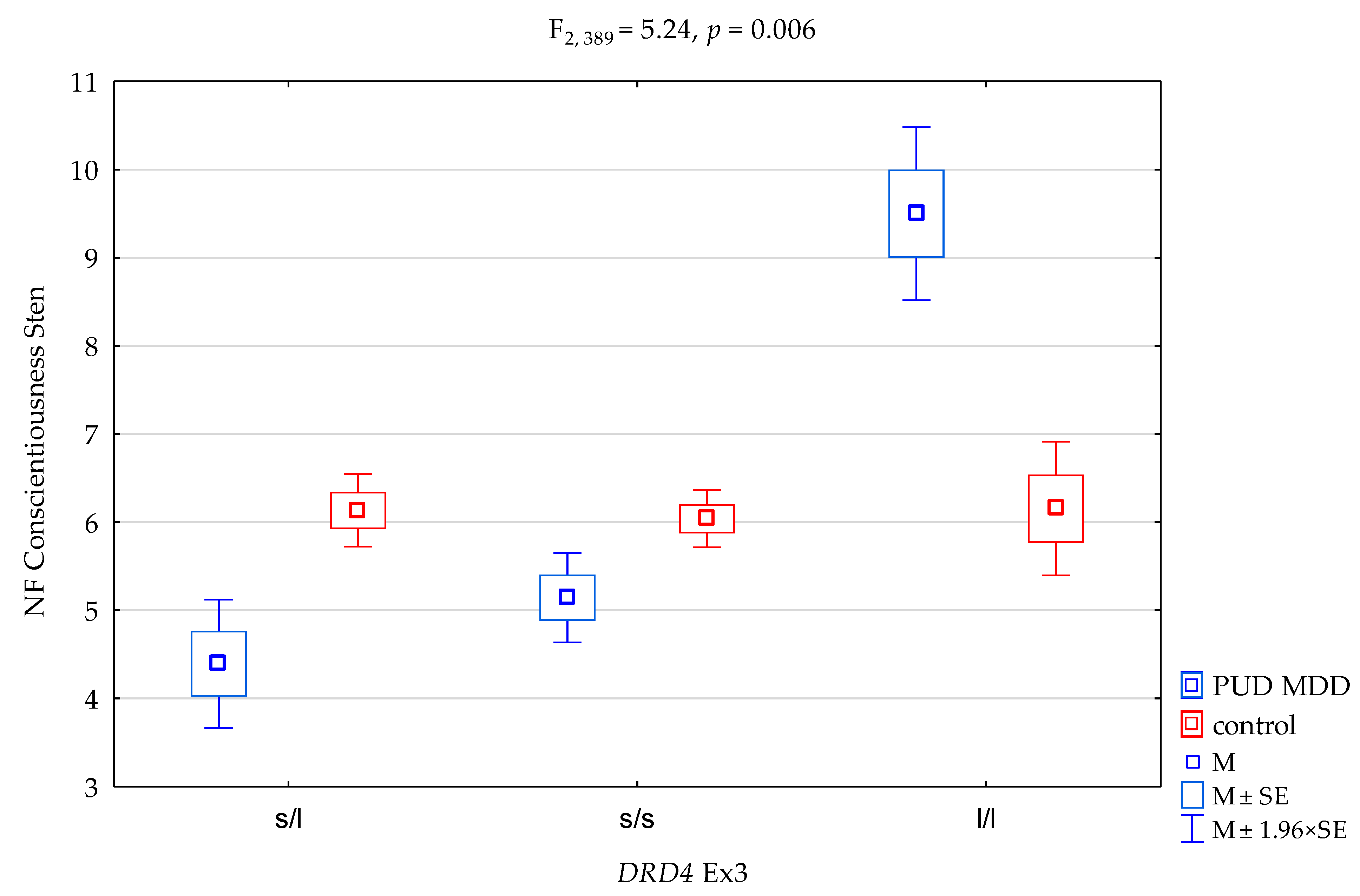
| DRD4 Ex3 | F2,389 = 5.09 (p = 0.007) * | 0.026 | 0.820 | ||||||
| PUD MDD/control × DRD4 Ex3 | F2,389 = 5.24 (p = 0.006) * | 0.026 | 0.831 | ||||||
| STAI/NEO Five Factor Inventory | DRD4 Ex3 | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PUD (n = 206) | Control (n = 301) | s/s (n = 304) | s/l (n = 169) | l/l (n = 34) | Factor | F (p-Value) | ɳ2 | Power (Alfa = 0.5) | |
| STAI trait/scale | 6.87 ± 2.25 | 5.16 ± 2.17 | 5.83 ± 2.49 | 6.00 ± 2.16 | 5.29 ± 2.14 | intercept | F1,501 = 1255.43 (p < 0.0001) * | 0.716 | 1.000 |
| PUD/control | F1,501 = 15.92 (p < 0.0001) * | 0.031 | 0.978 | ||||||
| DRD4 Ex3 | F2,501 = 0.98 (p = 0.375) | 0.004 | 0.221 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 1.52 (p = 0.219) | 0.006 | 0.324 | ||||||
| STAI state/scale | 5.54 ± 2.43 | 4.68 ± 2.14 | 4.99 ± 2.30 | 5.21 ± 2.27 | 4.50 ± 2.40 | intercept | F1,501 = 849.36 (p < 0.0001) * | 0.630 | 1.000 |
| PUD/control | F1,501 = 2.14 (p = 0.144) | 0.004 | 0.309 | ||||||
| DRD4 Ex3 | F2,501 = 1.41 (p = 0.245) | 0.006 | 0.303 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 1.21 (p = 0.299) | 0.005 | 0.264 | ||||||
| Neuroticism/scale | 6.45 ± 2.20 | 4.67 ± 2.01 | 5.46 ± 2.29 | 5.39 ± 2.16 | 4.67 ± 2.43 | intercept | F1,501 = 1196.67 (p < 0.0001) * | 0.705 | 1.000 |
| PUD/control | F1,501 = 27.48 (p < 0.0001) * | 0.052 | 0.999 | ||||||
| DRD4 Ex3 | F2,501 = 0.82 (p = 0.442) | 0.003 | 0.190 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 1.31 (p = 0.270) | 0.005 | 0.284 | ||||||
| Extraversion/scale | 5.90 ± 2.01 | 6.37 ± 1.97 | 6.15 ± 1.98 | 6.11 ± 2.03 | 6.76 ± 2.05 | intercept | F1,501 = 1803.32 (p < 0.0001) * | 0.782 | 1.000 |
| PUD/control | F1,501 = 0.73 (p = 0.391) | 0.001 | 0.137 | ||||||
| DRD4 Ex3 | F2,501 = 1.45 (p = 0.234) | 0.005 | 0.311 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 0.34 (p = 0.713) | 0.001 | 0.104 | ||||||
| Openness/scale | 4.80 ± 2.00 | 4.53 ± 1.61 | 4.51 ± 1.74 | 4.84 ± 1.82 | 4.85 ± 1.89 | intercept | F1,501 = 1335.71 (p < 0.0001) * | 0.727 | 1.000 |
| PUD/control | F1,501 = 3.99 (p = 0.046) | 0.008 | 0.514 | ||||||
| DRD4 Ex3 | F2,501 = 2.61 (p = 0.0740) | 0.010 | 0.520 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 0.99 (p = 0.372) | 0.003 | 0.222 | ||||||
| Agreeability/scale | 4.41 ± 1.90 | 5.60 ± 2.09 | 5.09 ± 2.12 | 5.22 ± 2.00 | 4.82 ± 2.25 | intercept | F1,501 = 993.33 (p < 0.0001) * | 0.665 | 1.000 |
| PUD/control | F1,501 = 33.04 (p < 0.0001) * | 0.061 | 0.999 | ||||||
| DRD4 Ex3 | F2,501 = 2.95 (p = 0.053) | 0.012 | 0.574 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 2.30 (p = 0.101) | 0.009 | 0.468 | ||||||
| Conscientiousness/scale | 5.85 ± 2.29 | 6.07 ± 2.15 | 5.93 ± 2.27 | 6.01 ± 2.14 | 6.26 ± 1.94 | intercept | F1,501 = 1357.97 (p < 0.0001) * | 0.730 | 1.000 |
| PUD/control | F1,501 = 0.002 (p = 0.961) | 0.00001 | 0.050 | ||||||
| DRD4 Ex3 | F2,501 = 0.53 (p = 0.587) | 0.002 | 0.138 | ||||||
| PUD/control x DRD4 Ex3 | F2,501 = 0.31 (p = 0.729) | 0.001 | 0.100 | ||||||
| DRD4Ex3 and NEO FFI Extraversion Scale | ||||||
| {1} M = 4.57 | {2} M = 5.71 | {3} M = 9.00 | {4} M = 6.28 | {5} M = 6.37 | {6} M = 6.73 | |
| PUD MDD DRD4 Ex3 s/l {1} | 0.0145 * | 0.0033 * | 0.0001 * | <0.0000 * | 0.0001 * | |
| PUD MDD DRD4 Ex3 s/s {2} | 0.0261 * | 0.0905 | 0.0290 * | 0.0339 * | ||
| PUD MDD DRD4 Ex3 l/l {3} | 0.0633 | 0.0720 | 0.1319 | |||
| control DRD4 Ex3 s/l {4} | 0.7060 | 0.3143 | ||||
| control DRD4 Ex3 s/s {5} | 0.4060 | |||||
| control DRD4 Ex3 l/l {6} | ||||||
| DRD4Ex3 and NEO FFI Conscientiousness Scale | ||||||
| {1} M = 4.39 | {2} M = 5.14 | {3} M = 9.50 | {4} M = 6.13 | {5} M = 6.04 | {6} M = 6.15 | |
| PUD MDD DRD4 Ex3 s/l {1} | 0.1210 | 0.0011 * | 0.0002 * | 0.0002 * | 0.0025 * | |
| PUD MDD DRD4 Ex3 s/s {2} | 0.0045 * | 0.0041 * | 0.0042 * | 0.0419 * | ||
| PUD MDD DRD4 Ex3 l/l {3} | 0.0271 * | 0.0226 * | 0.0325 * | |||
| control DRD4 Ex3 s/l {4} | 0.7281 | 0.9640 | ||||
| control DRD4 Ex3 s/s {5} | 0.7980 | |||||
| control DRD4 Ex3 l/l {6} | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielowiec, K.; Chmielowiec, J.; Masiak, J.; Czekaj, M.; Krawczyk, P.; Soroka, E.; Śmiarowska, M.; Musiał, W.; Pawłowski, T.; Grzywacz, A. DRD4 Exon 3 Gene Polymorphisms in Patients Diagnosed with Polysubstance Use Disorder and Co-Occurrence of a Depressive Episode. Genes 2021, 12, 1834. https://doi.org/10.3390/genes12111834
Chmielowiec K, Chmielowiec J, Masiak J, Czekaj M, Krawczyk P, Soroka E, Śmiarowska M, Musiał W, Pawłowski T, Grzywacz A. DRD4 Exon 3 Gene Polymorphisms in Patients Diagnosed with Polysubstance Use Disorder and Co-Occurrence of a Depressive Episode. Genes. 2021; 12(11):1834. https://doi.org/10.3390/genes12111834
Chicago/Turabian StyleChmielowiec, Krzysztof, Jolanta Chmielowiec, Jolanta Masiak, Małgorzata Czekaj, Piotr Krawczyk, Ewelina Soroka, Małgorzata Śmiarowska, Wojciech Musiał, Tomasz Pawłowski, and Anna Grzywacz. 2021. "DRD4 Exon 3 Gene Polymorphisms in Patients Diagnosed with Polysubstance Use Disorder and Co-Occurrence of a Depressive Episode" Genes 12, no. 11: 1834. https://doi.org/10.3390/genes12111834
APA StyleChmielowiec, K., Chmielowiec, J., Masiak, J., Czekaj, M., Krawczyk, P., Soroka, E., Śmiarowska, M., Musiał, W., Pawłowski, T., & Grzywacz, A. (2021). DRD4 Exon 3 Gene Polymorphisms in Patients Diagnosed with Polysubstance Use Disorder and Co-Occurrence of a Depressive Episode. Genes, 12(11), 1834. https://doi.org/10.3390/genes12111834






