Analysis of Long Noncoding RNA and mRNA Expression Profiles of Testes with High and Low Sperm Motility in Domestic Pigeons (Columba livia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sampling
2.3. LncRNA Library Construction and Sequencing
2.4. Quality Control and Mapping
2.5. LncRNA Identification
2.6. Different Expression Analysis of mRNAs and lncRNAs
2.7. Target Gene Prediction and Functional Analysis of LncRNAs
2.8. GO and KEGG Enrichment Analysis
2.9. qPCR Validation
3. Results
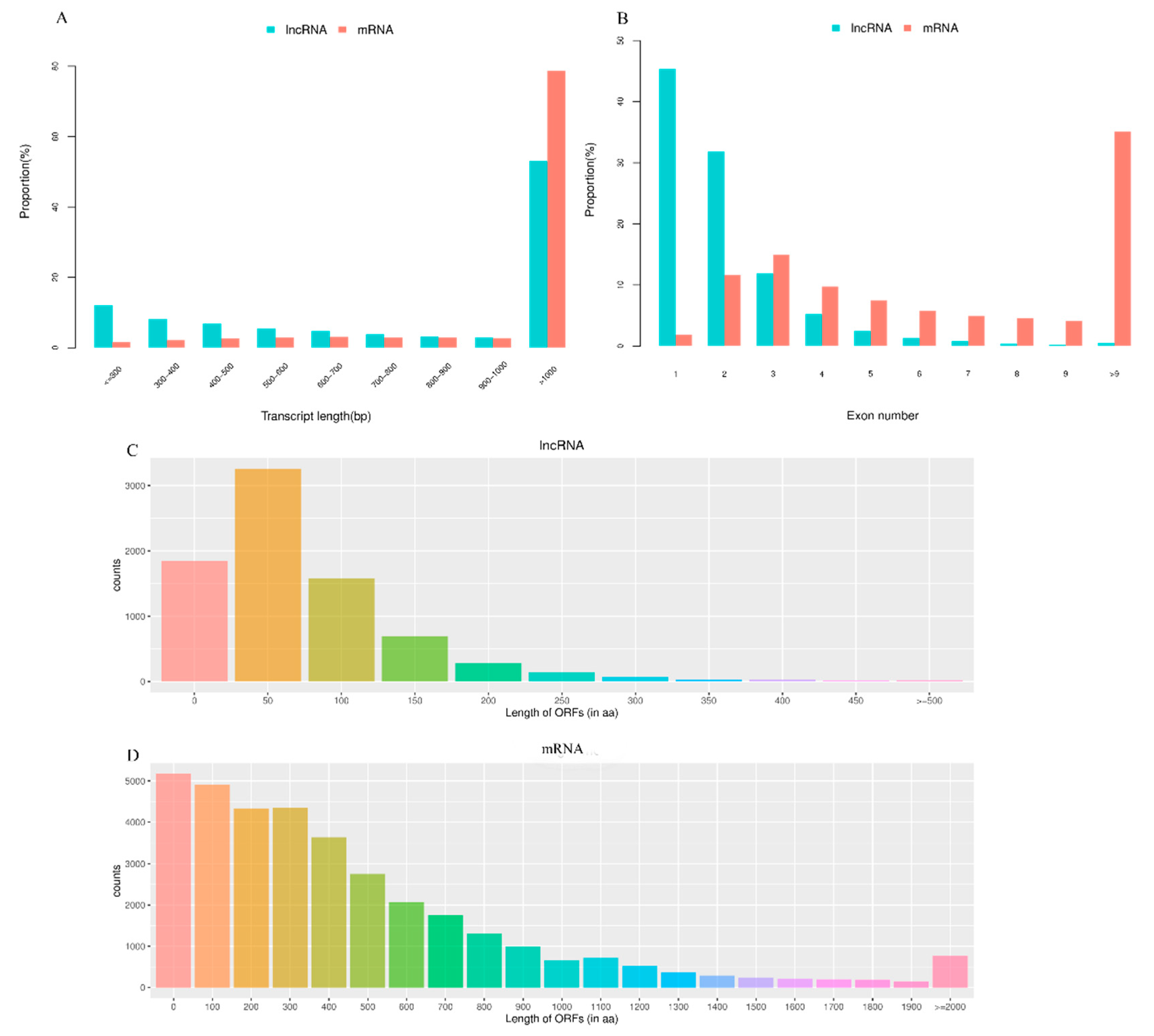
3.1. RNA Sequencing and Identification of LncRNAs and mRNAs in Pigeon Testes
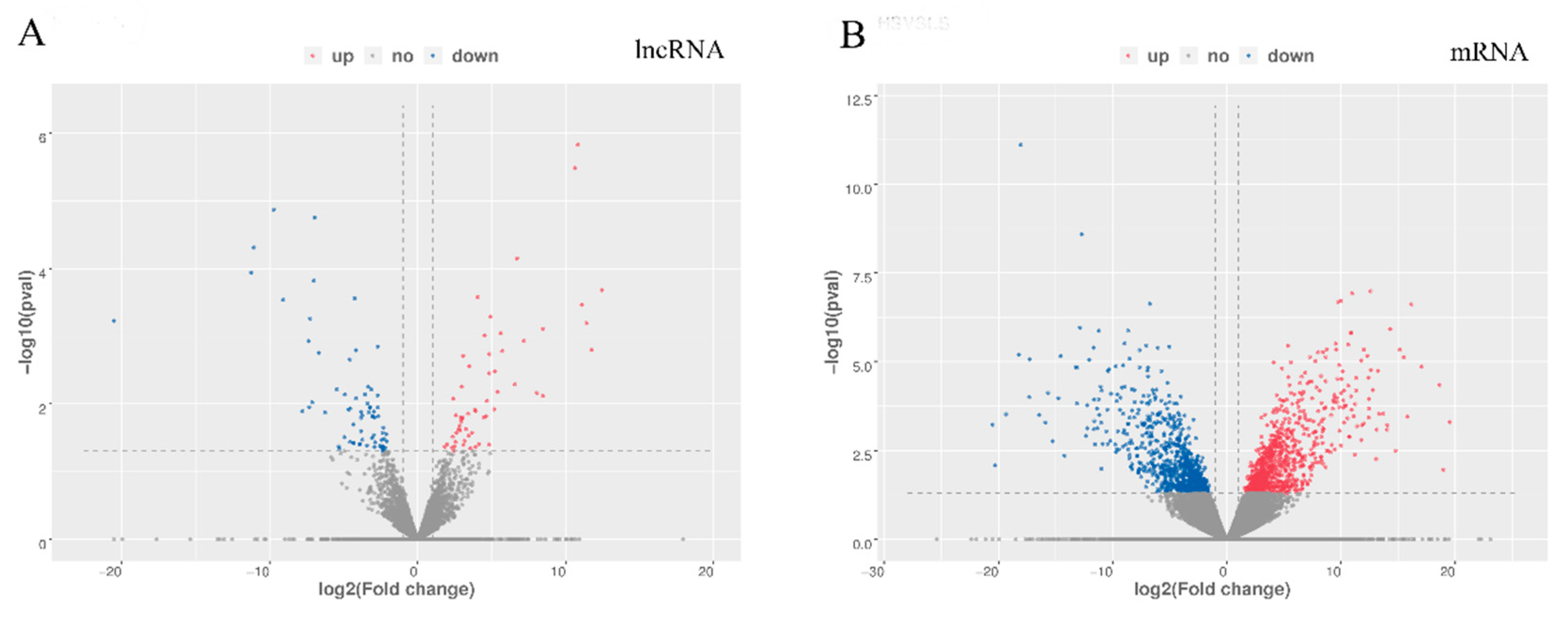
3.2. Differentially Expressed mRNAs and LncRNAs
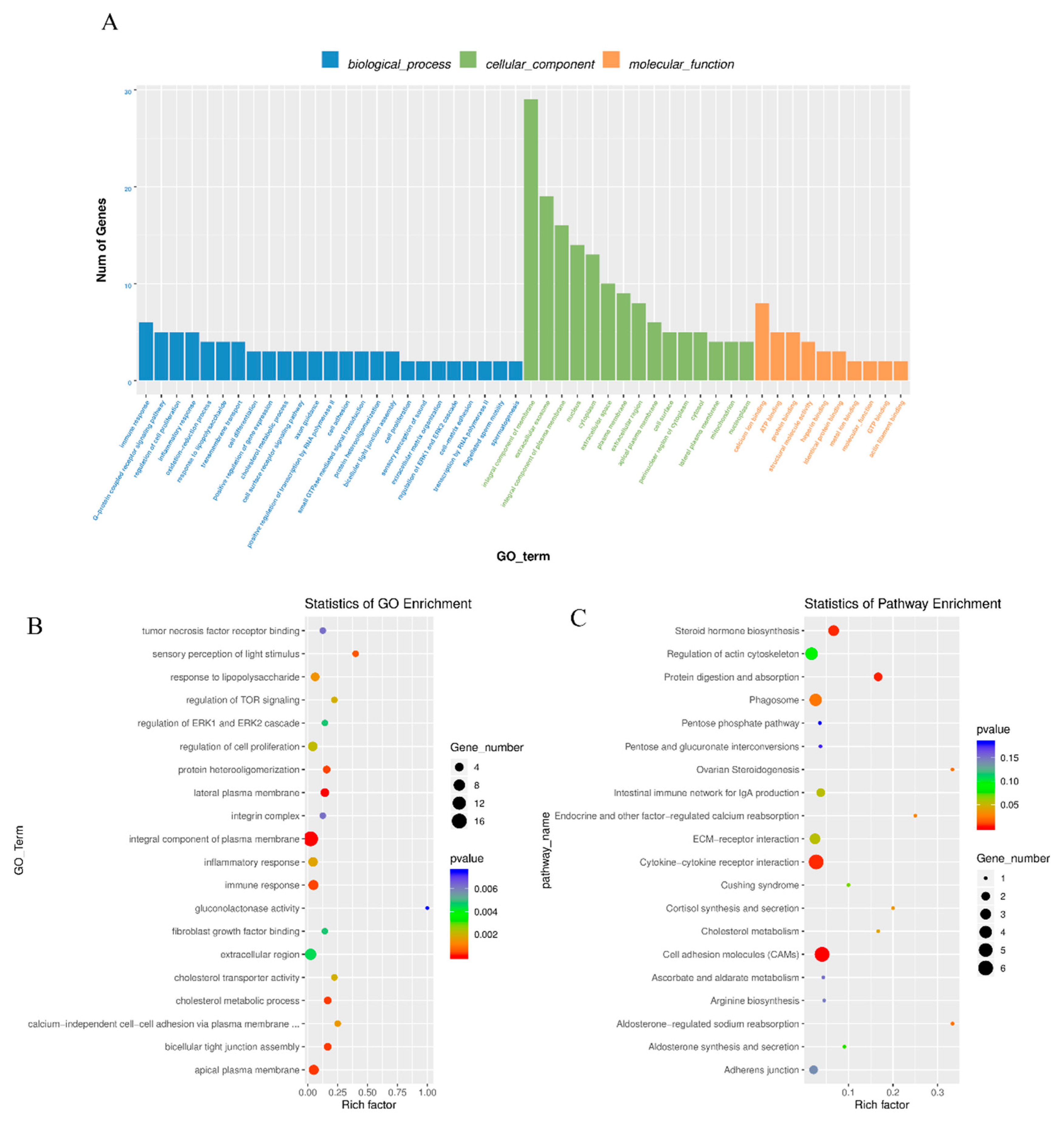
3.3. Enrichment Analysis of Differentially Expressed mRNAs
3.4. Cis-Regulatory Roles of Differentially Expressed LncRNAs in Testes
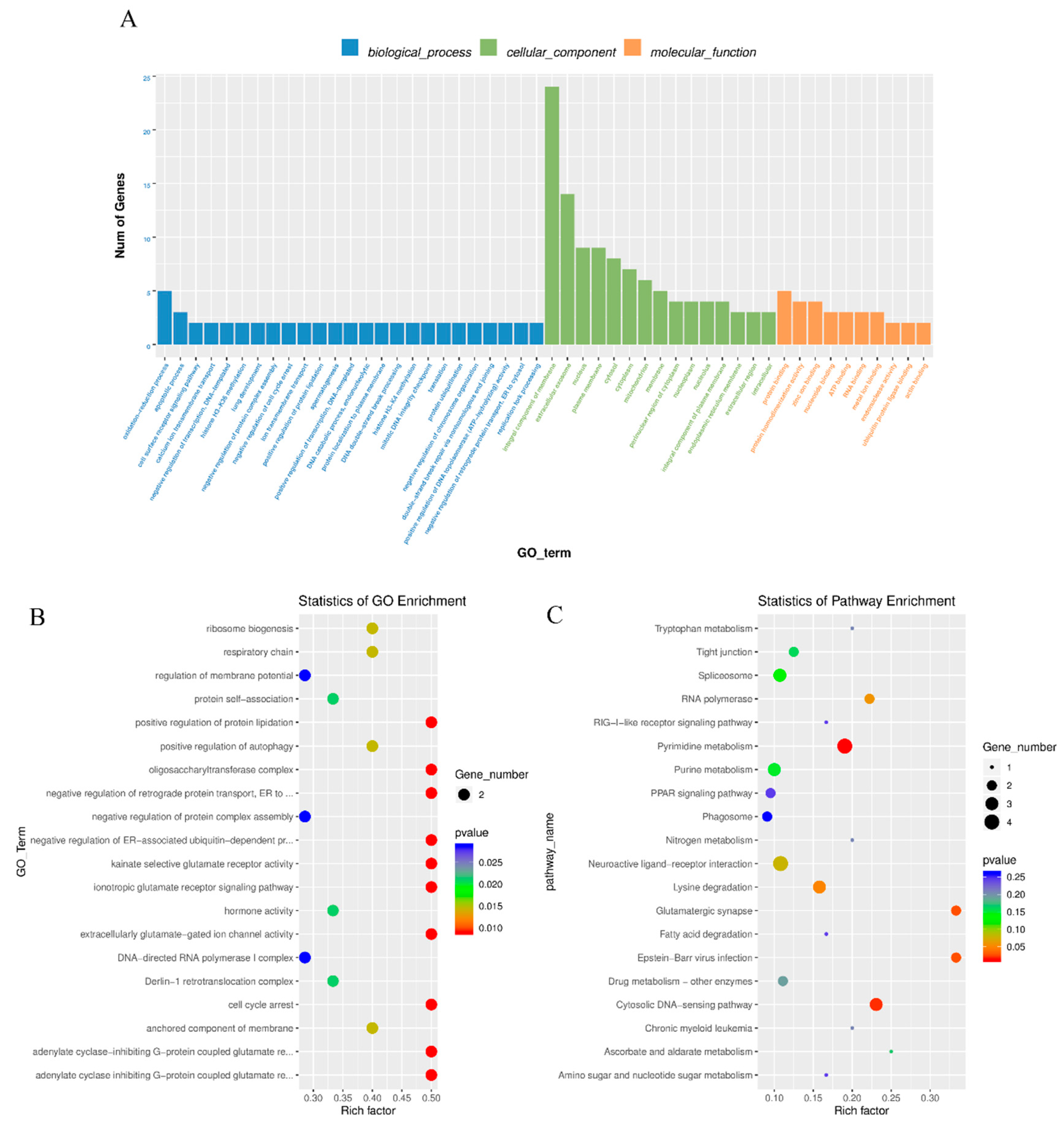
3.5. Co-Enriched GO Terms of DE LncRNA and mRNA
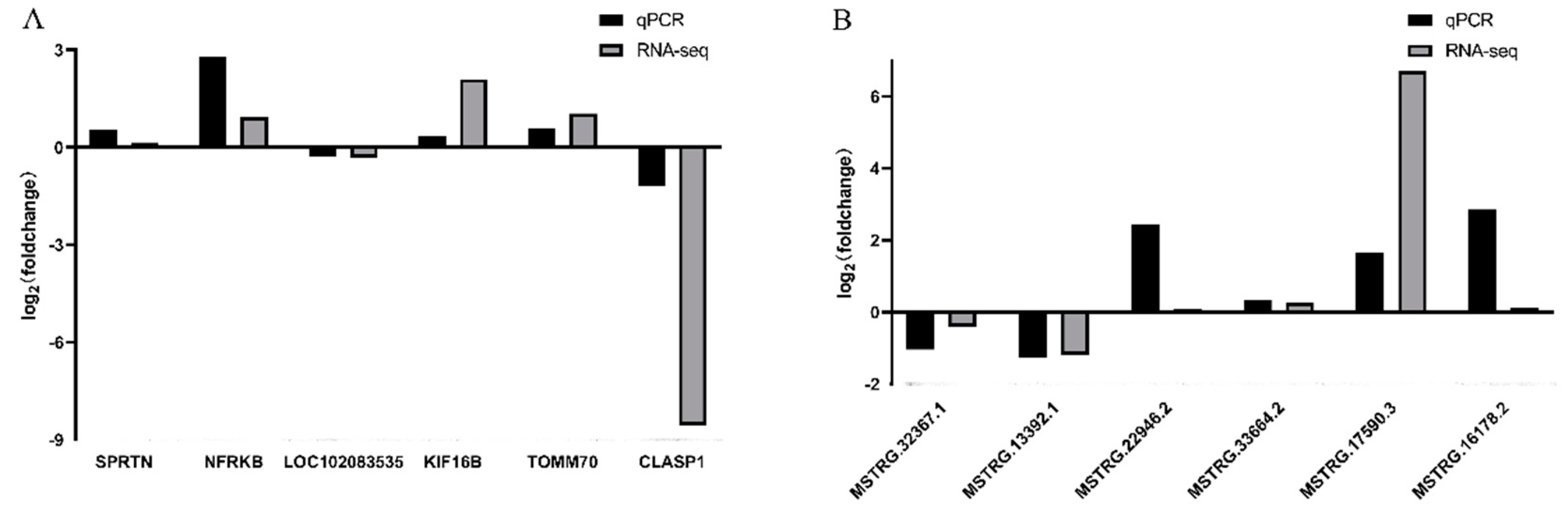
3.6. Verification of Differentially Expressed LncRNAs and mRNAs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Froman, D.P.; Feltmann, A.J. Sperm mobility: A quantitative trait of the domestic fowl (Gallus domesticus). Biol. Reprod. 1998, 58, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froman, D.P.; Pizzari, T.; Feltmann, A.J.; Castillo-Juarez, H.; Birkhead, T.R. Sperm mobility: Mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc. Biol. Sci. 2002, 269, 607–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.; Sa, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, H.; Wu, Y.; Zheng, X. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci. China Life Sci. 2013, 56, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.H.; Chu, E.T.; Spektor, R.; Soloway, P.D. Long non-coding RNA regulation of reproduction and development. Mol. Reprod. Dev. 2015, 82, 932–956. [Google Scholar] [CrossRef] [Green Version]
- Rolland, A.D.; Evrard, B.; Darde, T.A.; Le Beguec, C.; Le Bras, Y.; Bensalah, K.; Lavoue, S.; Jost, B.; Primig, M.; Dejucq-Rainsford, N.; et al. RNA profiling of human testicular cells identifies syntenic lncRNAs associated with spermatogenesis. Hum. Reprod. 2019, 34, 1278–1290. [Google Scholar] [CrossRef]
- Luk, A.C.; Chan, W.Y.; Rennert, O.M.; Lee, T.L. Long noncoding RNAs in spermatogenesis: Insights from recent high-throughput transcriptome studies. Reproduction 2014, 147, R131–R141. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Li, L.; Liao, Y.; Liang, M. LncRNA Gm2044 highly expresses in spermatocyte and inhibits Utf1 translation by interacting with Utf1 mRNA. Genes Genom. 2018, 40, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Yang, L.; Xiong, T.; Di, C.; Ma, D.; Wu, M.; Xue, Z.; Zhang, X.; Long, L.; Zhang, W.; et al. Critical roles of long noncoding RNAs in Drosophila spermatogenesis. Genome Res. 2016, 26, 1233–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Kyi-Tha-Thu, C.; Takizawa, T.; Naing, B.T.; Takizawa, T. 1700108J01Rik and 1700101O22Rik are mouse testis-specific long non-coding RNAs. Histochem. Cell Biol. 2018, 149, 517–527. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Aghaei, A. Effect of l-carnitine on sperm quality during liquid storage of chicken semen. Comp. Clin. Pathol. 2011, 21, 711–717. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Li, J.; Zhu, B.; Zhu, H.; Luo, Y.; Wang, Q.; Zuo, J. Analysis of long-non-coding RNAs associated with ethylene in tomato. Gene 2018, 674, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoov, B.; Ben-Barak, J.; Mayevsky, A.; Sneider, M.; Yogev, L.; Lightman, A. Sperm motility index: A new parameter for human sperm evaluation. Fertil. Steril. 1991, 56, 108–112. [Google Scholar] [CrossRef]
- Wishart, G.J.; Palmer, F.H. Correlation of the fertilising ability of semen from individual male fowls with sperm motility and ATP content. Br. Poult. Sci. 1986, 27, 97–102. [Google Scholar] [CrossRef]
- Kamar, G.A.R. The Influence of Semen Characteristics on Hatching Results of Chicken Eggs. Poult. Sci. 1960, 39, 188–192. [Google Scholar] [CrossRef]
- Liu, K.S.; Li, T.P.; Ton, H.; Mao, X.D.; Chen, Y.J. Advances of Long Noncoding RNAs-mediated Regulation in Reproduction. Chin. Med. J. (Engl.) 2018, 131, 226–234. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Xu, E.Y. LncRNA, a new component of expanding RNA-protein regulatory network important for animal sperm development. Semin. Cell Dev. Biol. 2016, 59, 110–117. [Google Scholar] [CrossRef]
- Sun, J.; Lin, Y.; Wu, J. Long non-coding RNA expression profiling of mouse testis during postnatal development. PLoS ONE 2013, 8, e75750. [Google Scholar] [CrossRef]
- Weng, B.; Ran, M.; Chen, B.; He, C.; Dong, L.; Peng, F. Genome-wide analysis of long non-coding RNAs and their role in postnatal porcine testis development. Genomics 2017, 109, 446–456. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Li, Y.; Bai, H.; Xue, F.; Xu, S.; Xu, H.; Shi, L.; Yang, N.; Chen, J. Analyses of Long Non-Coding RNA and mRNA profiling using RNA sequencing in chicken testis with extreme sperm motility. Sci. Rep. 2017, 7, 9055. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liang, Z.; Yang, J.; Wang, D.; Wang, H.; Zhu, M.; Geng, B.; Xu, E.Y. DAZL is a master translational regulator of murine spermatogenesis. Natl. Sci. Rev. 2019, 6, 455–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrans-Stassen, B.H.G.J.; Saunders, P.T.K.; Cooke, H.J.; de Rooij, D.G. Nature of the spermatogenic arrest in Dazl -/- mice. Biol. Reprod. 2001, 65, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kawano, N.; Yoshida, K. Control of sperm motility and fertility: Diverse factors and common mechanisms. Cell Mol. Life Sci. 2008, 65, 3446–3457. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D. Main signaling pathways involved in the control of fowl sperm motility. Poult. Sci. 2019, 98, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shingyoji, C. The roles of noncatalytic ATP binding and ADP binding in the regulation of dynein motile activity in flagella. Cell Motil. Cytoskelet. 2007, 64, 690–704. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Li, J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Cacciola, G.; Chianese, R.; Chioccarelli, T.; Fasano, S. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 279–291. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kolasa, M.; Podkowa, D.; Kloc, M.; Kubiak, J.Z. Tissue-specific knockout of E-cadherin (Cdh1) in developing mouse gonads causes germ cells loss. Reproduction 2019, 158, 147–157. [Google Scholar] [CrossRef]
- Silva, A.M.; Correia, S.; Casalta-Lopes, J.E.; Mamede, A.C.; Cavaco, J.E.; Botelho, M.F.; Socorro, S.; Maia, C.J. The protective effect of regucalcin against radiation-induced damage in testicular cells. Life Sci. 2016, 164, 31–41. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [Green Version]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Sinnar, S.A.; Small, C.L.; Evanoff, R.M.; Reinholdt, L.G.; Griswold, M.D.; Kopito, R.R.; Ryu, K.Y. Altered testicular gene expression patterns in mice lacking the polyubiquitin gene Ubb. Mol. Reprod. Dev. 2011, 78, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.C.; Yang, W.X. New insights to the ubiquitin-proteasome pathway (UPP) mechanism during spermatogenesis. Mol. Biol. Rep. 2013, 40, 3213–3230. [Google Scholar] [CrossRef]
- Sheng, K.; Liang, X.; Huang, S.; Xu, W. The role of histone ubiquitination during spermatogenesis. Biomed. Res. Int. 2014, 2014, 870695. [Google Scholar] [CrossRef]
- Brohi, R.D.; Huo, L.J. Posttranslational Modifications in Spermatozoa and Effects on Male Fertility and Sperm Viability. OMICS 2017, 21, 245–256. [Google Scholar] [CrossRef]
- Ryu, K.Y.; Sinnar, S.A.; Reinholdt, L.G.; Vaccari, S.; Hall, S.; Garcia, M.A.; Zaitseva, T.S.; Bouley, D.M.; Boekelheide, K.; Handel, M.A.; et al. The mouse polyubiquitin gene Ubb is essential for meiotic progression. Mol. Cell Biol. 2008, 28, 1136–1146. [Google Scholar] [CrossRef] [Green Version]

| High Sperm Motility Group | Low Sperm Motility Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | HS1 | HS2 | HS3 | HS4 | HS5 | Mean | LS1 | LS2 | LS3 | LS4 | LS5 | Mean |
| Sperm motility | 7 | 8.5 | 8.3 | 8.2 | 9 | 8.2 | 1.3 | 3.5 | 1 | 3 | 3 | 2.4 |
| Gene Name | lncRNA Transcript Name | Cis Location (bp) | Pearson Correlation Coefficient |
|---|---|---|---|
| CCSER1 | MSTRG.102.1 | 1k | 1 |
| BBS9 | MSTRG.18285.3 | 1K | 1 |
| SVIP | MSTRG.2435.3 | 1K | −0.29 |
| AGR3 | MSTRG.25354.1 | 1K | 1 |
| RPL34 | MSTRG.33554.2 | 1K | −0.26 |
| UBB | MSTRG.7787.5 | 1K | 1 |
| SSU72 | MSTRG.31781.3 | 10K | −0.38 |
| SRSF2 | MSTRG.9899.4 | 1K | 1 |
| GO Term | GO Function | p-Value |
|---|---|---|
| GO:0031225~anchored component of membrane | cellular_component | 0.014 |
| GO:0005179~hormone activity | molecular_function | 0.021 |
| GO:0005262~calcium channel activity | molecular_function | 0.037 |
| GO:0072686~mitotic spindle | cellular_component | 0.040 |
| GO:0005856~cytoskeleton | cellular_component | 0.042 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Tan, Y.; Mao, H.; Liu, H.; Dong, X.; Yin, Z. Analysis of Long Noncoding RNA and mRNA Expression Profiles of Testes with High and Low Sperm Motility in Domestic Pigeons (Columba livia). Genes 2020, 11, 349. https://doi.org/10.3390/genes11040349
Xu X, Tan Y, Mao H, Liu H, Dong X, Yin Z. Analysis of Long Noncoding RNA and mRNA Expression Profiles of Testes with High and Low Sperm Motility in Domestic Pigeons (Columba livia). Genes. 2020; 11(4):349. https://doi.org/10.3390/genes11040349
Chicago/Turabian StyleXu, Xiuli, Yuge Tan, Haiguang Mao, Honghua Liu, Xinyang Dong, and Zhaozheng Yin. 2020. "Analysis of Long Noncoding RNA and mRNA Expression Profiles of Testes with High and Low Sperm Motility in Domestic Pigeons (Columba livia)" Genes 11, no. 4: 349. https://doi.org/10.3390/genes11040349
APA StyleXu, X., Tan, Y., Mao, H., Liu, H., Dong, X., & Yin, Z. (2020). Analysis of Long Noncoding RNA and mRNA Expression Profiles of Testes with High and Low Sperm Motility in Domestic Pigeons (Columba livia). Genes, 11(4), 349. https://doi.org/10.3390/genes11040349




