Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification and Sequence Analysis of HSP90 Genes in C. farreri
2.2. Phylogenetic Analysis
2.3. Spatiotemporal Expression of HSP90 Genes in C. farreri
2.4. Expression Analysis of CfHSP90s under Heat Stress
3. Results
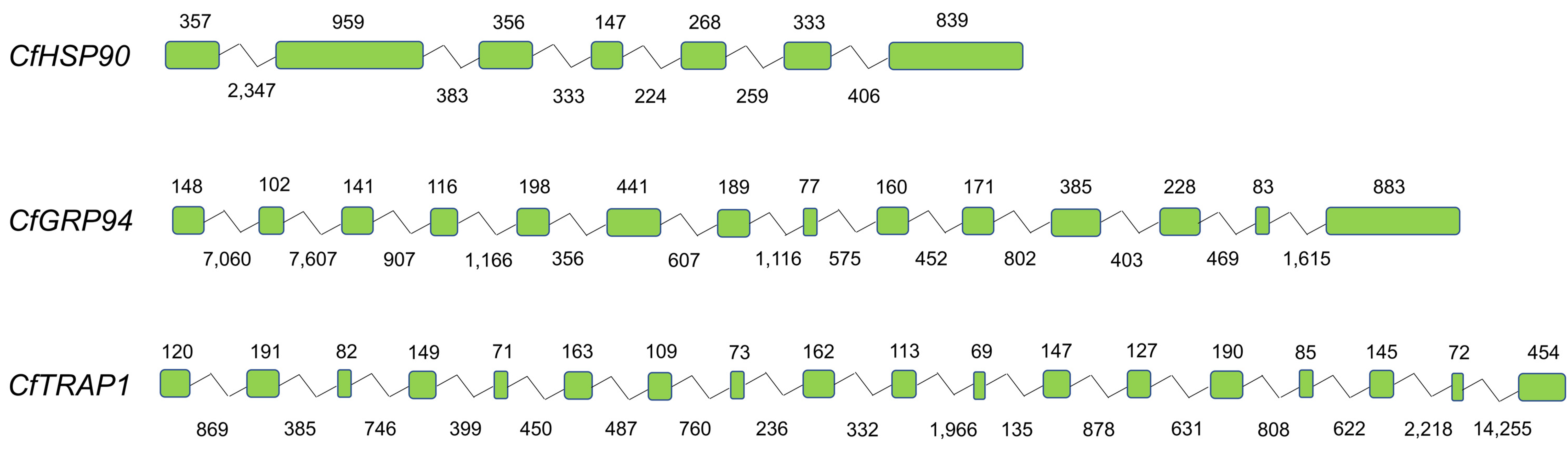
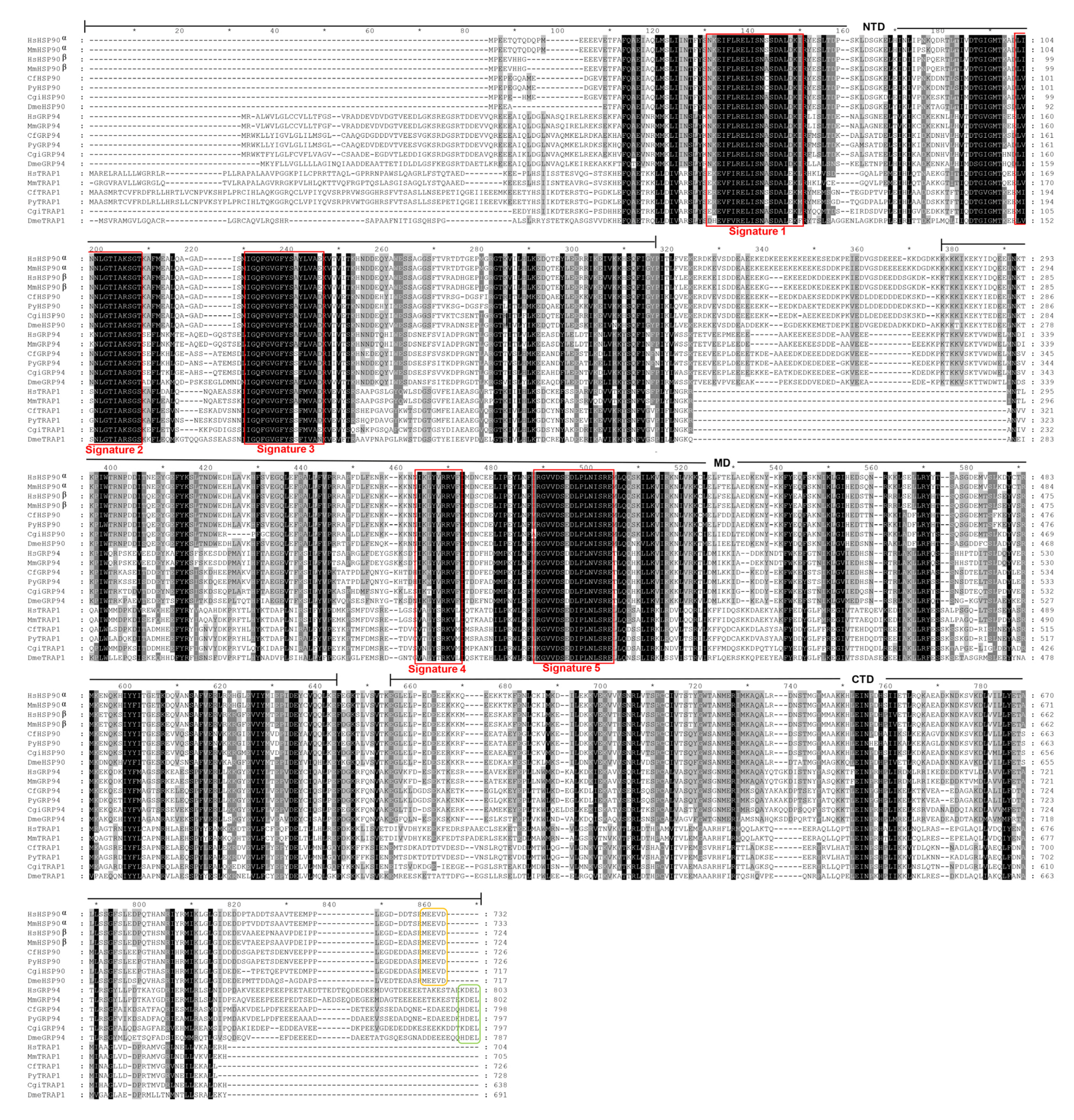
3.1. Sequence Identification and Analysis
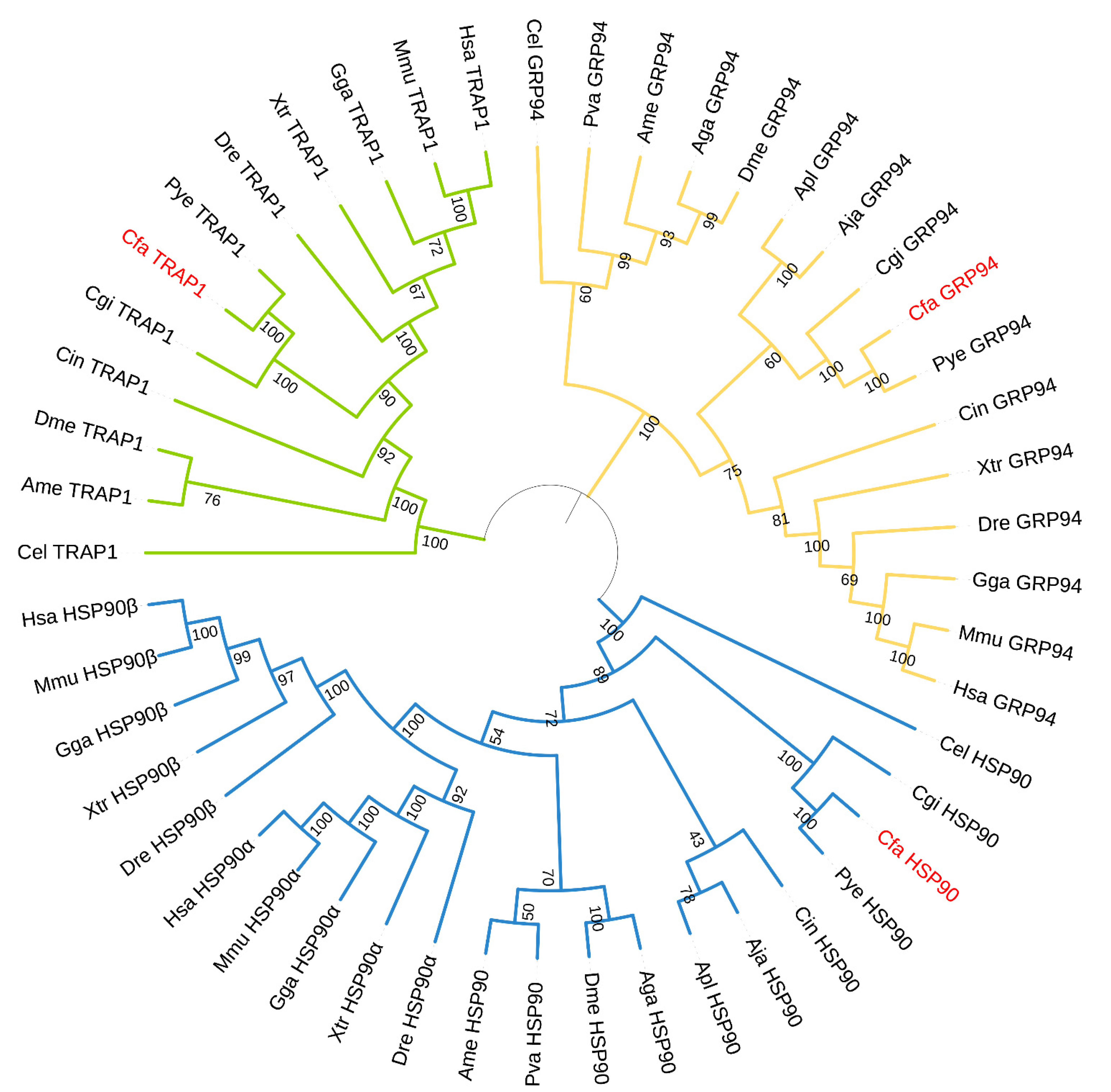
3.2. Phylogenetic Analysis
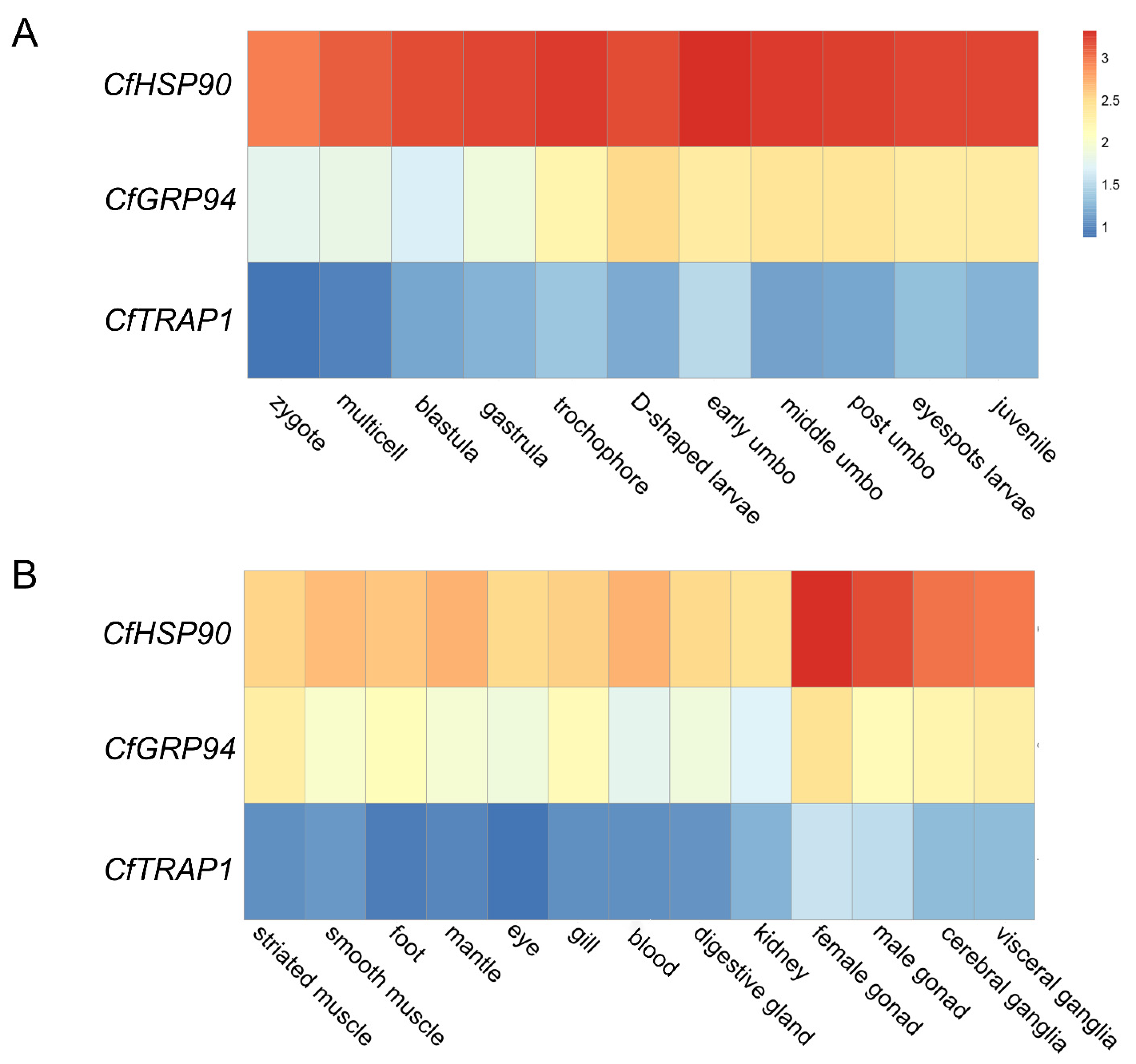
3.3. Spatiotemporal Expression of CfHSP90s
3.4. Expression Profiles of CfHSP90s under Heat Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Park, K.; Lee, J.S.; Kang, J.C.; Kim, J.W.; Kwak, I.S. Cascading effects from survival to physiological activities, and gene expression of heat shock protein 90 on the abalone Haliotis discus hannai responding to continuous thermal stress. Fish Shellfish. Immunol. 2015, 42, 233–240. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Qiu, L.; Zhang, H. Bivalve immunity. In Invertebrate Immunity; Springer: Boston, MA, USA, 2010; pp. 44–65. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming; IPCC: Geneva, Switzerland, 2018; Volume 1, pp. 1–9. [Google Scholar]
- Liu, S.; Jiang, X.; Hu, X.; Gong, J.; Hwang, H.; Mai, K. Effects of temperature on non-specific immune parameters in two scallop species: Argopecten irradians (Lamarck 1819) and Chlamys farreri (Jones & Preston 1904). Aquac. Res. 2004, 35, 678–682. [Google Scholar]
- Xiao, J.; Ford, S.E.; Yang, H.; Zhang, G.; Zhang, F.; Guo, X. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Hornstein, J.; Espinosa, E.P.; Cerrato, R.M.; Lwiza, K.M.; Allam, B. The influence of temperature stress on the physiology of the Atlantic surfclam, Spisula solidissima. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 222, 66–73. [Google Scholar] [CrossRef]
- Rahman, M.; Henderson, S.; Miller-Ezzy, P.; Li, X.; Qin, J. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish. Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Storey, K.B.; Dong, Y.-W. Adaptations to the mudflat: Insights from physiological and transcriptional responses to thermal stress in a burrowing bivalve Sinonovacula constricta. Sci. Total. Environ. 2020, 710, 136280. [Google Scholar] [CrossRef] [PubMed]
- Stillman, J.H. Acclimation capacity underlies susceptibility to climate change. Science 2003, 301, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [Green Version]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Putting the heat on tropical animals. Science 2008, 320, 1296. [Google Scholar] [CrossRef]
- Duarte, H.; Tejedo, M.; Katzenberger, M.; Marangoni, F.; Baldo, D.; Beltrán, J.F.; Martí, D.A.; Richter-Boix, A.; Gonzalez-Voyer, A. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob. Chang. Biol. 2012, 18, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Joly, A.-L.; Wettstein, G.; Mignot, G.; Ghiringhelli, F.; Garrido, C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J. Innate Immun. 2010, 2, 238–247. [Google Scholar] [CrossRef]
- Buchner, J.; Li, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2013, 36, 106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010, 277, 174–185. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.C.; Ramos, C.H. Protein folding assisted by chaperones. Protein Pept. Lett. 2005, 12, 257–261. [Google Scholar] [CrossRef]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Makhnevych, T.; Houry, W.A. The role of Hsp90 in protein complex assembly. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Houry, W.A. Molecular interaction network of the Hsp90 chaperone system. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks; Springer: New York, NY, USA, 2007; pp. 27–36. [Google Scholar]
- Lai, B.; Chin, N.; Stanek, A.; Keh, W.; Lanks, K. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol. Cell. Biol. 1984, 4, 2802–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees-Miller, S.P.; Anderson, C.W. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90α at two NH2-terminal threonine residues. J. Biol. Chem. 1989, 264, 17275–17280. [Google Scholar] [CrossRef]
- Liu, T.; Pan, L.; Cai, Y.; Miao, J.J.G. Molecular cloning and sequence analysis of heat shock proteins 70 (HSP70) and 90 (HSP90) and their expression analysis when exposed to benzo (a) pyrene in the clam Ruditapes philippinarum. Gene 2015, 555, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Wang, G.; Wu, C.; Li, J. Molecular cloning, sequencing and expression profiles of heat shock protein 90 (HSP90) in Hyriopsis cumingii exposed to different stressors: Temperature, cadmium and Aeromonas hydrophila. Aquac. Fish. 2017, 2, 59–66. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, H.; Zhang, H.; Tan, K.; Ye, T.; Ma, H.; Li, S.; Zheng, H. Effects of thermal stress on mortality and HSP90 expression levels in the noble scallops Chlamys nobilis with different total carotenoid content. Cell Stress Chaperones 2020, 25, 105–117. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and function. In Molecular Chaperones; Springer: Berlin/Heidelberg, Germany, 2012; pp. 155–240. [Google Scholar]
- Dollins, D.E.; Warren, J.J.; Immormino, R.M.; Gewirth, D.T. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol. Cell 2007, 28, 41–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, S.; Leskovar, A.; Reinstein, J.; Buchner, J. The ATPase cycle of the endoplasmic chaperone Grp94. J. Biol. Chem. 2007, 282, 35612–35620. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L.; Brown, C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones 2009, 14, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmbrecht, K.; Zeise, E.; Rensing, L. Chaperones in cell cycle regulation and mitogenic signal transduction: A review. Cell Prolif. 2000, 33, 341–365. [Google Scholar] [CrossRef]
- Queitsch, C.; Sangster, T.A.; Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 2002, 417, 618–624. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, J.; Song, L.; Qiu, L.; Yu, Y.; Zhang, H.; Ni, D. Molecular cloning, characterization and expression of heat shock protein 90 gene in the haemocytes of bay scallop Argopecten irradians. Fish Shellfish. Immunol. 2008, 24, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Boher, F.; Trefault, N.; Piulachs, M.-D.; Bellés, X.; Godoy-Herrera, R.; Bozinovic, F. Biogeographic origin and thermal acclimation interact to determine survival and hsp90 expression in Drosophila species submitted to thermal stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Jo, P.G.; An, K.W.; Park, M.S.; Youngchoi, C. mRNA expression of HSP90 and SOD, and physiological responses to thermal and osmotic stress in the Pacific oyster, Crassostrea gigas. Molluscan Res. 2008, 28, 158. [Google Scholar]
- Wang, S.; Li, X.; Li, T.; Wang, H.; Zhang, X.; Lou, J.; Xing, Q.; Hu, X.; Bao, Z. The GRP94 gene of Yesso scallop (Patinopecten yessoensis): Characterization and expression regulation in response to thermal and bacterial stresses. Fish Shellfish. Immunol. 2018, 80, 443–451. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Liu, C.; Zhou, Z.; Zhao, X.; Song, L. The polymorphisms in the promoter of HSP90 gene and their association with heat tolerance of bay scallop. Cell Stress Chaperones 2015, 20, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Yang, H. Analysis of the causes of mass mortality of farming Chlamys farreri in summer in coastal areas of Shandong, China. Mar. Sci. 1999, 1, 47. [Google Scholar]
- Li, Y.; Sun, X.; Hu, X.; Xun, X.; Zhang, J.; Guo, X.; Jiao, W.; Zhang, L.; Liu, W.; Wang, J. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Kolde, R.; Kolde, M.R. Package ‘Pheatmap’; R Package: Vienna, Austria, 2015; Volume 1, p. 790. [Google Scholar]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Song, L.; Ni, D.; Wu, L.; Zhang, H.; Chang, Y. cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop Chlamys farreri. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Jo, P.G.; Choi, C.Y. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the Pacific oyster, Crassostrea gigas. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, C.; Mai, K.; Chen, Q.; Xu, W. Molecular cloning, characterization and expression analysis of heat shock protein 90 from Pacific abalone, Haliotis discus hannai Ino in response to dietary selenium. Fish Shellfish. Immunol. 2011, 30, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, V.; Kaiser, C.J.; Richter, K. Hsp90 in non-mammalian metazoan model systems. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Knorr, E.; Vilcinskas, A. Post-embryonic functions of HSP90 in Tribolium castaneum include the regulation of compound eye development. Dev. Genes Exolution 2011, 221, 357–362. [Google Scholar] [CrossRef]
- Shumway, S.E.; Parsons, G.J. Scallops: Biology, Ecology, Aquaculture, and Fisheries; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mao, C.; Wang, M.; Luo, B.; Wey, S.; Dong, D.; Wesselschmidt, R.; Rawlings, S.; Lee, A.S. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS ONE 2010, 5, e10852. [Google Scholar] [CrossRef] [Green Version]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef]
- Barnes, J.; Smoak, I.W. Immunolocalization and heart levels of GRP94 in the mouse during post-implantation development. Anat. Embryol. 1997, 196, 335–341. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Huang, Z.; Wei, P.; Liu, Y.; Hao, J.; Zhao, L.; Zhang, F.; Tu, Y.; Wei, T. Aberrantly upregulated TRAP1 is required for tumorigenesis of breast cancer. Oncotarget 2015, 6, 44495. [Google Scholar] [CrossRef] [Green Version]
- Agorreta, J.; Hu, J.; Liu, D.; Delia, D.; Turley, H.; Ferguson, D.J.; Iborra, F.; Pajares, M.J.; Larrayoz, M.; Zudaire, I. TRAP1 regulates proliferation, mitochondrial function, and has prognostic significance in NSCLC. Mol. Cancer Res. 2014, 12, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Leav, I.; Plescia, J.; Goel, H.L.; Li, J.; Jiang, Z.; Cohen, R.J.; Languino, L.R.; Altieri, D.C. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am. J. Pathol. 2010, 176, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Li, X.; Jiang, Q.; Liu, Z.; Yang, H.; Wang, S.; Xie, S.; Liu, Q.; Liu, T.; Huang, J. Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of Southern China. J. Transl. Med. 2008, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Wang, X.; Gan, S.; Tang, X.; Kang, X.; Zhu, S. The Mitochondrial Chaperone TRAP1 as a Candidate Target of Oncotherapy. Front. Oncol. 2020, 10, 585047. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, L.; Zhou, F.; Huang, J.; Guo, Y.; Yang, K. Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol. Biol. Rep. 2009, 36, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, M.-Z.; Zheng, C.-J.; Liu, J.; Hu, H.-J. Identification of two hsp90 genes from the marine crab, Portunus trituberculatus and their specific expression profiles under different environmental conditions. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 465–473. [Google Scholar] [CrossRef]
- Lin, X.; Wu, X.; Liu, X. Temperature stress response of heat shock protein 90 (Hsp90) in the clam Paphia undulata. Aquac. Fish. 2018, 3, 106–114. [Google Scholar] [CrossRef]
- Fisher, D.; Mandart, E.; Doree, M. Hsp90 is required for c-Mos activation and biphasic MAP kinase activation in Xenopus oocytes. EMBO J. 2000, 19, 1516–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, Z.; Szymik, N.; Wiśniewski, J. Expression of hsp70-related gene in developing and degenerating rat testis. Mol. Biol. Rep. 1987, 12, 35–41. [Google Scholar] [CrossRef]
- Vamvakopoulos, N.O. Tissue-specific expression of heat shock proteins 70 and 90: Potential implication for differential sensitivity of tissues to glucocorticoids. Mol. Cell. Biol. 1993, 98, 49–54. [Google Scholar] [CrossRef]
- Itoh, H.; Toyoshima, I.; Mizunuma, H.; Kobayashi, R.; Tashima, Y. Three-step purification method and characterization of the bovine brain 90-kDa heat shock protein. Arch. Biochem. Biophys. 1990, 282, 290–296. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, L.; Qin, J.; Wu, P.; Zhang, F.; Li, E.; Tang, B. MnHSP90 cDNA characterization and its expression during the ovary development in oriental river prawn, Macrobrachium nipponense. Mol. Biol. Rep. 2011, 38, 1399–1406. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S. Induced thermotolerance and expression of heat shock protein 70 in sea cucumber Apostichopus japonicus. Fish. Sci. 2008, 74, 573–578. [Google Scholar] [CrossRef]
- Peng, G.; Zhao, W.; Shi, Z.; Chen, H.; Liu, Y.; Wei, J.; Gao, F. Cloning HSP70 and HSP90 genes of kaluga (Huso dauricus) and the effects of temperature and salinity stress on their gene expression. Cell Stress Chaperones 2016, 21, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Ahn, I.-Y.; Kim, H.; Cheon, J.; Park, H. Molecular characterization and induction of heat shock protein 90 in the Antarctic bivalve Laternula elliptica. Cell Stress Chaperones 2009, 14, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Zhang, L.; Li, L.; Que, H.; Zhang, G. Expression characterization of stress genes under high and low temperature stresses in the Pacific oyster, Crassostrea gigas. Mar. Biotechnol. 2016, 18, 176–188. [Google Scholar] [CrossRef]

| HSP90 | GRP94 | TRAP1 | |
| Total length (bp) | 7211 | 26,457 | 28,699 |
| 5’UTR length (bp) | 357 | 96 | — |
| 3’UTR length (bp) | 726 | 841 | 358 |
| ORF length (bp) | 2181 | 2397 | 2181 |
| Amino acids length | 726 | 798 | 726 |
| Weight (kDa) | 83.274 | 91.120 | 82.862 |
| Theoretical pI | 4.80 | 4.72 | 5.84 |
| Number of exons | 7 | 14 | 18 |
| Number of introns | 6 | 13 | 17 |
| Number of alpha helixes | 33 | 34 | 39 |
| Number of beta strands | 33 | 26 | 38 |
| Number of coils | 38 | 45 | 50 |
| Number of turns | 28 | 34 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Yang, Z.; Sui, M.; Cui, C.; Hu, Y.; Hou, X.; Xing, Q.; Huang, X.; Bao, Z. Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri. Genes 2021, 12, 1592. https://doi.org/10.3390/genes12101592
Yu H, Yang Z, Sui M, Cui C, Hu Y, Hou X, Xing Q, Huang X, Bao Z. Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri. Genes. 2021; 12(10):1592. https://doi.org/10.3390/genes12101592
Chicago/Turabian StyleYu, Haitao, Zujing Yang, Mingyi Sui, Chang Cui, Yuqing Hu, Xiujiang Hou, Qiang Xing, Xiaoting Huang, and Zhenmin Bao. 2021. "Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri" Genes 12, no. 10: 1592. https://doi.org/10.3390/genes12101592
APA StyleYu, H., Yang, Z., Sui, M., Cui, C., Hu, Y., Hou, X., Xing, Q., Huang, X., & Bao, Z. (2021). Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri. Genes, 12(10), 1592. https://doi.org/10.3390/genes12101592





