The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Instruments (Genetic Associations with Testosterone)
2.2. Genetic Association with Outcomes
2.3. Statistical Analysis
3. Results
3.1. Genetic Instruments
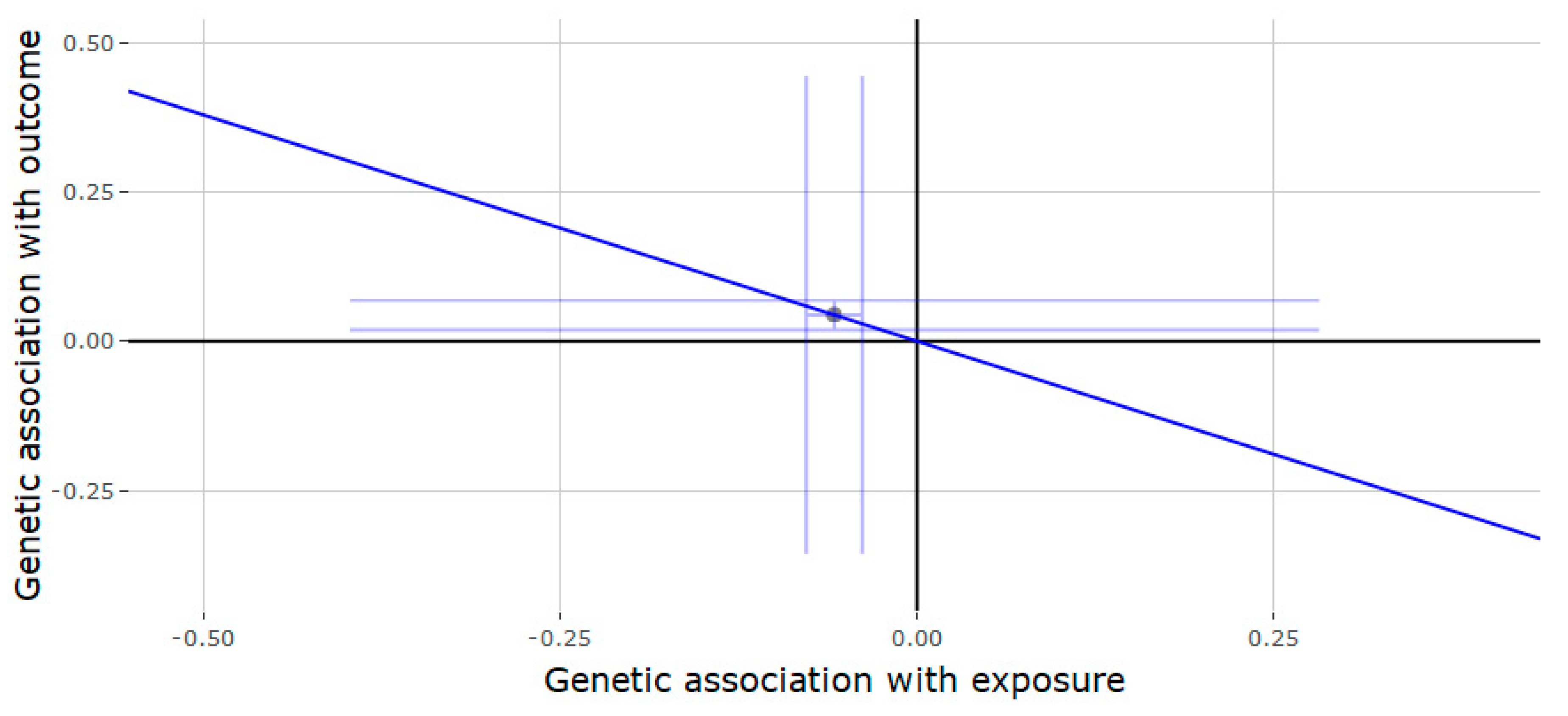
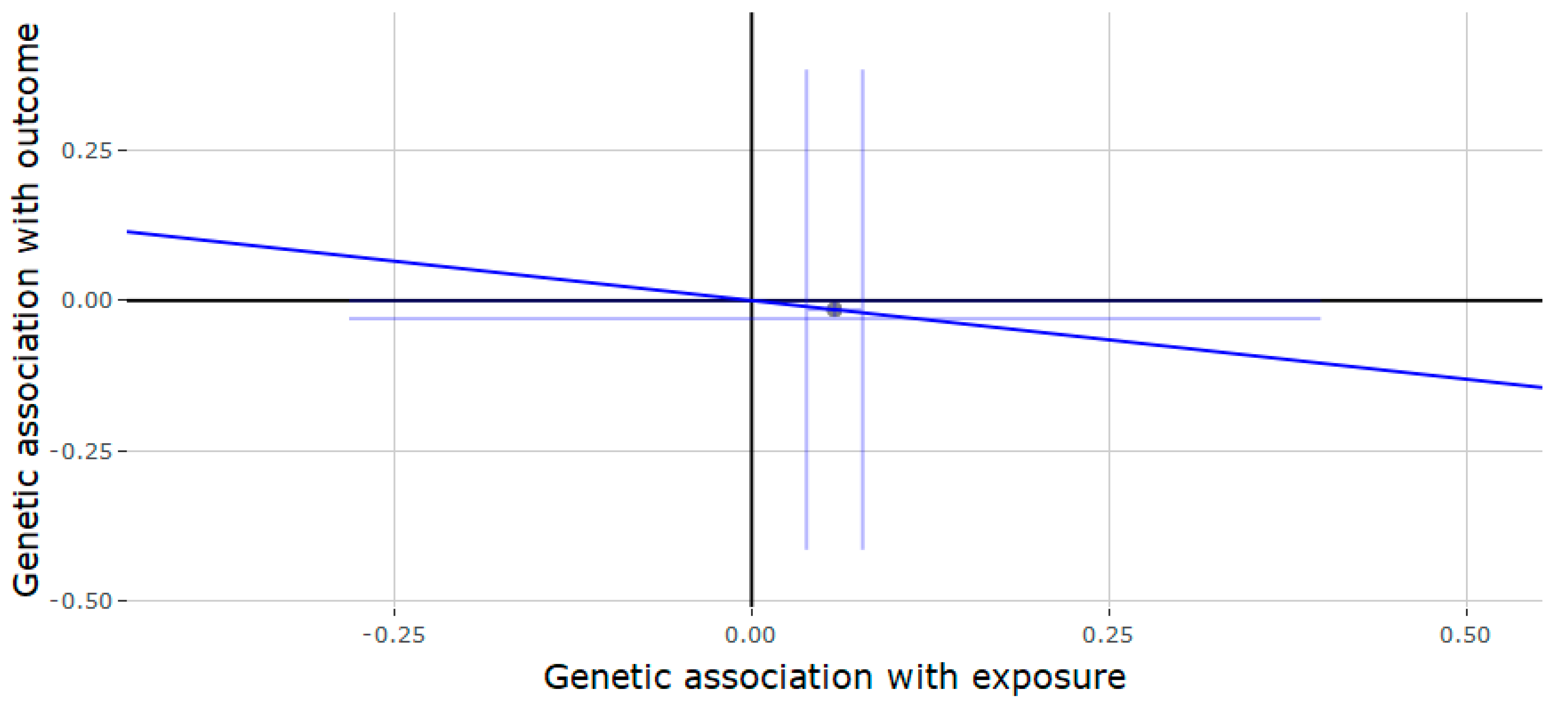
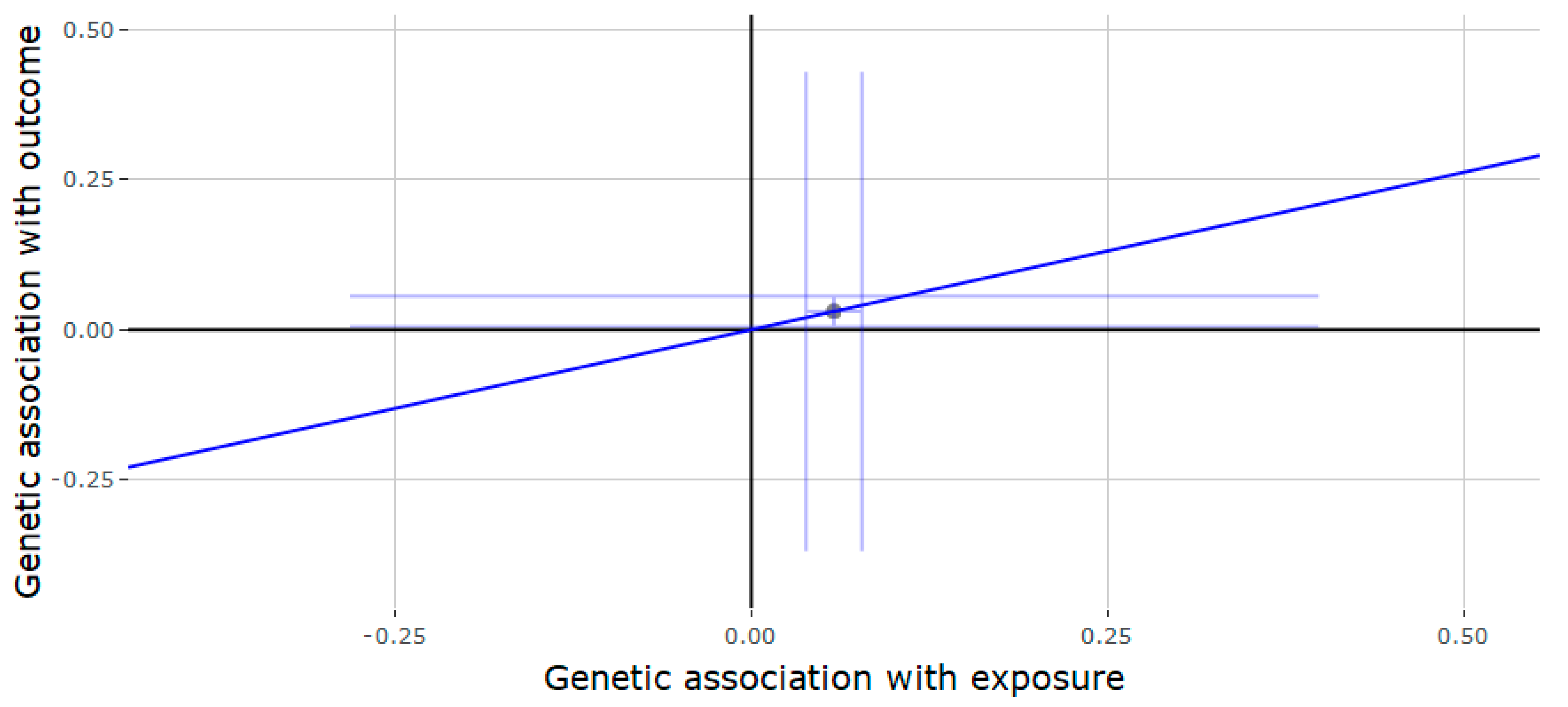
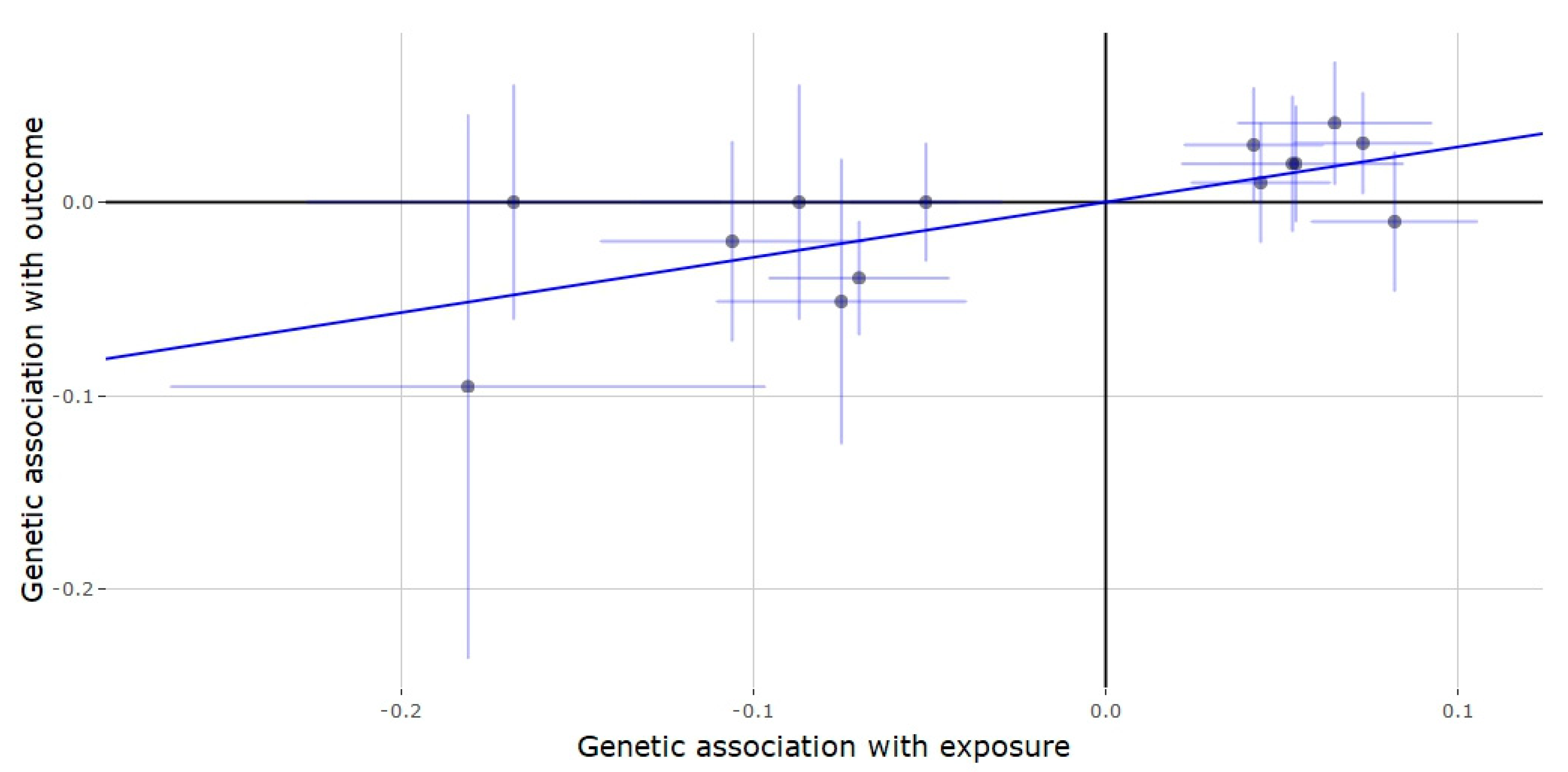
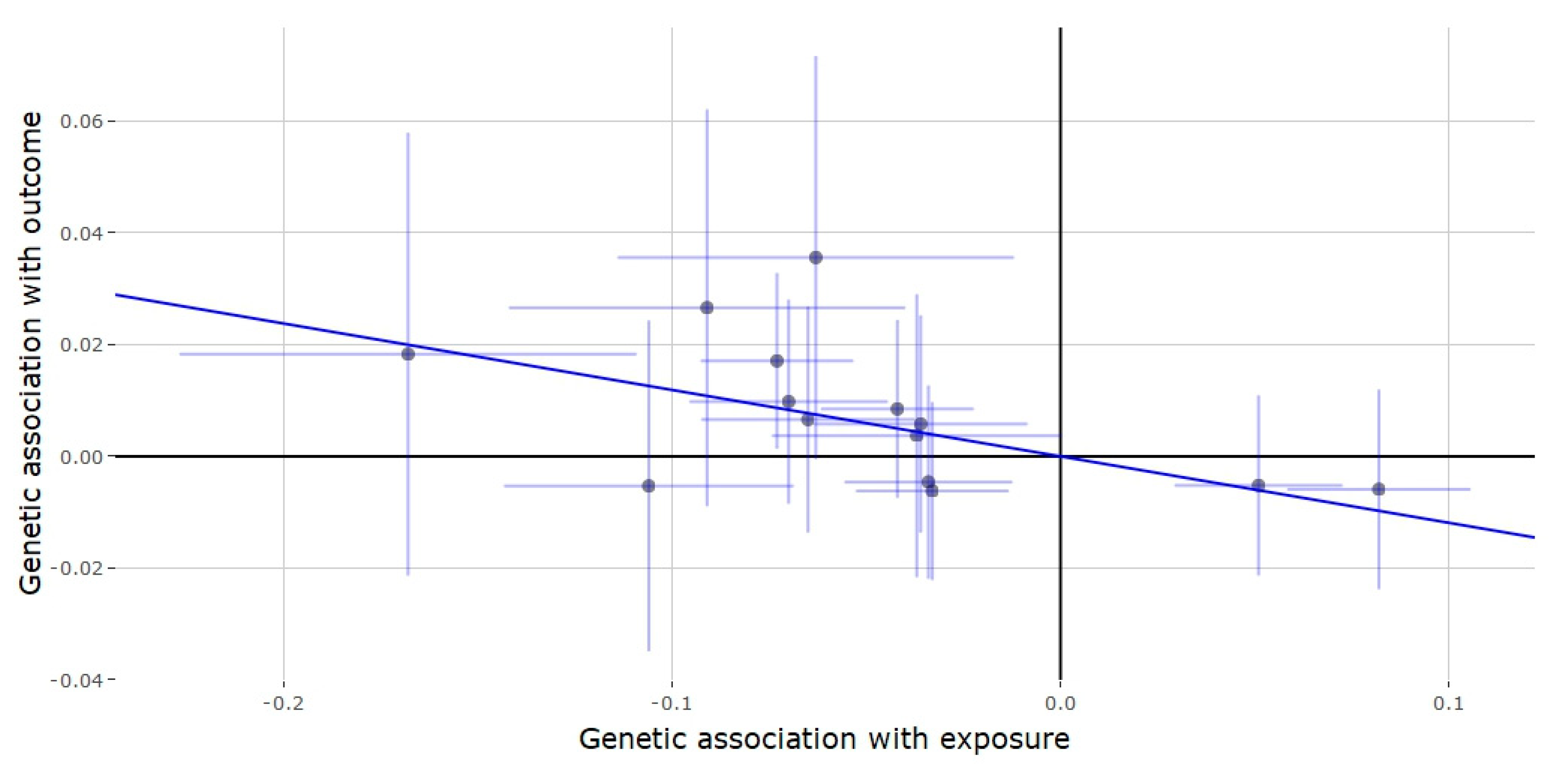
3.2. Mendelian Randomization of the JMJD1C Locus
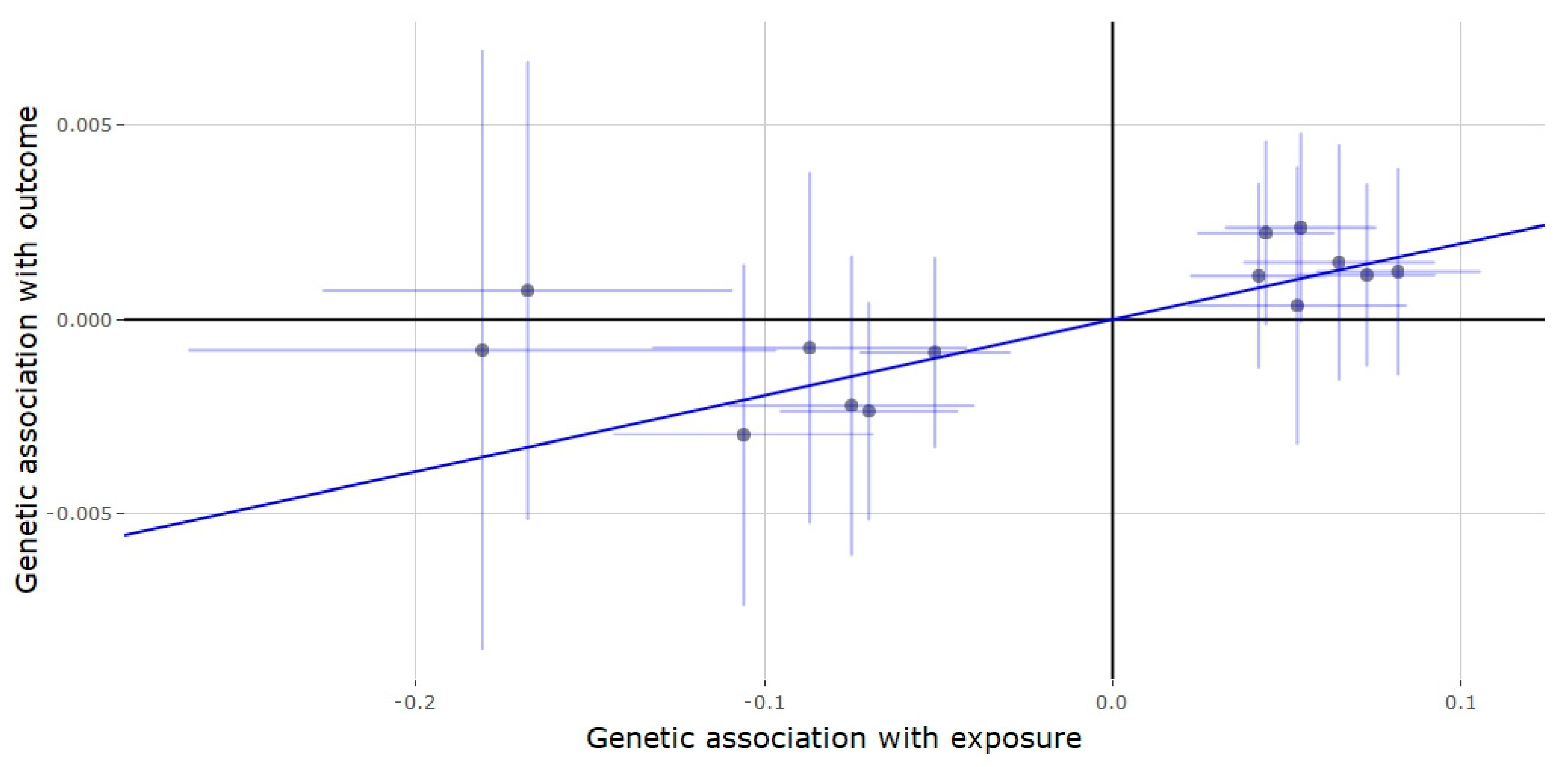
3.3. Mendelian Randomization of the SHBG Locus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological Actions of Androgens. Endocr. Rev. 1987, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Bergstrom, J. Low Serum Testosterone and Mortality in Older Men. J. Clin. Endocrinol. Metab. 2008, 93, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Shores, M.M.; Matsumoto, A.M.; Sloan, K.L.; Kivlahan, D.R. Low Serum Testosterone and Mortality in Male Veterans. Arch. Intern. Med. 2006, 166, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Cobiello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Harman, S.M.; Meteer, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Wu, F.; Tajar, A.; Pye, S.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.; Forti, G.; Giwercman, A.; et al. Hypothalamic-Pituitary-Testicular Axis Disruptions in Older Men Are Differentially Linked to Age and Modifiable Risk Factors: The European Male Aging Study. J. Clin. Endocrinol. Metab. 2008, 93, 2737–2745. [Google Scholar] [CrossRef]
- Mulligan, T.; Frick, M.F.; Zuraw, Q.C.; Stemhagen, A.; McWhirter, C. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int. J. Clin. Pract. 2008, 60, 762–769. [Google Scholar] [CrossRef]
- Layton, J.B.; Li, N.; Meier, C.R.; Sharpless, J.L.; Stürmer, T.; Jick, S.; Brookhart, M.A. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J. Clin. Endocrinol. Metab. 2014, 99, 835–842. [Google Scholar] [CrossRef]
- Elliott, J.; E Kelly, S.; Millar, A.C.; Peterson, J.; Chen, L.; Johnston, A.; Kotb, A.; Skidmore, B.; Bai, Z.; Mamdani, M.; et al. Testosterone therapy in hypogonadal men: A systematic review and network meta-analysis. BMJ Open 2017, 7, e015284. [Google Scholar]
- Canguven, O.; Talib, R.A.; El Ansari, W.; Yassin, D.; Salman, M.; Al-Ansari, A. Testosterone therapy has positive effects on anthropometric measures, metabolic syndrome components (obesity, lipid profile, Diabetes Mellitus control), blood indices, liver enzymes, and prostate health indicators in elderly hypogonadal men. Andrologia 2017, 49, e12768. [Google Scholar] [CrossRef]
- Gagliano-Jucá, T.; Basaria, S. Testosterone replacement therapy and cardiovascular risk. Nat. Rev. Cardiol. 2019, 16, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Finkle, W.D.; Greenland, S.; Ridgeway, G.; Adams, J.L.; Frasco, M.; Cook, M.B.; Fraumeni, J.F.; Hoover, R.N. Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men. PLoS ONE 2014, 9, e85805. [Google Scholar] [CrossRef] [PubMed]
- Vigen, R.; O’Donnell, C.I.; Barón, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of Testosterone Therapy with Mortality, Myocardial Infarction, and Stroke in Men With Low Testosterone Levels. JAMA 2013, 310, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.M.; Archer, J. Testosterone and human behavior: The role of individual and contextual variables. Curr. Opin. Psychol. 2018, 19, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.-C.; Dunne, S.; Pazderska, A.; Frodl, T.; Nolan, J.J.; O’Doherty, J.P. Testosterone causes both prosocial and antisocial status-enhancing behaviors in human males. Proc. Natl. Acad. Sci. USA 2016, 113, 11633–11638. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.T.; Hildreth, K.L.; Pelak, V.S. Effects of Testosterone Therapy on Cognitive Function in Aging. Cogn. Behav. Neurol. 2016, 29, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Mannucci, E.; Ricca, V.; Lotti, F.; Boddi, V.; Bandini, E.; Balercia, G.; Forti, G.; Maggi, M. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int. J. Androl. 2009, 32, 720–728. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Gann, P.H.; Kopp, P.; Colangelo, L.; Longcope, C.; Liu, K. Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1041–1047. [Google Scholar]
- Jensen, M.B.; Andersson, A.-M.; Jørgensen, N.; Andersen, A.-G.; Carlsen, E.; Petersen, J.H.; Skakkebæk, N.E. Body mass index in relation to semen quality and reproductive hormonesamong 1558 Danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef]
- Svartberg, J.; Von Mühlen, D.; Sundsfjord, J.; Jorde, R. Waist circumference and testosterone levels in community dwelling men. The Tromsø study. Eur. J. Epidemiol. 2004, 19, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.V.; Jiang, C.; Lam, T.H.; Liu, B.; Cheng, K.; Xu, L.; Yeung, S.L.A.; Zhang, W.; Leung, G.; Schooling, M. Genetically predicted testosterone and cardiovascular risk factors in men: A Mendelian randomization analysis in the Guangzhou Biobank Cohort Study. Int. J. Epidemiol. 2013, 43, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.V.; Jiang, C.; Lam, T.H.; Liu, B.; Cheng, K.; Xu, L.; Yeung, S.L.A.; Zhang, W.; Leung, G.; Schooling, M. Genetically Predicted Testosterone and Systemic Inflammation in Men: A Separate-Sample Mendelian Randomization Analysis in Older Chinese Men. PLoS ONE 2015, 10, e0126442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.V.; Lam, T.H.; Jiang, C.; Cherny, S.S.; Liu, B.; Cheng, K.K.; Zhang, W.; Leung, G.; Schooling, M. A Mendelian randomization study of testosterone and cognition in men. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- Schooling, M.; Luo, S.; Yeung, S.L.A.; Thompson, D.J.; Karthikeyan, S.; Bolton, T.R.; Mason, A.M.; Ingelsson, E.; Burgess, S. Genetic predictors of testosterone and their associations with cardiovascular disease and risk factors: A Mendelian randomization investigation. Int. J. Cardiol. 2018, 267, 171–176. [Google Scholar] [CrossRef]
- Paruk, I.M.; Pirie, F.J.; Nkwanyana, N.M.; Motala, A.A. Prevalence of low serum testosterone levels among men with type 2 diabetes mellitus attending two outpatient diabetes clinics in KwaZulu-Natal Province, South Africa. S. Afr. Med. J. 2019, 109, 963–970. [Google Scholar] [CrossRef]
- Pui, K.; Waddell, C.; Dalbeth, N. Early onset of hyperuricaemia and gout following treatment for female to male gender reassignment. Rheumatology 2008, 47, 1840–1841. [Google Scholar] [CrossRef]
- Pikwer, M.; Giwercman, A.; Bergström, U.; Nilsson, J.; Jacobsson, L.T.H.; Turesson, C. Association between testosterone levels and risk of future rheumatoid arthritis in men: A population-based case–control study. Ann. Rheum. Dis. 2013, 73, 573–579. [Google Scholar] [CrossRef]
- Amiaz, R.; Seidman, S.N. Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Nead, K.T. Androgens and depression. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wooderson, S.C.; Gallagher, P.; Watson, S.; Young, A.H. An exploration of testosterone levels in patients with bipolar disorder. BJPsych Open 2015, 1, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Du, N.; Liu, Y.; Fan, X.; Wang, Y.; Jia, X.; Hou, X.; Wang, B. Low Testosterone Level and Risk of Alzheimer’s Disease in the Elderly Men: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2015, 53, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Sun, J.; Kim, S.-T.; Feng, J.; Wang, Z.; Tao, S.; Chen, Z.; Purcell, L.; Smith, S.; Isaacs, W.B.; et al. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Hum. Mol. Genet. 2012, 21, 5222–5228. [Google Scholar] [CrossRef]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2013, 506, 376–381. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.-K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1–10. [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Kim, C.; Halter, J.B. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr. Cardiol. Rep. 2014, 16, 467. [Google Scholar] [CrossRef] [PubMed]
- Yialamas, M.A.; Dwyer, A.A.; Hanley, E.; Lee, H.; Pitteloud, N.; Hayes, F.J. Acute Sex Steroid Withdrawal Reduces Insulin Sensitivity in Healthy Men with Idiopathic Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2007, 92, 4254–4259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, P.M.; Kelly, D.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Senmaru, T.; Fukuda, T.; Yamazaki, M.; Shinomiya, K.; Ueno, M.; Kinoshita, S.; Kitawaki, J.; Katsuyama, M.; Tsujikawa, M.; et al. Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 2016, 51, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241–253. [Google Scholar]
- Cai, Z.; Yan, L.-J.; Li, K.; Quazi, S.H.; Zhao, B. Roles of AMP-activated Protein Kinase in Alzheimer’s Disease. Neuromol. Med. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Mol. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Caronia, L.M.; Dwyer, A.A.; Hayden, U.; Amati, F.; Pitteloud, N.; Hayes, F.J. Abrupt decrease in serum testosterone levels after an oral glucose load in men: Implications for screening for hypogonadism. Clin. Endocrinol. 2013, 78, 291–296. [Google Scholar] [CrossRef]
- Seidell, J.C.; Björntorp, P.; Sjöström, L.; Kvist, H.; Sannerstedt, R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990, 39, 897–901. [Google Scholar] [CrossRef]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2013, 220, R37–R55. [Google Scholar] [CrossRef]
- Martens, H.F.; Sheets, P.K.; Tenover, J.S.; E Dugowson, C.; Bremner, W.J.; Starkebaum, G. Decreased testosterone levels in men with rheumatoid arthritis: Effect of low dose prednisone therapy. J. Rheumatol. 1994, 21, 1427–1431. [Google Scholar] [PubMed]
- Spector, T.D.; A Perry, L.; Tubb, G.; Silman, A.J.; Huskisson, E.C. Low free testosterone levels in rheumatoid arthritis. Ann. Rheum. Dis. 1988, 47, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Geenen, R.; Van Middendorp, H.; Bijlsma, J.W. The Impact of Stressors on Health Status and Hypothalamic-Pituitary-Adrenal Axis and Autonomic Nervous System Responsiveness in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2006, 1069, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, M.; Noori, A.; Oveisi, S.; Kheirkhah, M. Association of serum testosterone and dehydroepiandrosterone sulfate with rheumatoid arthritis: A case control study. Electron. Physician 2018, 10, 6500–6505. [Google Scholar] [CrossRef][Green Version]
- Seidman, S.N.; Walsh, B.T. Testosterone and depression in aging men. Am. J. Geriatr. Psychiatry 1999, 7, 18–33. [Google Scholar] [CrossRef]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 2013, 35, 42–57. [Google Scholar] [CrossRef]
- Zarrouf, F.A.; Artz, S.; Griffith, J.; Sirbu, C.; Kommor, M. Testosterone and Depression. Am. J. Geriatr. Psychiatry 2009, 15, 289–305. [Google Scholar] [CrossRef]
| JMJD1CLocus | ||||||
| SNP | Position | Beta | Beta (se) | p-Value | EA | OA |
| rs10822184 | 65007159 | −0.058 | 0.01 | 1.12 × 10−8 | T | C |
| SHBGLocus | ||||||
| SNP | Position | Beta | Beta (se) | p-Value | EA | OA |
| rs727428 | 7478517 | −0.073 | 0.01 | 1.26 × 10−12 | T | C |
| rs1799941 | 7474148 | 0.082 | 0.012 | 1.39 × 10−12 | A | G |
| rs17806566 | 7292887 | −0.168 | 0.03 | 2.61 × 10−8 | C | T |
| rs9913778 | 7474626 | −0.106 | 0.019 | 3.05 × 10−8 | T | C |
| rs9900162 | 7387788 | −0.07 | 0.013 | 6.13 × 10−8 | G | A |
| rs35894069 | 7335900 | 0.054 | 0.011 | 5.74 × 10−7 | A | G |
| rs9908275 | 7367048 | −0.065 | 0.014 | 1.59 × 10−6 | T | C |
| rs4511593 | 7396260 | 0.051 | 0.011 | 1.73 × 10−6 | C | T |
| rs55784804 | 7477185 | −0.075 | 0.018 | 2.25 × 10−5 | T | G |
| rs3853818 | 7287026 | −0.044 | 0.01 | 2.36 × 10−5 | T | C |
| rs12944954 | 7425855 | −0.181 | 0.043 | 2.88 × 10−5 | G | A |
| rs55894190 | 7323962 | −0.042 | 0.01 | 6.90 × 10−5 | C | T |
| rs8069501 | 7335692 | −0.087 | 0.023 | 1.52 × 10−4 | G | A |
| rs858517 | 7474996 | −0.091 | 0.026 | 4.44 × 10−4 | C | T |
| rs2955611 | 7490299 | −0.053 | 0.016 | 9.08 × 10−4 | C | A |
| rs12942088 | 7423503 | −0.033 | 0.01 | 1.59 × 10−3 | C | T |
| rs2302762 | 7299585 | −0.034 | 0.011 | 3.06 × 10−3 | T | C |
| rs12936934 | 7441490 | −0.036 | 0.014 | 9.74 × 10−3 | T | C |
| rs4968211 | 7399786 | −0.063 | 0.026 | 1.4 × 10−2 | A | G |
| rs4796305 | 7276779 | −0.037 | 0.019 | 4.6 × 10−2 | G | T |
| Outcome | OR | Estimate (se) | p-Value |
|---|---|---|---|
| Alzheimer’s Disorder | 0.875 | −0.133 (0.145) | 0.359 |
| Bipolar Disorder | 1.402 | 0.338 (0.234) | 0.149 |
| Schizophrenia | 1.132 | 0.124 (0.182) | 0.496 |
| Depression | 0.984 | −0.016 (0.020) | 0.437 |
| Rheumatoid Arthritis | 1.690 | 0.525 (0.226) | 0.020 |
| Gout | 0.469 | −0.757 (0.221) | 0.001 |
| Type 2 Diabetes | 0.769 | −0.262 (0.133) | 0.048 |
| Outcome | OR | Beta (se) | p-Value | MR-Egger Intercept Estimate (p-Value) |
|---|---|---|---|---|
| Alzheimer’s Disorder | 0.998 | −0.002 (0.029) | 0.935 | 0.013 (0.015) |
| Bipolar Disorder | 0.991 | −0.009 (0.085) | 0.920 | 0.007 (0.664) |
| Schizophrenia | 1.038 | 0.038 (0.053) | 0.176 | −0.004 (0.672) |
| Depression | 1.02 | 0.020 (0.006) | 0.001 | 0.002 (0.092) |
| Rheumatoid Arthritis | 1.329 | 0.285 (0.060) | <0.001 | 0.022 (0.120) |
| Gout | 0.971 | −0.029 (0.064) | 0.649 | 0.005 (0.683) |
| Type 2 Diabetes | 0.887 | −0.119 (0.030) | 0.003 | −0.027 (0.607) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, A.A.S.; He, L.; Shi, Y. The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Randomization Study. Genes 2020, 11, 721. https://doi.org/10.3390/genes11070721
Syed AAS, He L, Shi Y. The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Randomization Study. Genes. 2020; 11(7):721. https://doi.org/10.3390/genes11070721
Chicago/Turabian StyleSyed, Ali Alamdar Shah, Lin He, and Yongyong Shi. 2020. "The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Randomization Study" Genes 11, no. 7: 721. https://doi.org/10.3390/genes11070721
APA StyleSyed, A. A. S., He, L., & Shi, Y. (2020). The Potential Effect of Aberrant Testosterone Levels on Common Diseases: A Mendelian Randomization Study. Genes, 11(7), 721. https://doi.org/10.3390/genes11070721






