BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway
Abstract
1. Introduction
2. Results
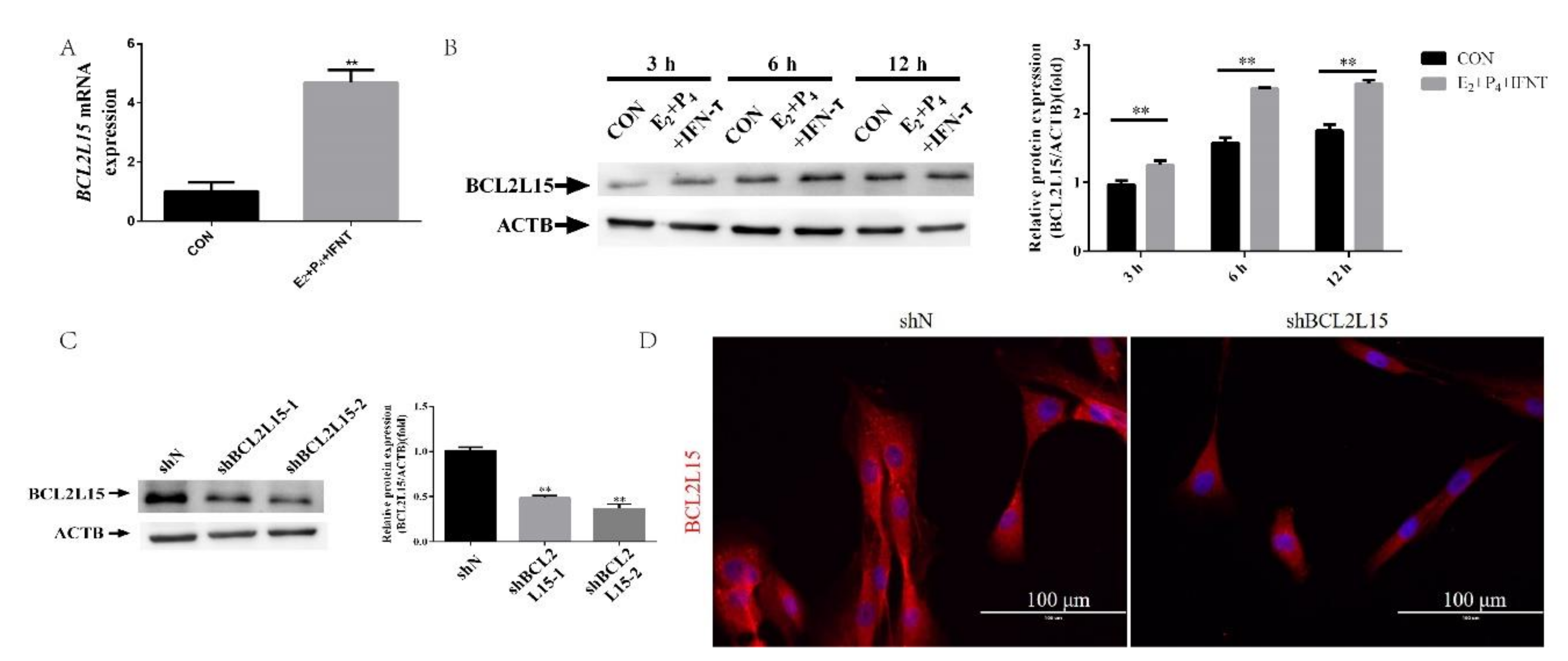
2.1. Hormone and IFN-τ Treatment Triggered BCL2L15
2.2. Knockdown of BCL2L15 Impaired Endometrial Receptivity
2.3. BCL2L15 Knockdown Activated STAT1 and STAT3 Pathways
2.4. Fludarabine Restored the Effect of Silencing BCL2L15 on Endometrial Receptivity
3. Materials and Methods
3.1. Cell Culture and Drug Treatment
3.2. Cell Transfection
3.3. Spheroid Co-Culture Assay
3.4. RNA Extraction and Real-Time Quantitative PCR
3.5. Western Blot Analysis
3.6. Immunofluorescent Staining
3.7. Measurement of Cell Viability
3.8. EdU Proliferation Assay
3.9. SEM Analysis
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vries, A. Economic value of pregnancy in dairy cattle. J. Dairy Sci. 2006, 89, 3876–3885. [Google Scholar] [CrossRef]
- Kuriakose, S.; Onyilagha, C.; Singh, R.; Olayinka-Adefemi, F.; Jia, P.; Uzonna, J.E. TLR-2 and MyD88-Dependent Activation of MAPK and STAT Proteins Regulates Proinflammatory Cytokine Response and Immunity to Experimental Trypanosoma congolense Infection. Front. Immunol. 2019, 10, 2673. [Google Scholar] [CrossRef] [PubMed]
- Beach, K.M.; Wang, J.; Otteson, D.C. Regulation of Stem Cell Properties of Muller Glia by JAK/STAT and MAPK Signaling in the Mammalian Retina. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Javadian, M.; Babaloo, Z.; Baradaran, B. Janus kinase inhibitors: A therapeutic strategy for cancer and autoimmune diseases. J. Cell. Physiol. 2020, 235, 5903–5924. [Google Scholar] [CrossRef] [PubMed]
- Rosario, G.X.; Stewart, C.L. The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Pirochta, J.; Tufano, S.Y.; Teixeira, J.M. Gain-of-function β-catenin in the uterine mesenchyme leads to impaired implantation and decidualization. J. Endocrinol. 2017, 233, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A. Interferons and uterine receptivity. Semin. Reprod. Med. 2009, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Haeger, J.D.; Pfarrer, C. IFNtau mediates chemotaxis, motility, metabolism and CK18 downregulation in bovine trophoblast cells in vitro via STAT1 and MAPK42/44 signaling. Placenta 2018, 64, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.M.; Burghardt, R.C.; Geisert, R.D.; Burghardt, J.R.; Hooper, R.N.; Ross, J.W.; Ashworth, M.D.; Johnson, G.A. Pig conceptuses secrete estrogen and interferons to differentially regulate uterine STAT1 in a temporal and cell type-specific manner. Endocrinology 2007, 148, 4420–4431. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-Stimulated Genes by Conceptus-Derived Exosomes during the Attachment Period. PLoS ONE 2016, 11, e0158278. [Google Scholar] [CrossRef]
- Ozoren, N.; Inohara, N.; Nunez, G. A putative role for human BFK in DNA damage-induced apoptosis. Biotechnol. J. 2009, 4, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Pujianto, D.A.; Damdimopoulos, A.E.; Sipila, P.; Jalkanen, J.; Huhtaniemi, I.; Poutanen, M. Bfk, a novel member of the bcl2 gene family, is highly expressed in principal cells of the mouse epididymis and demonstrates a predominant nuclear localization. Endocrinology 2007, 148, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Coultas, L.; Pellegrini, M.; Visvader, J.E.; Lindeman, G.J.; Chen, L.; Adams, J.M.; Huang, D.C.; Strasser, A. Bfk: A novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ. 2003, 10, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dempsey, C.E.; Dive, C.; Fletcher, D.J.; Barnes, F.A.; Lobo, A.; Bingle, C.D.; Whyte, M.K.; Renshaw, S.A. Expression of pro-apoptotic Bfk isoforms reduces during malignant transformation in the human gastrointestinal tract. FEBS Lett. 2005, 579, 3646–3650. [Google Scholar] [CrossRef]
- Ragusa, S.; Cheng, J.; Ivanov, K.I.; Zangger, N.; Ceteci, F.; Bernier-Latmani, J.; Milatos, S.; Joseph, J.M.; Tercier, S.; Bouzourene, H.; et al. PROX1 promotes metabolic adaptation and fuels outgrowth of Wnt(high) metastatic colon cancer cells. Cell Rep. 2014, 8, 1957–1973. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Ma, L.; Zhou, Z.; Song, Y.; Cao, B. The developmental transcriptome landscape of receptive endometrium during embryo implantation in dairy goats. Genes 2017, 633, 82–95. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, T.; Liu, J.; Hong, J.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Hormone regulates endometrial function via cooperation of endoplasmic reticulum stress and mTOR-autophagy. J. Cell. Physiol. 2018, 233, 6644–6659. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, T.; Liu, J.; Zhang, B.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. CREB3 regulatory factor -mTOR-autophagy regulates goat endometrial function during early pregnancy. Biol. Reprod. 2018, 98, 713–721. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, B.; Wang, Z.; Zhang, L.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Lin, P.; Jin, Y. COPS5 negatively regulates goat endometrial function via the ERN1 and mTOR-autophagy pathways during early pregnancy. J. Cell. Physiol. 2019, 234, 18666–18678. [Google Scholar] [CrossRef]
- Sucurovic, S.; Nikolic, T.; Brosens, J.J.; Mulac-Jericevic, B. Analysis of heart and neural crest derivatives-expressed protein 2 (HAND2)-progesterone interactions in peri-implantation endometrium. Biol. Reprod. 2020, 102, 1111–1121. [Google Scholar] [CrossRef]
- Oestreich, A.K.; Chadchan, S.B.; Medvedeva, A.; Lydon, J.P.; Jungheim, E.S.; Moley, K.H.; Kommagani, R. The autophagy protein, FIP200 (RB1CC1) mediates progesterone responses governing uterine receptivity and decidualization. Biol. Reprod. 2020, 102, 843–851. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Che, S.; Cui, J.; Ma, X.; An, X.; Cao, B.; Song, Y. Endometrial Epithelial Cell Apoptosis Is Inhibited by a ciR8073-miR181a-Neurotensis Pathway during Embryo Implantation. Mol. Ther.-Nucleic Acids 2019, 14, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wang, A.H.; Wu, Q.X.; Sheng, H.X.; Jin, Y.P. Establishment and Characteristics of Immortal Goat Endometrial Epithelial Cells and Stromal Cells with hTERT. J. Anim. Vet. Adv. 2010, 9, 2738–2747. [Google Scholar] [CrossRef]
- Dong, F.; Huang, Y.; Li, W.; Zhao, X.; Zhang, W.; Du, Q.; Zhang, H.; Song, X.; Tong, D. The isolation and characterization of a telomerase immortalized goat trophoblast cell line. Placenta 2013, 34, 1243–1250. [Google Scholar] [CrossRef]
- Wang, X.; Lin, P.; Yin, Y.; Zhou, J.; Lei, L.; Zhou, X.; Jin, Y.; Wang, A. Brucella suis vaccine strain S2-infected immortalized caprine endometrial epithelial cell lines induce non-apoptotic ER-stress. Cell Stress Chaperones 2015, 20, 399–409. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Tang, K.; Zhou, D.; Wang, A.; et al. Apoptosis inducing factor gene depletion inhibits zearalenone-induced cell death in a goat Leydig cell line. Reprod. Toxicol. 2017, 67, 129–139. [Google Scholar] [CrossRef]
- Antoniotti, G.S.; Coughlan, M.; Salamonsen, L.A.; Evans, J. Obesity associated advanced glycation end products within the human uterine cavity adversely impact endometrial function and embryo implantation competence. Hum. Reprod. 2018, 33, 654–665. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Wang, A.; Jin, Y. Knock-down of apoptosis inducing factor gene protects endoplasmic reticulum stress-mediated goat granulosa cell apoptosis. Theriogenology 2017, 88, 89–97. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Lin, P.; Jiang, T.; Wang, N.; Zhao, F.; Chen, H.; Tang, K.; Zhou, D.; Wang, A.; et al. An immortalized steroidogenic goat granulosa cell line as a model system to study the effect of the endoplasmic reticulum (ER)-stress response on steroidogenesis. J. Reprod. Dev. 2017, 63, 27–36. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Ma, X.; Zhou, Z.; Song, Y.; Cao, B. miR-26a promoted endometrial epithelium cells (EECs) proliferation and induced stromal cells (ESCs) apoptosis via the PTEN-PI3K/AKT pathway in dairy goats. J. Cell. Physiol. 2018, 233, 4688–4706. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M.B.H.; Niknafs, B.; Seghinsara, A.M.; Shokrzadeh, N.; Alivand, M.R. Administration of dexamethasone disrupts endometrial receptivity by alteration of expression of miRNA 223, 200a, LIF, Muc1, SGK1, and ENaC via the ERK1/2-mTOR pathway. J. Cell. Physiol. 2019, 234, 19629–19639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chauhan, L.; Barry, A.T.; Abudureyimu, A.; Oguejiofor, C.F.; Chen, X.; Wathes, D.C. Acute bovine viral diarrhoea virus infection inhibits expression of interferon tau-stimulated genes in bovine endometrium. Biol. Reprod. 2017, 96, 1142–1153. [Google Scholar] [CrossRef]
- Imakawa, K.; Imai, M.; Sakai, A.; Suzuki, M.; Nagaoka, K.; Sakai, S.; Lee, S.R.; Chang, K.T.; Echternkamp, S.E.; Christenson, R.K. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol. Reprod. Dev. 2006, 73, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, R.; Hayashi, K.G.; Fujii, S.; Kanahara, H.; Hosoe, M.; Furusawa, T.; Kizaki, K. Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy. Int. J. Mol. Sci. 2017, 18, 742. [Google Scholar] [CrossRef]
- Kaneko, Y.; Day, M.L.; Murphy, C.R. Uterine epithelial cells: Serving two masters. Int. J. Biochem. Cell Biol. 2013, 45, 359–363. [Google Scholar] [CrossRef]
- Monsivais, D.; Clementi, C.; Peng, J.; Titus, M.M.; Barrish, J.P.; Creighton, C.J.; Lydon, J.P.; DeMayo, F.J.; Matzuk, M.M. Uterine ALK3 is essential during the window of implantation. Proc. Natl. Acad. Sci. USA 2016, 113, E387–E395. [Google Scholar] [CrossRef]
- Boruszewska, D.; Kowalczyk-Zieba, I.; Sinderewicz, E.; Grycmacher, K.; Staszkiewicz, J.; Woclawek-Potocka, I. The effect of lysophosphatidic acid together with interferon tau on the global transcriptomic profile in bovine endometrial cells. Theriogenology 2017, 92, 111–120. [Google Scholar] [CrossRef]
- Rarani, F.Z.; Borhani, F.; Rashidi, B. Endometrial pinopode biomarkers: Molecules and microRNAs. J. Cell. Physiol. 2018, 233, 9145–9158. [Google Scholar] [CrossRef]
- Liang, Y.X.; Liu, L.; Jin, Z.Y.; Liang, X.H.; Fu, Y.S.; Gu, X.W.; Yang, Z.M. The high concentration of progesterone is harmful for endometrial receptivity and decidualization. Sci. Rep. 2018, 8, 712. [Google Scholar] [CrossRef]
- Banerjee, P.; Malik, A.; Malhotra, S.S.; Gupta, S.K. Role of STAT signaling and autocrine action of chemokines during H2 O 2 induced HTR-8/SVneo trophoblastic cells invasion. J. Cell. Physiol. 2019, 234, 1380–1397. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, Q.; Liu, K.; Li, Z.; Fu, F.; Zhang, K.; Zhang, H.; Zheng, M.; Zhao, Y.; Zhang, S. FGFR2/STAT3 Signaling Pathway Involves in the Development of MMTV-Related Spontaneous Breast Cancer in TA2 Mice. Front. Oncol. 2020, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Sousa, F.L.; Chaiwangyen, W.; Morales-Prieto, D.M.; Ospina-Prieto, S.; Weber, M.; Photini, S.M.; Sass, N.; Daher, S.; Schleussner, E.; Markert, U.R. Involvement of STAT1 in proliferation and invasiveness of trophoblastic cells. Reprod. Biol. 2017, 17, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hindupur, S.V.; Schmid, S.C.; Koch, J.A.; Youssef, A.; Baur, E.M.; Wang, D.; Horn, T.; Slotta-Huspenina, J.; Gschwend, J.E.; Holm, P.S.; et al. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 1106. [Google Scholar] [CrossRef] [PubMed]



| shRNA | Sequence (Loop in Bold Letters) (5′ to 3′) |
|---|---|
| shBCL2L15-1 | GATCCGGTATCGAACACCAACTAAGCCTCGAGGCTTAGTTGGTGTTCGATACCTTTTTG AATTCAAAAAGCTGGGTGACAAATTCAATGGCTCGAGCCATTGAATTTGTCACCCAGCG |
| shBCL2L15-2 | GATCCATTGGAAGCTTCTGCCAGAAACTCGAGTTTCTGGCAGAAGCTTCCAATTTTTTG AATTCAAAAAATTGGAAGCTTCTGCCAGAAACTCGAGTTTCTGGCAGAAGCTTCCAATG |
| shN | GATCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG AATTCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAG |
| Gene | Sequences (5′→3′) | References or GenBank Accession Number |
|---|---|---|
| BCL2L15 | Forward: ctgtcctgccacgttaggat Reverse: tctctcagcaatgcctggt | XM_018045934.1 |
| ISG15 | Forward: ggtgaggaacgacaagggtc Reverse: cagaattggtccgcttgcac | XM_005690795.3 |
| CXCL10 | Forward: ggttttcttattttctgccttat Reverse: atccattactgatctcgatgc | NM_001285721.1 |
| RSAD2 | Forward: tgcttggtgcccgagtctaac Reverse: tccgcccatttctacagttca | XM_018055702.1 |
| PGR | Forward: aagccagccagagcccacagt Reverse: tgcaatcgtttcttccagcacata | XM_018059880.1 |
| ESR1 | Forward: atcaactgggcaaagagggtg Reverse: aggttgggagcaaataggagc | XM_018053363.1 |
| IFNAR1 | Forward: aacctccttcctctgttgacg Reverse: ttgggaattgtactcttcgtg | XM_018046572.1 |
| IFNAR2 | Forward: cagcctcgtatttggtatttc Reverse: cagtccttgacgaccttcata | XM_018046609.1 |
| ITGB1 | Forward: tccctaagtcagcggtaggaa Reverse: tccggtaatttgctgtcctcc | NM_001285667.1 |
| ITGB3 | Forward: acggtgagcttcagcattga Reverse: acaccccacactcaaaggtc | XM_018047091.1 |
| ITGB5 | Forward: cccacgagaaggctacttgg Reverse: ttcaacaggcgtctcgatcc | XM_018047092.1 |
| SPP1 | Forward: tgagaattgcagtgatttgc Reverse: tgagatgggtcaggctttag | XM_005680968.3 |
| HOXA10 | Forward: cttccaaaggcgaaaacgca Reverse: gtctggtgcttggtgtaggg | XM_018047091.1 |
| HOXA11 | Forward: cagattcgggagctagagcg Reverse: cggtcagtgaggttgagcat | XM_018047092.1 |
| LIF | Forward: cttccccaacaacctgga Reverse: gcgatgatgcgatacagc | XM_005691625.3 |
| GAPDH | Forward: gatggtgaaggtcggagtgaac Reverse: gtcattgatggcgacgatgt | XM_005680968.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Liu, A.; Wu, Y.; Li, B.; Nan, S.; Yin, R.; Zhu, H.; Chen, J.; Ding, Y.; Ding, M. BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway. Genes 2020, 11, 816. https://doi.org/10.3390/genes11070816
Yang D, Liu A, Wu Y, Li B, Nan S, Yin R, Zhu H, Chen J, Ding Y, Ding M. BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway. Genes. 2020; 11(7):816. https://doi.org/10.3390/genes11070816
Chicago/Turabian StyleYang, Diqi, Ai Liu, Yanqin Wu, Bin Li, Sha Nan, Ruiling Yin, Hongmei Zhu, Jianguo Chen, Yi Ding, and Mingxing Ding. 2020. "BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway" Genes 11, no. 7: 816. https://doi.org/10.3390/genes11070816
APA StyleYang, D., Liu, A., Wu, Y., Li, B., Nan, S., Yin, R., Zhu, H., Chen, J., Ding, Y., & Ding, M. (2020). BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway. Genes, 11(7), 816. https://doi.org/10.3390/genes11070816





