Preliminary Evidence of Endotoxin Tolerance in Dairy Cows during the Transition Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Bovine PBMCs Isolation and In Vitro Proliferation
2.3. In Vitro LPS Stimulation of Bovine PBMCs and Gene Expression Analysis
2.4. Statistical Analysis
3. Results
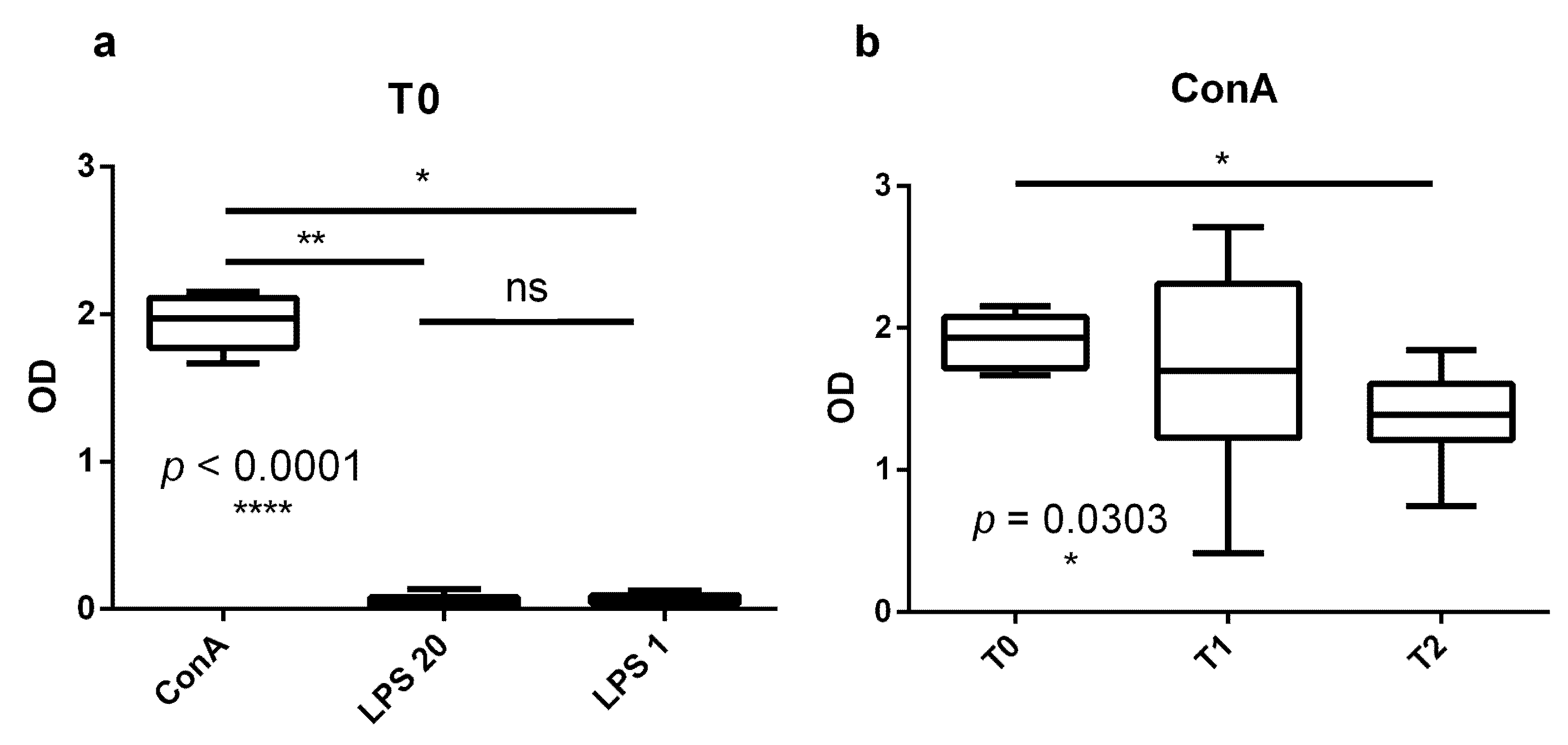
3.1. Proliferation Response of Bovine PBMCs Is Higher in the Dry Period Than at the End of Transition Period
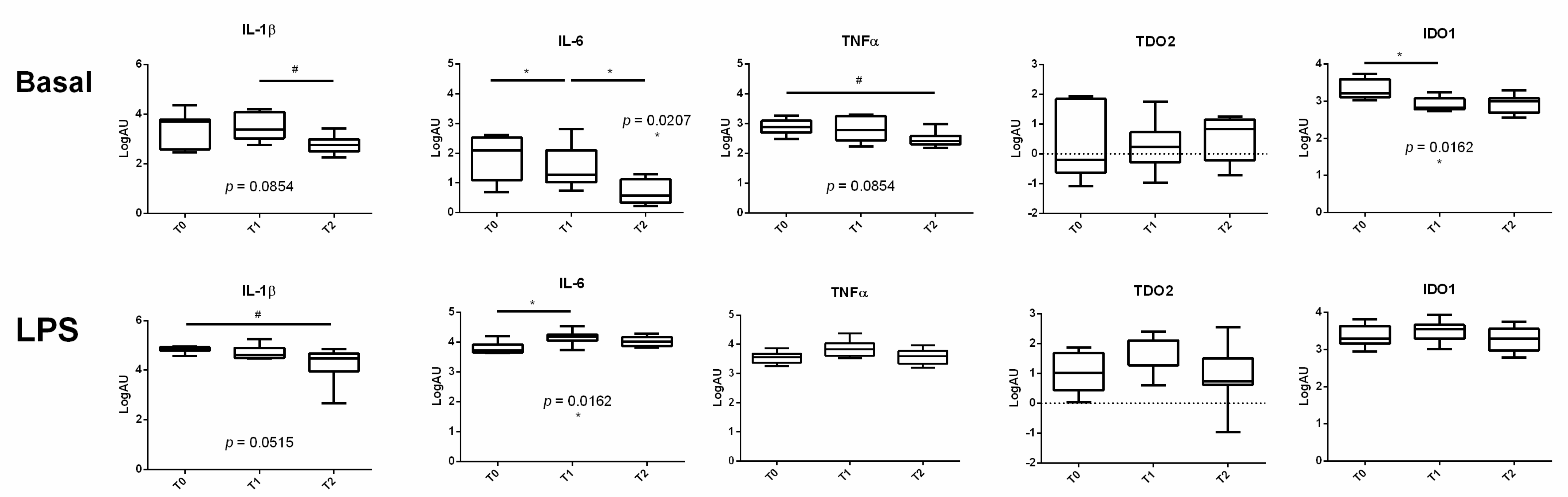
3.2. The Expression of Proinflammatory Cytokines Varied during the Transition Period
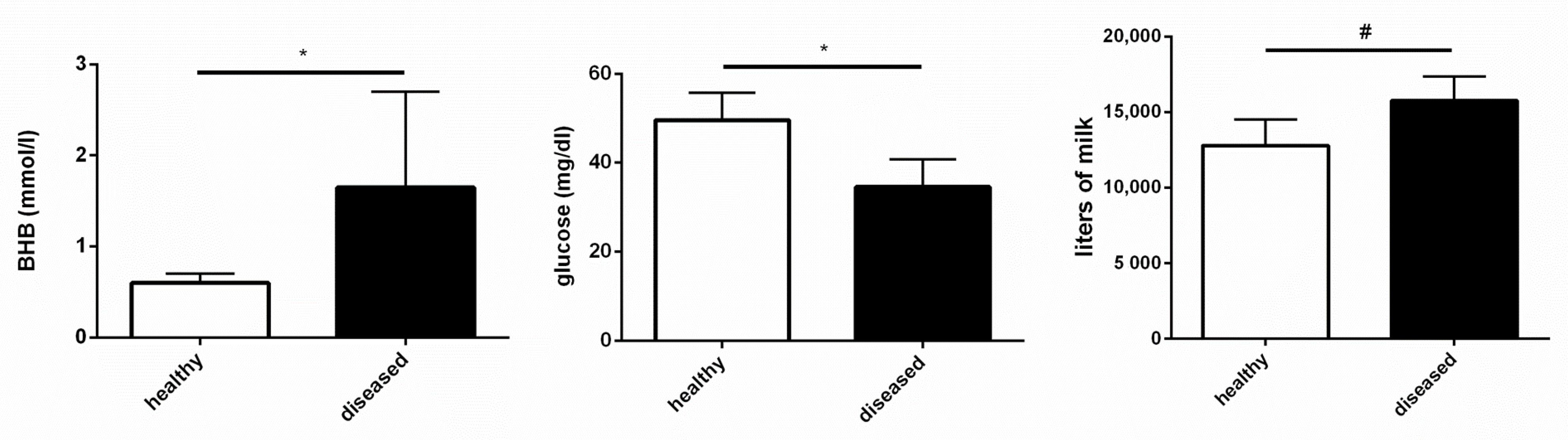
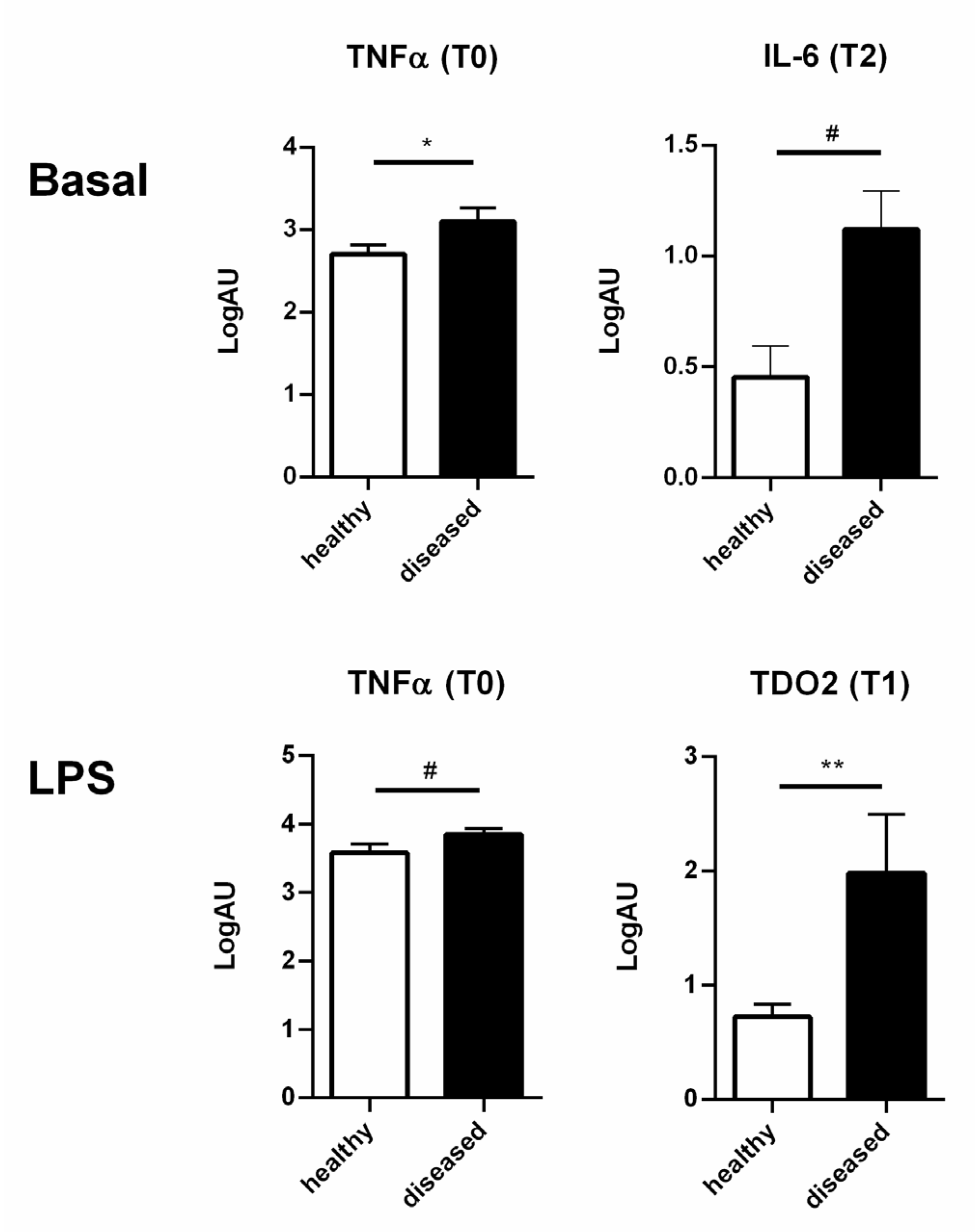
3.3. Healthy and Diseased Animals Developed Diverging Responses to High Doses of LPS
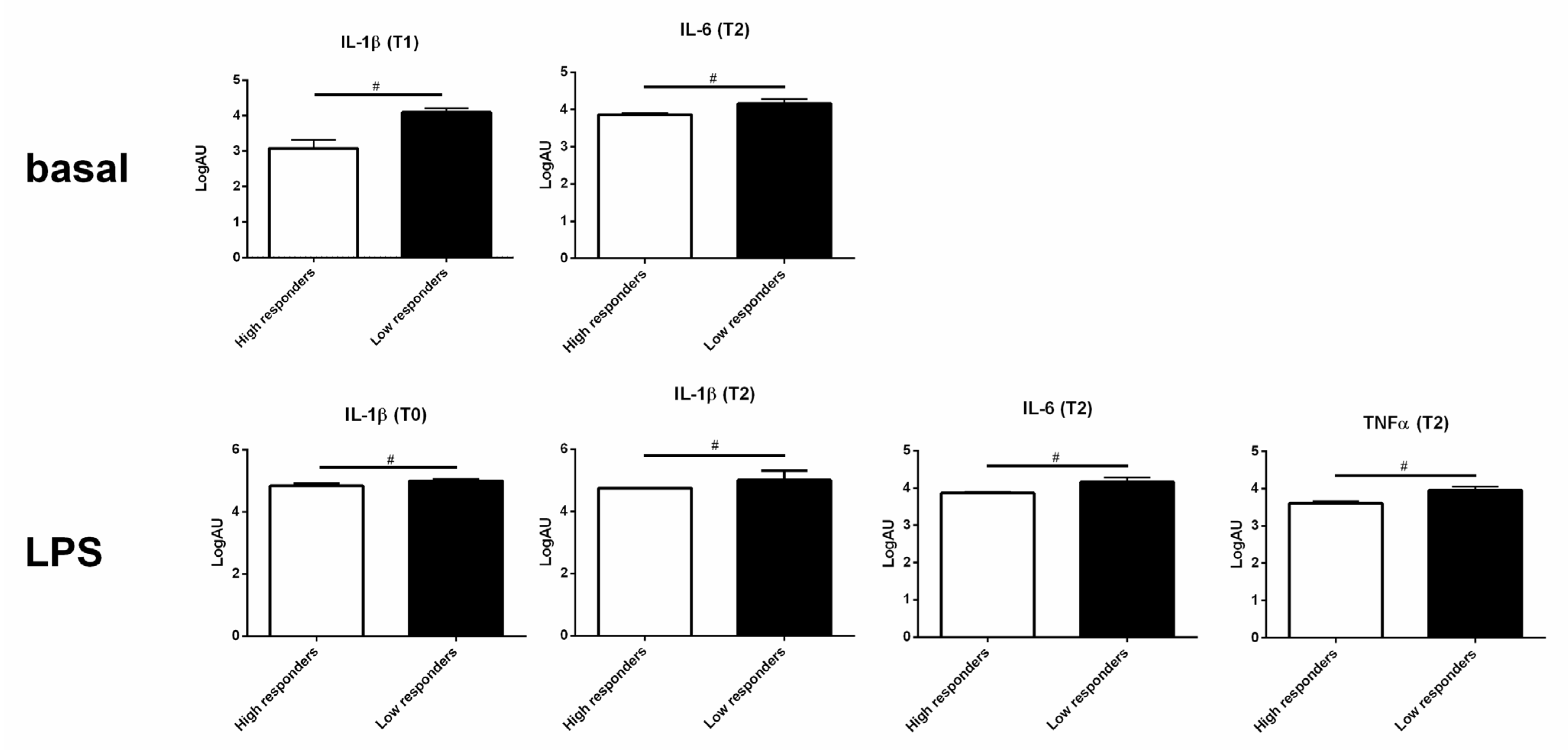
3.4. High and Low Responders Differently Expressed Cytokine Genes
3.5. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muul, L.M.; Silvin, C.; James, S.P.; Candotti, F. Measurement of Proliferative Responses of Cultured Lymphocytes. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Abraham, C.; Tal, Y.; Gershon, H. Reducèd in vitro response to concanavalin A and lipopolysaccharide in senescent mice: A function of reduced number of responding cells. Eur. J. Immunol. 1977, 7, 301–304. [Google Scholar] [CrossRef]
- Amadori, M.; Soares-Filipe, J.F.; Riva, F.; Vitali, A.; Ruggeri, J.; Lacetera, N. Characterization of the blastogenic response to LPS of bovine peripheral blood mononuclear cells. PLoS ONE 2018, 13, e204827. [Google Scholar] [CrossRef] [Green Version]
- Catalani, E.; Amadori, M.; Vitali, A.; Lacetera, N. Short communication: Lymphoproliferative response to lipopolysaccharide and incidence of infections in periparturient dairy cows. J. Dairy Sci. 2013, 96, 7077–7081. [Google Scholar] [CrossRef] [Green Version]
- Trevisi, E.; Minuti, A. Assessment of the innate immune response in the periparturient cow. Res. Vet. Sci. 2018, 116, 47–54. [Google Scholar] [CrossRef]
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018, 116, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.J.; Woodman, S.; Pennelegion, C.; Patterson, R.; Stuart, E.; Hosker, N.; Siviter, P.; Douglas, C.; Whitehouse, J.; Wilkinson, W.; et al. Differential macrophage function in Brown Swiss and Holstein Friesian cattle. Vet. Immunol. Immunopathol. 2016, 181, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, P.B.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Funakoshi, H.; Nakamura, T.; Schwarcz, R.; Muchowski, P.J. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 2345–2354. [Google Scholar] [CrossRef] [Green Version]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 117864691769193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Ospina, P.A.; Oetzel, G.R. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 2011, 94, 6011–6020. [Google Scholar] [CrossRef] [Green Version]
- Riva, F.; Rahman, M.M.; Turin, L.; Ceciliani, F.; Russo, S.; Tribbioli, G.; Lecchi, C. TIR8 receptor expression in bovine tissues. Vet. Immunol. Immunopathol. 2010, 136, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bond, J.A.; Newsholme, E.A. Fatty acid inhibition of lipopolysaccharide-stimulated B lymphocyte proliferation. Biochem. Soc. Trans. 1990, 18, 904–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menge, C.; Dean-Nystrom, E.A. Dexamethasone Depletes γδT Cells and Alters the Activation State and Responsiveness of Bovine Peripheral Blood Lymphocyte Subpopulations. J. Dairy Sci. 2008, 91, 2284–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebisch, G.; Neumaier-Wagner, P.M.; Huch, R.; von Mandach, U. Maternal serum interleukin-1β, -6 and -8 levels and potential determinants in pregnancy and peripartum. J. Perinat. Med. 2004, 32, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Trevisi, E.; Jahan, N.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-inflammatory cytokine profile in dairy cows: Consequences for new lactation. Ital. J. Anim. Sci. 2015, 14, 285–292. [Google Scholar] [CrossRef]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Amadori, M.; Bionaz, M.; Trevisi, E. The role of altered immune function during the dry period in promoting the development of subclinical ketosis in early lactation. J. Dairy Sci. 2019, 102, 9241–9258. [Google Scholar] [CrossRef]
- Ogata, H.; Su, I.; Miyake, K.; Nagai, Y.; Akashi, S.; Mecklenbrauker, I.; Rajewsky, K.; Kimoto, M.; Tarakhovsky, A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 2000, 192, 23–29. [Google Scholar] [CrossRef] [Green Version]
| Gene | Abbreviation | Sequence |
|---|---|---|
| Interleukin-1 beta | IL-1β | F: CTG TTA TTT GAG GCT GAT GAC C R: TTG TTG TAG AAC TGG TGA GAA ATC |
| Tumor Necrosis Factor alpha | TNF-α | F: TCT TCT CAA GCC TCA AGT AAC AAG T R: CCA TGA GGG CAT TGG CAT AC |
| Interleukin-6 | IL-6 | F: CAC TCC AGA GAA AAC CGA AGC R: GAA GCA TCC CGT CCT TTT CCT C |
| Toll-like receptor 4 | TLR4 | F: CTGCGGCTCTGATCCCAG R: TTAGGAACAACCTGTACGCAAGG |
| Indoleamine 2,3-dioxygenase | IDO1 | F: GGG TCA AGG CGA TGG AGA C R: ACA GCG ATA TTG CTT GGC AA |
| Tryptophan 2,3-dioxygenase | TDO2 | F: TTG AGG CAT GGC TGG AAA G R: AGT TAA ATC CAT GCG GCT CTA AAC |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | F: GGCGTGAACCACGAGAAGTATAA R: CCCTCCACGATGCCAAAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipe, J.; Inglesi, A.; Amadori, M.; Guarneri, F.; Menchetti, L.; Curone, G.; Brecchia, G.; Vigo, D.; Riva, F. Preliminary Evidence of Endotoxin Tolerance in Dairy Cows during the Transition Period. Genes 2021, 12, 1801. https://doi.org/10.3390/genes12111801
Filipe J, Inglesi A, Amadori M, Guarneri F, Menchetti L, Curone G, Brecchia G, Vigo D, Riva F. Preliminary Evidence of Endotoxin Tolerance in Dairy Cows during the Transition Period. Genes. 2021; 12(11):1801. https://doi.org/10.3390/genes12111801
Chicago/Turabian StyleFilipe, Joel, Alessia Inglesi, Massimo Amadori, Flavia Guarneri, Laura Menchetti, Giulio Curone, Gabriele Brecchia, Daniele Vigo, and Federica Riva. 2021. "Preliminary Evidence of Endotoxin Tolerance in Dairy Cows during the Transition Period" Genes 12, no. 11: 1801. https://doi.org/10.3390/genes12111801
APA StyleFilipe, J., Inglesi, A., Amadori, M., Guarneri, F., Menchetti, L., Curone, G., Brecchia, G., Vigo, D., & Riva, F. (2021). Preliminary Evidence of Endotoxin Tolerance in Dairy Cows during the Transition Period. Genes, 12(11), 1801. https://doi.org/10.3390/genes12111801










