Immune Profiling of Medullary Thyroid Cancer—An Opportunity for Immunotherapy
Abstract
:1. Introduction
2. Results
2.1. Characteristics at Presentation and Primary Treatment
2.2. Response to Therapy
2.3. Expression of 395 Genes in Tumor and Control Tissues
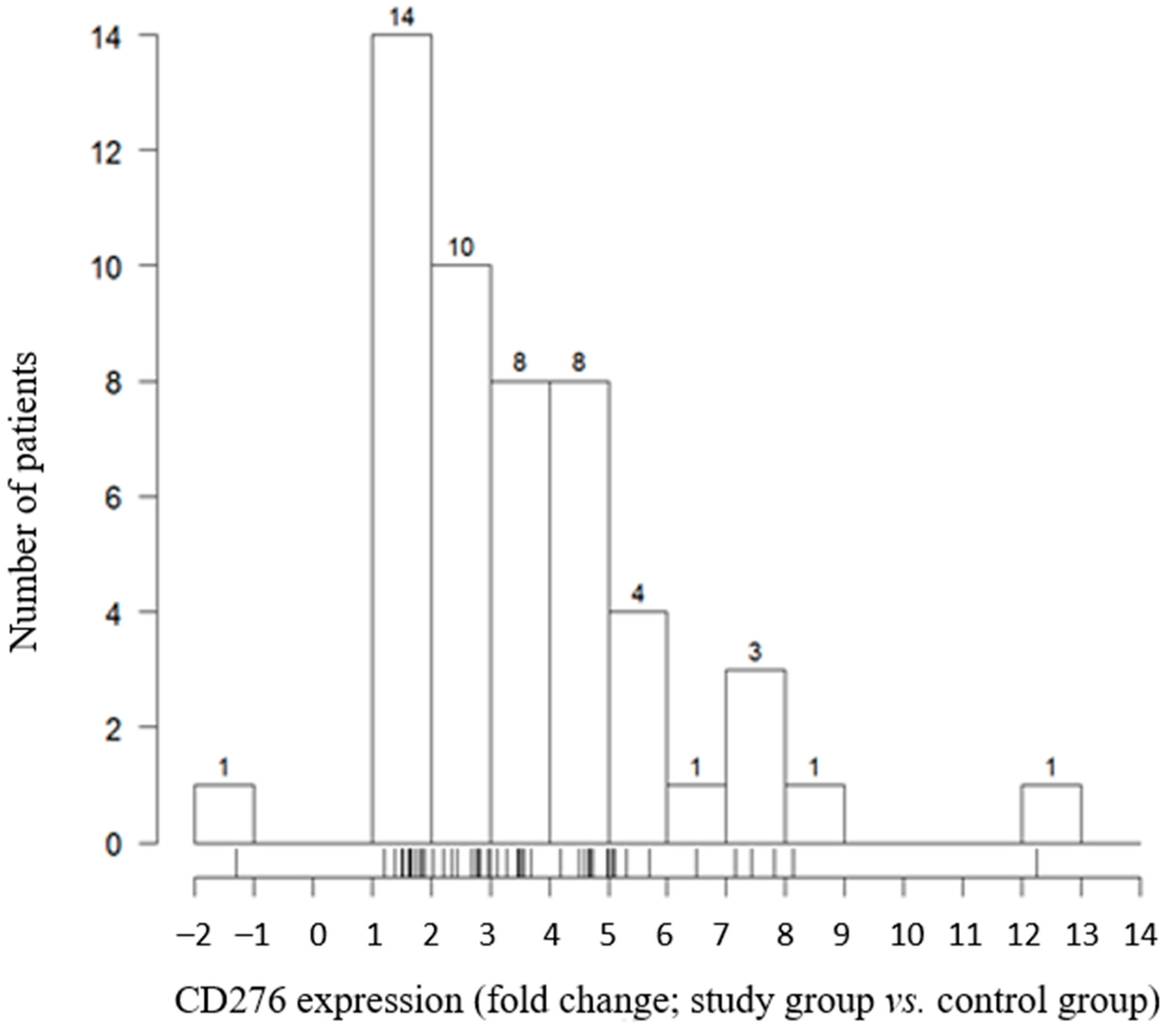
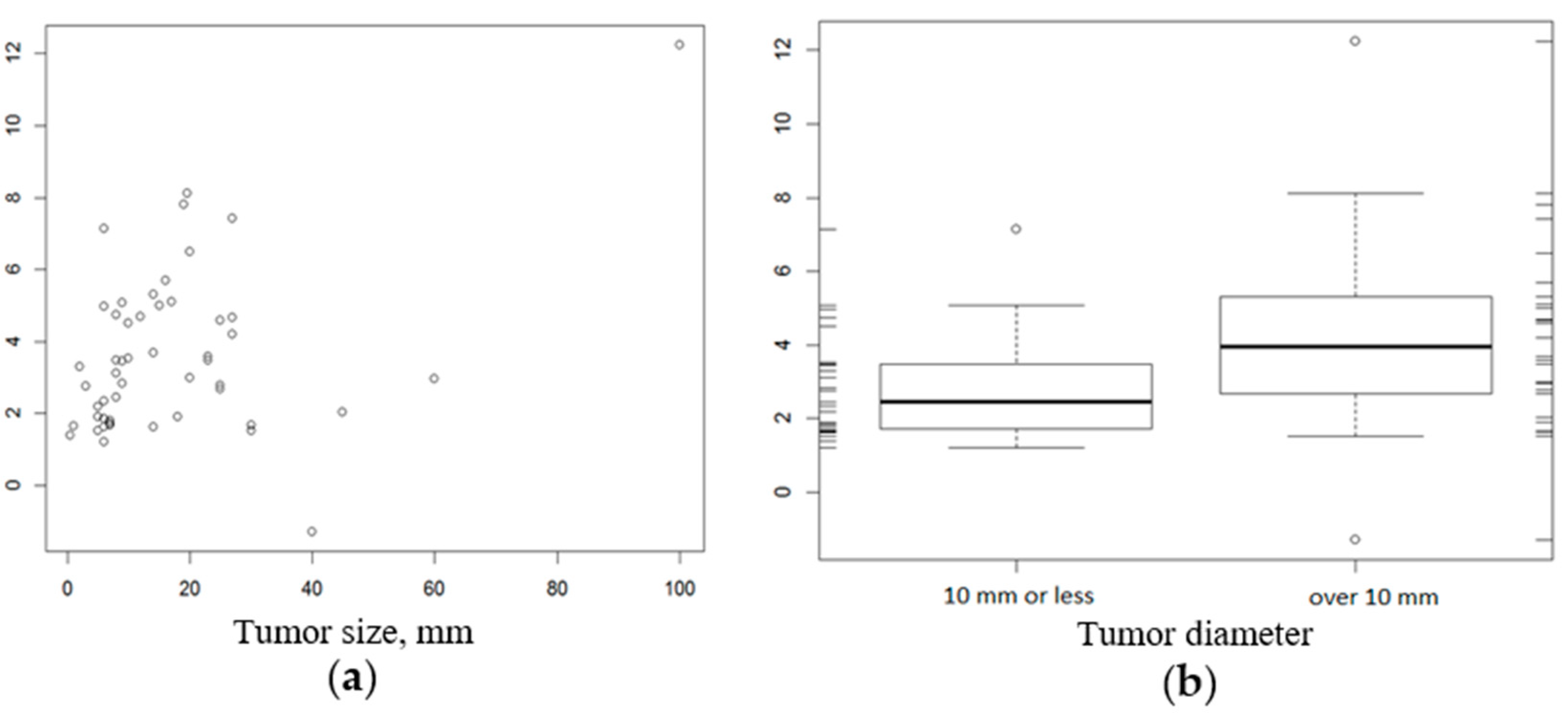
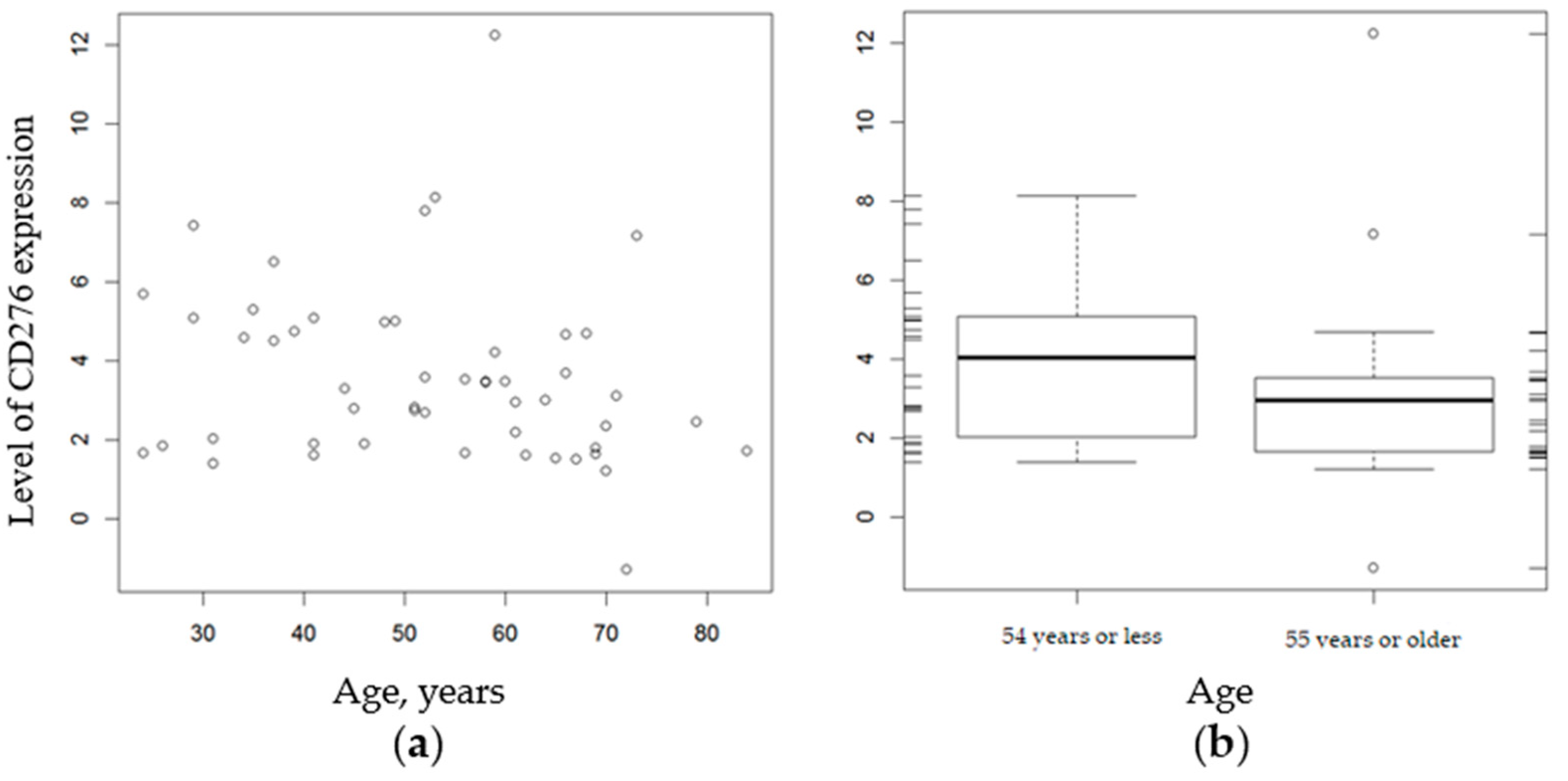
2.4. Relationship between CD276 Expression and Histopathologic Factors
2.5. Potential Therapeutic Targets for MTC
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Isolation of RNA from Formalin-Fixed, Paraffin-Embedded (FFPE) Samples
4.3. Reverse Transcription
4.4. Oncomine Immune Response Research Assay
4.5. Emulsion PCR and Sequencing
4.6. Bioinformatics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Hadoux, J.; Pacini, F.; Tuttle, R.M.; Schlumberger, M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol. 2016, 4, 64–71. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Renzini, G.; Molinaro, E.; Agate, L.; Vivaldi, A.; Faviana, P.; Basolo, F.; et al. Prognostic Significance of SomaticRETOncogene Mutations in Sporadic Medullary Thyroid Cancer: A 10-Year Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 682–687. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, L.M.; Kwok, J.B.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Mole, S.E.; Moore, J.K.; Papi, L.; et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef]
- Marsh, D.J.; Learoyd, D.L.; Andrew, S.D.; Krishnan, L.; Pojer, R.; Richardson, A.-L.; Delbridge, L.; Eng, C.; Robinson, B.G. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin. Endocrinol. 1996, 44, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Jiao, Y.; Sausen, M.; Leary, R.; Bettegowda, C.; Roberts, N.J.; Bhan, S.; Ho, A.S.; Khan, Z.; Bishop, J.; et al. Exomic Sequencing of Medullary Thyroid Cancer Reveals Dominant and Mutually Exclusive Oncogenic Mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013, 98, E364–E369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, M.M.; Cavaco, B.M.; Pinto, A.E.; Leite, V. High Prevalence of RASMutations in RET-Negative Sporadic Medullary Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2011, 96, E863–E868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boichard, A.; Croux, L.; Al Ghuzlan, A.; Broutin, S.; Dupuy, C.; Leboulleux, S.; Schlumberger, M.; Bidart, J.M.; Lacroix, L. SomaticRASMutations Occur in a Large Proportion of SporadicRET-Negative Medullary Thyroid Carcinomas and Extend to a Previously Unidentified Exon. J. Clin. Endocrinol. Metab. 2012, 97, E2031–E2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2016, 161, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadu, R.; Bagheri-Yarmand, R.; Ringel, M.D.; Grubbs, E.G.; Zafereo, M.; Cote, G.; Gagel, R.F.; Robinson, B.G.; Shaw, K.R.; I Hu, M. Hereditary endocrine tumours: Current state-of-the-art and research opportunities: The state of science in medullary thyroid carcinoma: Current challenges and unmet needs. Endocr Relat. Cancer 2020, 27, T27–T39. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarząb, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarząb, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- ESMO LOXO-292 Demonstrates Promising Anti-Tumour Activity in RET-Altered Thyroid Cancer. Available online: https://www.esmo.org/oncology-news/loxo-292-demonstrates-promising-anti-tumour-activity-in-ret-altered-thyroid-cancer (accessed on 7 April 2021).
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Castellone, M.D.; Melillo, R.M. RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr. Relat. Cancer 2018, 25, T105–T119. [Google Scholar] [CrossRef] [Green Version]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koperek, O.; Bergner, O.; Pichlhofer, B.; Oberndorfer, F.; A Hainfellner, J.; Kaserer, K.; Horvat, R.; Harris, A.L.; Niederle, B.; Birner, P. Expression of hypoxia-associated proteins in sporadic medullary thyroid cancer is associated with desmoplastic stroma reaction and lymph node metastasis and may indicate somatic mutations in the VHL gene. J. Pathol. 2011, 225, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torphy, R.J.; Schulick, R.D.; Zhu, Y. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2642. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.W.; A Barbie, D.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewski, T.F.; Meng, Y.; Harlin, H. Immune Suppression in the Tumor Microenvironment. J. Immunother. 2006, 29, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front. Oncol. 2018, 8, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.R.; Purvis, I.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66–75. [Google Scholar]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Fodstad, O. B7-H3 and its relevance in cancer immunological and non-immunological perspectives. Front. Biosci. 2011, E3, 989–993. [Google Scholar] [CrossRef]
- Seaman, S.; Zhu, Z.; Saha, S.; Zhang, X.M.; Yang, M.Y.; Hilton, M.B.; Morris, K.; Szot, C.; Morris, H.; Swing, D.A.; et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017, 31, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, D.-C.; Zhu, X.-G.; Gan, W.-J.; Li, Z.; Xiong, F.; Zhang, Z.-X.; Zhang, G.-B.; Zhang, X.-G.; Zhao, H. B7-H3 overexpression in pancreatic cancer promotes tumor progression. Int. J. Mol. Med. 2012, 31, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Arigami, T.; Narita, N.; Mizuno, R.; Nguyen, L.; Ye, X.; Chung, A.; Giuliano, A.E.; Hoon, D.S.B. B7-H3 Ligand Expression by Primary Breast Cancer and Associated with Regional Nodal Metastasis. Ann. Surg. 2010, 252, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Chen, Y.; Zhou, H.; Wang, B.; Du, Q.; Chen, Y. B7-H3 expression and its correlation with clinicopathologic features, angiogenesis, and prognosis in intrahepatic cholangiocarcinoma. APMIS 2018, 126, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, V.A.; Boye, K.; Tekle, C.; Nesland, J.M.; Flatmark, K.; Fodstad, Ø. B7-H3 expression in colorectal cancer: Nuclear localization strongly predicts poor outcome in colon cancer. Int. J. Cancer 2012, 131, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Sullivan, P.S.; A Soslow, R.; Waitz, R.; E Reuter, V.; Wilton, A.; Thaler, H.T.; Arul, M.; Slovin, S.F.; Wei, J.; et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 2010, 23, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Sakakibara, R.; Ninomiya, H.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer 2016, 103, 44–51. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zhao, J.; Gu, M.; Giscombe, R.; Lefvert, A.K.; Wang, X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006, 53, 143–151. [Google Scholar] [CrossRef]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-T.; Chen, C.-H.; Ku, K.-L.; Hsiao, M.; Chiang, C.-P.; Hsu, T.-L.; Chen, M.-H.; Wong, C.-H. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc. Natl. Acad. Sci. USA 2015, 112, 13057–13062. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Zhang, H.; Ye, D.; Dai, B.; Zhu, Y.; Shi, G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. OncoTargets Ther. 2013, 6, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, J.; Kelly, J.; Gu, G.; Hou, J.; Zhou, Y.; Redmond, H.P.; Wang, J.H.; Zhang, X. B7-H3 Augments the Inflammatory Response and Is Associated with Human Sepsis. J. Immunol. 2010, 185, 3677–3684. [Google Scholar] [CrossRef]
- Chen, X.; Quinn, E.M.; Ni, H.; Wang, J.; Blankson, S.; Redmond, H.P.; Wang, J.H.; Feng, X. B7-H3 Participates in the Development of Experimental Pneumococcal Meningitis by Augmentation of the Inflammatory Response via a TLR2-Dependent Mechanism. J. Immunol. 2012, 189, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stöckl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Enoblituzumab Overview—Creative Biolabs. Available online: https://www.creativebiolabs.net/enoblituzumab-overview.htm (accessed on 20 May 2021).
- DeSantes, K.; Maris, J.M.; McDowell, K.; Mackall, C.; Shankar, S.; Vasselli, J.; Chen, F.; Loo, D.; Moore, P.A.; Wigginton, J.M.; et al. A phase 1, open-label, dose escalation study of enoblituzumab (MGA271) in pediatric patients with B7-H3-expressing relapsed or refractory solid tumors. J. Clin. Oncol. 2017, 35, TPS2596. [Google Scholar] [CrossRef]
- Enoblituzumab (Anti-B7-H3). MacroGenics. Available online: https://macrogenics.com/ (accessed on 30 June 2021).
- Masonic Cancer Center. University of Minnesota Intraperitoneal FATE FT516 and Interleukin-2 (IL-2) with Intravenous Enoblituzumab in Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. 2021. Available online: https://clinicaltrials.gov (accessed on 30 June 2021).
- Shenderov, E.; DeMarzo, A.; Boudadi, K.; Allaf, M.; Wang, H.; Chapman, C.; Pavlovich, C.; Bivalacqua, T.; O’Neal, T.S.; Harb, R.; et al. Phase II neoadjuvant and immunologic study of B7-H3 targeting with enoblituzumab in localized intermediate- and high-risk prostate cancer. J. Clin. Oncol. 2018, 36, TPS5099. [Google Scholar] [CrossRef]
| Feature | Total (n = 51) |
|---|---|
| Gender, n (%) | |
| Female | 36 (70.6%) |
| Male | 15 (29.4%) |
| Median age at diagnosis, years (Q1–Q3; range) | 53.0 (41.0, 65.5; 24–84) |
| Median tumor size, mm (Q1–Q3; range) | 12.0 (6.5, 23.0; 0.5–100) |
| Multifocality, n (%) | |
| No | 36 (70.6%) |
| Yes | 17 (33.3%) |
| Extrathyroidal extension, n (%) | |
| No | 47 (92.2%) |
| Yes | 4 (7.8%) |
| Angioinvasion, n (%) | |
| No | 45 (88.2%) |
| Yes | 6 (11.8%) |
| Tumor stage, n (%) | |
| T1a | 16 (31.4%) |
| T1am | 8 (15.7%) |
| T1b | 9 (17.6%) |
| T1bm | 3 (5.9%) |
| T2 | 6 (11.8%) |
| T2m | 4 (7.8%) |
| T3 | 3 (5.9%) |
| T3m | 2 (3.9%) |
| Node stage, n (%) | |
| N0 | 31 (60,7,%) |
| N1a | 6 (11.8%) |
| N1b | 14 (27.5%) |
| Distant metastasis, n (%) | |
| M0 | 49 (96,1%) |
| M1 | 2 (3.9%) |
| Initial Response to Therapy | n (%) |
| Excellent | 27 (52.9%) |
| Biochemical incomplete response | 18 (35.3%) |
| Structural incomplete response | 2 (3.9%) |
| Death | n (%) |
| No | 47 (92.2%) |
| MTC-unrelated | 2 (3.9%) |
| MTC-related | 2 (3.9%) |
| CD276 Expression | Feature | p-Value | |
|---|---|---|---|
| Tumor Size, mm | |||
| 10 mm or less (n = 25) | Over 10 mm (n = 26) | ||
| 0.0205 | |||
| Mean (SD) | 2.89 (1.47) | 4.27 (2.71) | |
| Median (Q1, Q3) | 2.44 (1.72, 3.47) | 3.94 (2.71, 5.25) | |
| Range | 1.21–7.15 | −1.29–12.24 | |
| Multifocality | |||
| No (n = 36) | Yes (n = 15) | ||
| 0.2265 | |||
| Mean (SD) | 3.88 (2.32) | 2.91 (2.08) | |
| Median (Q1, Q3) | 3.46 (2.23, 4.99) | 2.20 (1.69, 4.62) | |
| Range | 1.21–12.24 | −1.29–7.42 | |
| Angioinvasion | |||
| No (n = 45) | Yes (n = 6) | ||
| 0.5201 | |||
| Mean (SD) | 3.48 (1.85) | 4.42 (4.55) | |
| Median (Q1, Q3) | 3.00 (1.90, 4.66) | 4.29 (2.12, 5.25) | |
| Range | 1.21–8.13 | −1.29–12.24 | |
| Central Lymph Nodes | |||
| No (n = 45) | Yes (n = 6) | ||
| 0.3571 | |||
| Mean (SD) | 3.71 (2.19) | 2.71 (2.95) | |
| Median (Q1, Q3) | 3.29 (1.90, 4.74) | 1.96 (1.73, 3.88) | |
| Range | 1.21–12.24 | −1.29–7.42 | |
| Lateral Lymph Nodes | |||
| No (n = 37) | Yes (n = 14) | ||
| 0.1542 | |||
| Mean (SD) | 3.25 (1.93) | 4.49 (2.89) | |
| Median (Q1, Q3) | 2.83 (1.72, 4.69) | 3.50 (2.75, 5.14) | |
| Range | −1.29–7.80 | 1.61–12.24 | |
| Node Stage | |||
| N0 (n = 31) | N1 (n = 20) | ||
| 0.4873 | |||
| Mean (SD) | 3.35 (1.72) | 3.96 (2.95) | |
| Median (Q1, Q3) | 3.00 (1.75, 4.71) | 3.48 (1.90, 4.82) | |
| Range | 1.21–7.80 | −1.29–12.24 | |
| Level CD276 Expression | Response to Initial Therapy, n (%) | p-Value | ||||
|---|---|---|---|---|---|---|
| Remission (n = 27) | Biochemical Persistent Disease (n = 18) | Structural Persistent Disease (n = 2) | Death MTC-Related (n = 2) | Death MTC-Unrelated (n = 2) | ||
| Mean (SD) | 3.27 (1.73) | 2.89 (1.57) | 5.24 (3.62) | 10.19 (2.91) | 5.95 (1.70) | 0.457 |
| Median (Q1, Q3) | 3.00 (1.65, 4.60) | 2.90 (1.90, 3.52) | ||||
| Range | 1.21–7.42 | −1.29–5.30 | 2.68–7.80 | 8.13–12.24 | 4.74–7.15 | |
| Level CD276 Expression | Gender | p-Value | ||
|---|---|---|---|---|
| 10 mm or Less (n = 25) | Over 10 mm (n = 26) | Total (n = 51) | ||
| Mean (SD) | 3.47 (2.40) | 3.89 (2.00) | 3.59 (2.28) | 0.4821 |
| Median (Q1, Q3) | 2.88 (1.82, 4.80) | 3.47 (2.81, 4.62) | 3.12 (1.88, 4.71) | |
| Range | −1.29–12.24 | 1.51–8.13 | −1.29–12.24 | |
| Level CD276 Expression | Age, Years | p-Value | |
|---|---|---|---|
| 54 Years or Less (n = 26) | 55 Years or Older (n = 25) | ||
| Mean (SD) | 4.04 (2.02) | 3.12 (2.47) | 0.0511 |
| Median (Q1, Q3) | 4.04 (2.19, 5.09) | 2.96 (1.67, 3.53) | |
| Range | 1.39–8.13 | −1.29–12.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hińcza-Nowak, K.; Kowalik, A.; Walczyk, A.; Pałyga, I.; Gąsior-Perczak, D.; Płusa, A.; Kopczyński, J.; Chrapek, M.; Góźdź, S.; Kowalska, A. Immune Profiling of Medullary Thyroid Cancer—An Opportunity for Immunotherapy. Genes 2021, 12, 1534. https://doi.org/10.3390/genes12101534
Hińcza-Nowak K, Kowalik A, Walczyk A, Pałyga I, Gąsior-Perczak D, Płusa A, Kopczyński J, Chrapek M, Góźdź S, Kowalska A. Immune Profiling of Medullary Thyroid Cancer—An Opportunity for Immunotherapy. Genes. 2021; 12(10):1534. https://doi.org/10.3390/genes12101534
Chicago/Turabian StyleHińcza-Nowak, Kinga, Artur Kowalik, Agnieszka Walczyk, Iwona Pałyga, Danuta Gąsior-Perczak, Agnieszka Płusa, Janusz Kopczyński, Magdalena Chrapek, Stanisław Góźdź, and Aldona Kowalska. 2021. "Immune Profiling of Medullary Thyroid Cancer—An Opportunity for Immunotherapy" Genes 12, no. 10: 1534. https://doi.org/10.3390/genes12101534
APA StyleHińcza-Nowak, K., Kowalik, A., Walczyk, A., Pałyga, I., Gąsior-Perczak, D., Płusa, A., Kopczyński, J., Chrapek, M., Góźdź, S., & Kowalska, A. (2021). Immune Profiling of Medullary Thyroid Cancer—An Opportunity for Immunotherapy. Genes, 12(10), 1534. https://doi.org/10.3390/genes12101534








