Genetic Knockouts Indicate That the ABCC2 Protein in the Bollworm Helicoverpa zea Is Not a Major Receptor for the Cry1Ac Insecticidal Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. ABCC2 Transporter Gene Sequence
2.2. Guide RNA Design
2.3. Insect Colony Maintenance and Egg Collection
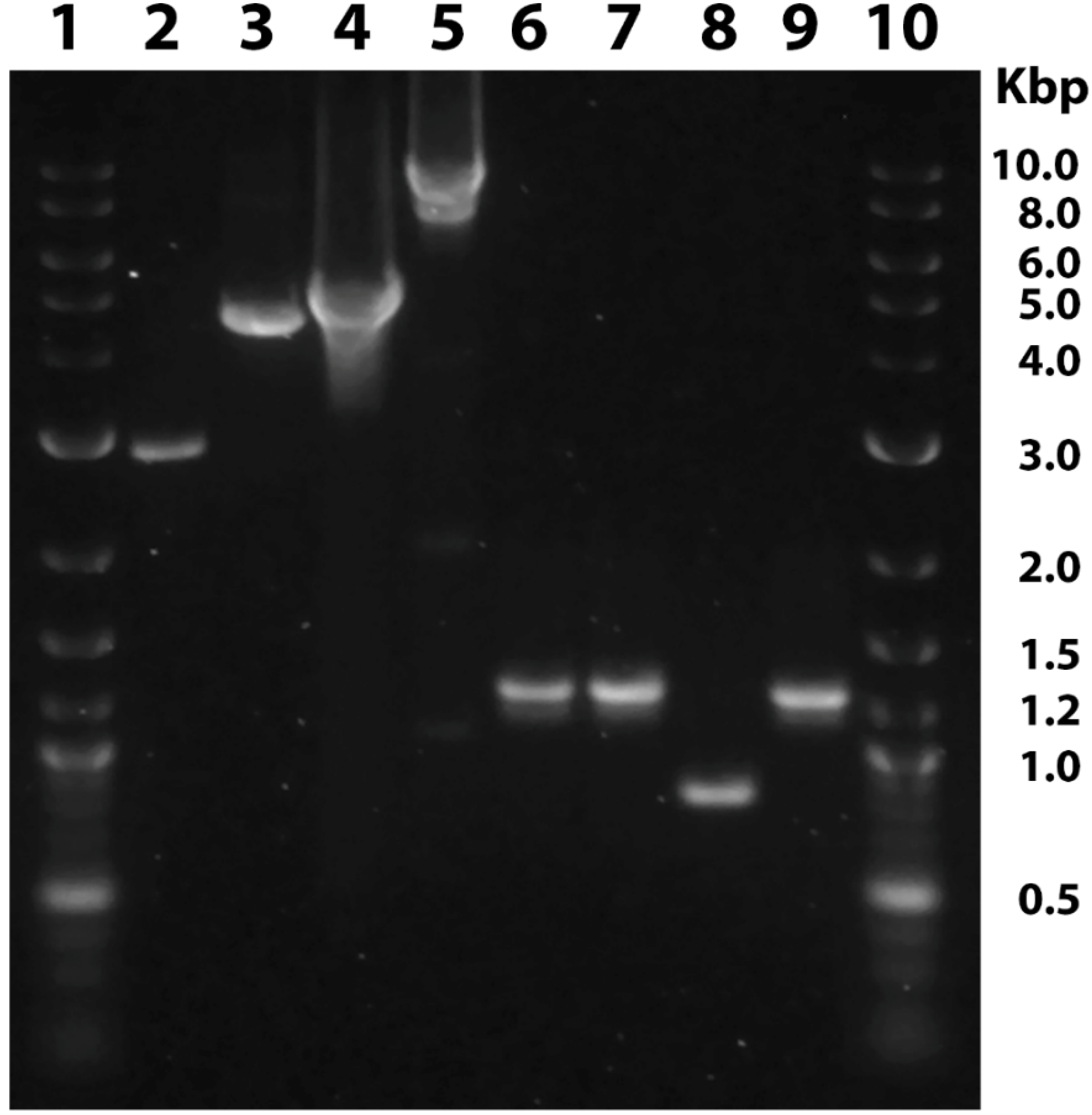
2.4. Microinjections
2.5. Genotyping and DNA Analysis
2.6. Insect Husbandry
2.7. Validation of Gene Knockouts by mRNA and Genomic DNA Sequencing
2.8. Off-Target Sequence Analysis
2.9. Bioassays
2.10. Brush Border Membrane Vesicle (BBMV) Preparation
2.11. Cry1Ac Radioiodination and Binding Assays
3. Results
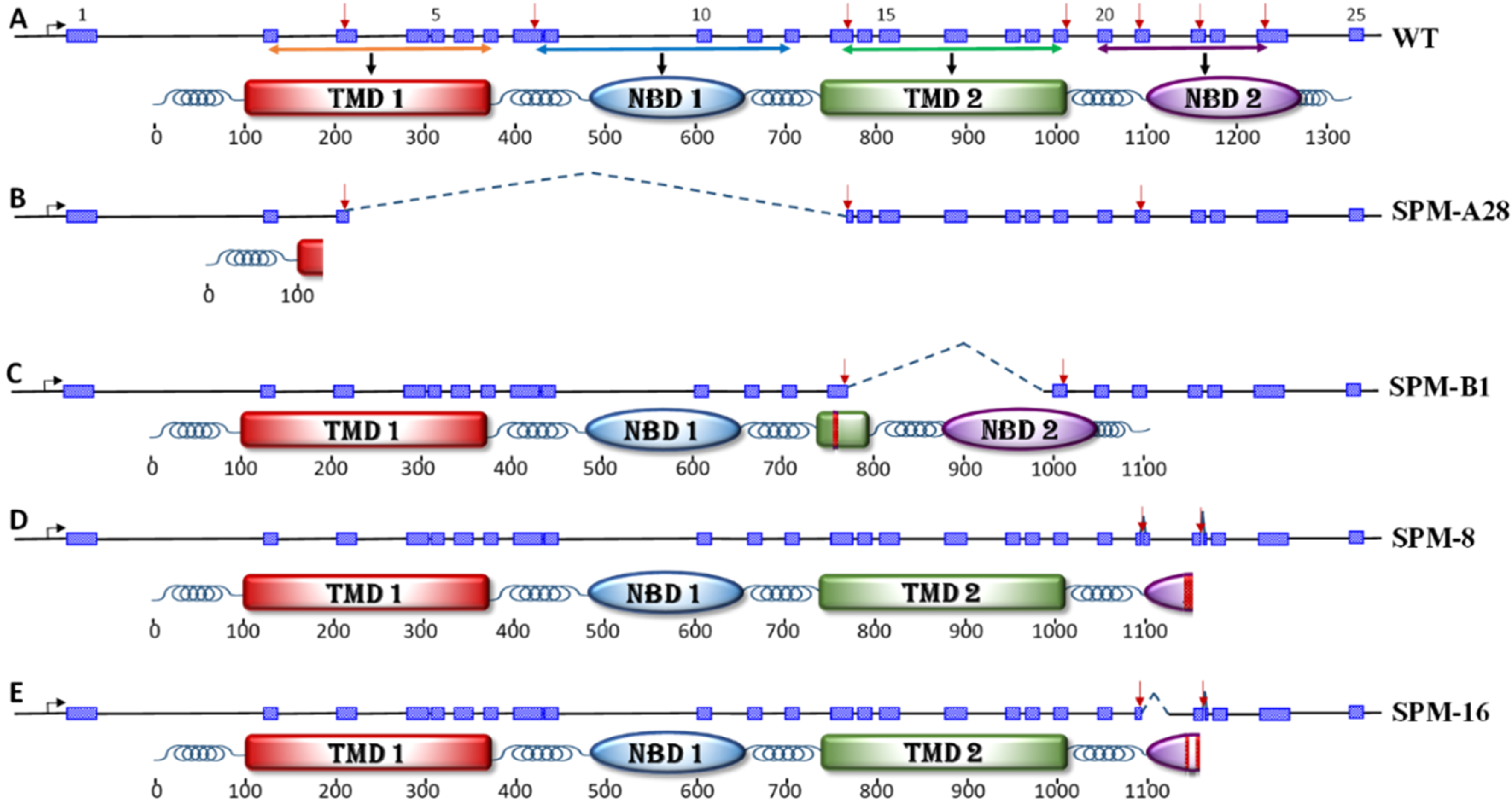
3.1. Genomic Organization of ABCC2 Transporter Gene in Helicoverpa zea
3.2. Embryo Injections Egg Hatch and Mutation Rates
3.3. Off-Target Analysis
3.4. Bioassays
3.5. Saturation Cry1Ac Binding Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perlak, F.J.; Oppenhuizen, M.; Gustafson, K.; Voth, R.; Sivasupramaniam, S.; Heering, D.; Carey, B.; Ihrig, R.A.; Roberts, J.K. Development and commercial use of Bollgard cotton in the USA—early promises versus today’s reality. Plant J. 2001, 27, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GM Approval Database [Internet]. 2021. Available online: http://www.isaaa.org/gmapprovaldatabase/ (accessed on 10 August 2021).
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carrière, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef]
- Dhurua, S.; Gujar, G.T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 2011, 67, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Ponnuraj, J.; Singh, A.; Tanwar, R.K.; Unnithan, G.C.; Yelich, A.J.; Li, X.; Carriere, Y.; Tabashnik, B.E. Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PLoS ONE 2014, 9, e97900. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Sree, K.S.; Sachdev, B.; Rashmi, M.; Ravi, K.; Suresh, P.; Mohan, K.S.; Bhatnagar, R.K. Analysis of resistance to Cry1Ac in field-collected pink bollworm, Pectinophora gossypiella (Lepidoptera: Gelechiidae), populations. GM Crops Food 2014, 5, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.L.; Nunziata, S.O.; Guo, R.; Tabashnik, B.E.; Carrière, Y. Mutations in a novel cadherin gene associated with Bt resistance in Helicoverpa zea. G3 Genes Genomes Genet. 2020, 10, 1563–1574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wei, J.; Ni, X.; Zhang, J.; Jurat-Fuentes, J.L.; Fabrick, J.A.; Carriere, Y.; Tabashnik, B.E.; Li, X. Decreased Cry1Ac activation by midgut proteases associated with Cry1Ac resistance in Helicoverpa zea. Pest Manag. Sci. 2019, 75, 1099–1106. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Heckel, D.; Ferré, J. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Ann. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef]
- Tanaka, S.; Miyamoto, K.; Noda, H.; Jurat-Fuentes, J.L.; Yoshizawa, Y.; Endo, H.; Sato, R. The ATP-binding cassette transporter subfamily C member 2 in Bombyx mori larvae is a functional receptor for Cry toxins from Bacillus thuringiensis. FEBS J. 2013, 280, 1782–1794. [Google Scholar] [CrossRef]
- Gahan, L.J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010, 6, e1001248. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhang, T.; Liu, C.; Heckel, D.G.; Li, X.; Tabashnik, B.E.; Wu, K. Mis-splicing of the ABCC2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci. Rep. 2014, 4, 6184. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, S.; Chen, D.; Wu, Q.; Wang, S.; Xie, W.; Zhu, X.; Baxter, S.W.; Zhou, X.; Jurat-Fuentes, J.L.; et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 2015, 11, e1005124. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Sun, D.; Kang, S.; Zhou, J.; Gong, L.; Qin, J.; Guo, L.; Zhu, L.; Bai, Y.; Luo, L.; et al. CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect. Biochem. Mol. Biol. 2019, 107, 31–38. [Google Scholar] [CrossRef]
- Banerjee, R.; Hasler, J.; Meagher, R.; Nagoshi, R.; Hietala, L.; Huang, F.; Narva, K.; Jurat-Fuentes, J.L. Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci. Rep. 2017, 7, 10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinos, D.; Martínez-Solís, M.; Herrero, S.; Ferré, J.; Hernández-Martínez, P. The Spodoptera exigua ABCC2 acts as a Cry1A receptor independently of its nucleotide binding domain II. Toxins 2019, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.L.; Jiang, W.L.; Ma, Y.J.; Hu, H.Y.; Ma, X.Y.; Ma, Y.; Li, G.Q. The Spodoptera exigua (Lepidoptera: Noctuidae) ABCC2 mediates Cry1Ac cytotoxicity and, in conjunction with cadherin, contributes to cnhance Cry1Ca toxicity in Sf9 cells. J. Econ. Entomol. 2016, 109, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Ocelotl, J.; Sánchez, J.; Gómez, I.; Tabashnik, B.E.; Bravo, A.; Soberón, M. ABCC2 is associated with Bacillus thuringiensis Cry1Ac toxin oligomerization and membrane insertion in diamondback moth. Sci. Rep. 2017, 7, 2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanikolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef] [PubMed]
- Perera, O.P.; Little, N.S.; Pierce, C.A., III. CRISPR/Cas9 mediated high efficiency knockout of the eye color gene Vermillion in Helicoverpa zea (Boddie). PLoS ONE 2018, 13, e0197567. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.; Adamczyk, J.J.; Blanco, C.A. Selective feeding of tobacco budworm and bollworm (Lepidoptera: Noctuidae) on meridic diet with different concentrations of Bacillus thuringiensis proteins. J. Econ. Entomol. 2005, 98, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Mali, P.; Aach, J.; Stranges, P.B.; Esvelt, K.M.; Moosburner, M.; Kosuri, S.; Yang, L.; Church, G.M. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013, 31, 833–838. [Google Scholar] [CrossRef]
- Little, N.S.; Elkins, B.H.; Mullen, R.M.; Perera, O.P.; Parys, K.A.; Allen, K.C.; Boykin, D.L. Differences between two populations of bollworm, Helicoverpa zea (Lepidoptera: Noctuidae), with variable measurements of laboratory susceptibilities to Bt toxins exposed to non-Bt and Bt cottons in large field cages. PLoS ONE 2019, 14, e0212567. [Google Scholar] [CrossRef] [PubMed]
- Finney, D. The adjustment for a natural response rate in probit analysis. Ann. Appl. Biol. 1949, 36, 187–195. [Google Scholar] [CrossRef] [PubMed]
- SAS-Institute. SAS Software, version 9.4; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Wolfersberger, M.; Luthy, P.; Maurer, A.; Parenti, P.; Sacchi, V.F.; Giordana, B.; Hanozet, G.M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Phys. B 1987, 86, 301–308. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Gould, F.L.; Adang, M.J. Altered Glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microb. 2002, 68, 5711–5717. [Google Scholar] [CrossRef] [Green Version]
- Jurat-Fuentes, J.L.; Karumbaiah, L.; Jakka, S.R.; Ning, C.; Liu, C.; Wu, K.; Jackson, J.; Gould, F.; Blanco, C.; Portilla, M.; et al. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis. PLoS ONE 2011, 6, e17606. [Google Scholar] [CrossRef] [Green Version]
- Perera, O.P.; Willis, J.D.; Adang, M.J.; Jurat-Fuentes, J.L. Cloning and characterization of the Cry1Ac-binding alkaline phosphatase (HvALP) from Heliothis virescens. ICES J. Mar. Sci. J. Du Cons. 2009, 39, 294–302. [Google Scholar] [CrossRef]
- Gouffon, C.; Van Vliet, A.; Van Rie, J.; Jansens, S.; Jurat-Fuentes, J.L. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl. Environ. Microb. 2011, 77, 3182–3188. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Heckel, D.G. The essential and enigmatic role of ABC transporters in Bt resistance of noctuids and other insect pests of agriculture. Insects 2021, 12, 389. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Z.; Liu, Y.; Liang, G.; Shu, C.; Song, F.; Zhou, X.; Bravo, A.; Soberón, M.; Zhang, J. Identification of ABCC2 as a binding protein of Cry1Ac on brush border membrane vesicles from Helicoverpa armigera by an improved pull-down assay. MicrobiologyOpen 2016, 5, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ma, H.; Zhao, S.; Huang, J.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Functional redundancy of two ABC transporter proteins in mediating toxicity of Bacillus thuringiensis to cotton bollworm. PLoS Pathog. 2020, 16, e1008427. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, S.; Ma, X.; Baxter, S.W.; Vasseur, L.; Xiong, L.; Huang, Y.; Yang, G.; You, S.; You, M. Resistance to Bacillus thuringiensis Cry1Ac toxin requires mutations in two Plutella xylostella ATP-binding cassette transporter paralogs. PLoS Pathog. 2020, 16, e1008697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, M.; Yang, Y.; Zhang, J.; Yang, Y.; Liu, K.; Soberón, M.; Bravo, A.; Xiao, Y.; Wu, K. Synergistic resistance of Helicoverpa armigera to Bt toxins linked to cadherin and ABC transporters mutations. Insect. Biochem. Mol. Biol. 2021, 137, 103635. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Yang, Y.; Xiao, Y.; Liu, C.; Ma, Y.; Soberon, M.; Bravo, A.; Yang, Y.; Liu, K. A single amino acid polymorphism in ABCC2 loop 1 is responsible for differential toxicity of Bacillus thuringiensis Cry1Ac toxin in different Spodoptera (Noctuidae) species. Insect. Biochem. Mol. Biol. 2018, 100, 59–65. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, M.; Wang, L.; Wang, H.; Xia, Z.; Yang, Y.; Bravo, A.; Soberón, M.; Xiao, Y.; Liu, K. SfABCC2 transporter extracellular loops 2 and 4 are responsible for the Cry1Fa insecticidal specificity against Spodoptera frugiperda. Insect. Biochem. Mol. Biol. 2021, 135, 103608. [Google Scholar] [CrossRef]
- Tanaka, S.; Endo, H.; Adegawa, S.; Iizuka, A.; Imamura, K.; Kikuta, S.; Sato, R. Bombyx mori ABC transporter C2 structures responsible for the receptor function of Bacillus thuringiensis Cry1Aa toxin. Insect. Biochem. Mol. Biol. 2017, 91, 44–54. [Google Scholar] [CrossRef]
- Flagel, L.; Lee, Y.; Wanjugi, H.; Swarup, S.; Brown, A.; Wang, J.; Kraft, E.; Greenplate, J.; Simmons, J.; Adams, N. Mutational disruption of the ABCC2 gene in fall armyworm, Spodoptera frugiperda, confers resistance to the Cry1Fa and Cry1A. 105 insecticidal proteins. Sci. Rep. 2018, 8, 7255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Zhang, M.; Liang, G.; Li, X. Alkaline phosphatase 2 is a functional receptor of Cry1Ac but not Cry2Ab in Helicoverpa zea. Insect. Mol. Biol. 2019, 28, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Xiao, Y.; Yang, Y.; Liu, C.; Peng, R.; Yang, Y.; Bravo, A.; Soberón, M.; Liu, K. The cadherin Cry1Ac binding-region is necessary for the cooperative effect with ABCC2 transporter enhancing insecticidal activity of Bacillus thuringiensis Cry1Ac toxin. Toxins 2019, 11, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| crRNA Name | crRNA Sequence | Strand | Target |
|---|---|---|---|
| Hz_Ex3 | GCTACTGTCGTACTGGTCGGTGG | + | Exon 3 |
| Hz_Ex8 | TTAACAAAGTAAGCGCATCGTGG | + | Exon 8 |
| Hz_Ex13 | CTGCCGACTATTGGTTGAGTTGG | − | Exon 13 |
| Hz_Ex19 | TTGCTCCATATTGGTCTCCGTGG | − | Exon 19 |
| Hz_Ex21 | CGGCAAGTCATCGCTCATCGCGG | + | Exon 21 |
| Hz_Ex22 | ACAGCGACGACGATATTTGGAGG | + | Exon 22 |
| Hz_Ex24 | GGTCATGGACCAGGGCGAAGTGG | + | Exon 24 |
| sgRNA/Scaffold | Position | Sequence | Strand |
|---|---|---|---|
| sgRNA Exon 13 | ACTCAACCAATAGTCGGCAGTGG | ||
| KZ118710.1 | 289298-289320 | CAAT...T............TGG | + |
| sgRNA Ex19 | TTGCTCCATATTGGTCTCCGTGG | ||
| KZ117297.1 | 190397-190417 | G..TGATTC...........TGG | − |
| 204522-204544 | GCTAGTA..T...T......TGG | - | |
| KZ117617.1 | 70340-70362 | C.TACT.T.A..........TGG | - |
| Insect Line | ABCC2 Domain Knockout | LC50 µg/mL (Lower-Upper) | RR | LC90 µg/mL (Lower-Upper) | Slope ± SE | Χ2 Slope |
|---|---|---|---|---|---|---|
| SPM-A28C a | Complete | 21.87 † (13.92–33.77) | 9.2 | 251.65 (141.04–576.35) | 1.22 ± 0.14 | 74.00 p < 0.0001 |
| SPM-B1 b | TM2 to NBD2 | 94.32 ‡ (65.75–137.50) | 39.8 | 455.69 (282.81–946.91) | 1.87 ± 0.25 | 54.58 p < 0.0001 |
| SPM-16 c | NBD2 (insertion) | 42.58 † (24.71–74.62) | 18.0 | 1118.00 (491.32–3876.00) | 0.90 ± 0.11 | 65.79 p < 0.0001 |
| SPM-8 d | NBD2 | 17.35 †,‡ (10.82–29.13) | 7.3 | 355.42 (156.54–1357.00) | 0.98 ± 0.13 | 52.79 p < 0.0001 |
| Control e | None | 2.37 § (1.75–3.20) | 1 | 22.31 (14.85–37.31) | 1.32 ± 0.10 | 162.63 p < 0.0001 |
| Strain | Kd ± SE | P | Bmax ± SE | P | R2 |
|---|---|---|---|---|---|
| SIMRU | 1.55 ± 0.37 | 0.0002 | 49.61 ± 8.08 | 0.0024 | 0.9978 |
| SPM-8 | 0.16 ± 0.09 | 0.1094 | 5.20 ± 0.84 | 0.0002 | 0.9883 |
| SPM-B1 | 0.04 ± 0.05 | 0.4244 | 3.07 ± 0.50 | 0.0002 | 0.7669 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, O.P.; Little, N.S.; Abdelgaffar, H.; Jurat-Fuentes, J.L.; Reddy, G.V.P. Genetic Knockouts Indicate That the ABCC2 Protein in the Bollworm Helicoverpa zea Is Not a Major Receptor for the Cry1Ac Insecticidal Protein. Genes 2021, 12, 1522. https://doi.org/10.3390/genes12101522
Perera OP, Little NS, Abdelgaffar H, Jurat-Fuentes JL, Reddy GVP. Genetic Knockouts Indicate That the ABCC2 Protein in the Bollworm Helicoverpa zea Is Not a Major Receptor for the Cry1Ac Insecticidal Protein. Genes. 2021; 12(10):1522. https://doi.org/10.3390/genes12101522
Chicago/Turabian StylePerera, Omaththage P., Nathan S. Little, Heba Abdelgaffar, Juan Luis Jurat-Fuentes, and Gadi V. P. Reddy. 2021. "Genetic Knockouts Indicate That the ABCC2 Protein in the Bollworm Helicoverpa zea Is Not a Major Receptor for the Cry1Ac Insecticidal Protein" Genes 12, no. 10: 1522. https://doi.org/10.3390/genes12101522
APA StylePerera, O. P., Little, N. S., Abdelgaffar, H., Jurat-Fuentes, J. L., & Reddy, G. V. P. (2021). Genetic Knockouts Indicate That the ABCC2 Protein in the Bollworm Helicoverpa zea Is Not a Major Receptor for the Cry1Ac Insecticidal Protein. Genes, 12(10), 1522. https://doi.org/10.3390/genes12101522









