Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains
2.2. Activation of Transgenes Using the Gal4/UAS System
2.3. Genetic Ablation and Dual Gal4/LexA Transactivation System
2.4. Immunohistochemistry and Statistics
2.5. Test for Adult Wing Phenotypes
3. Results
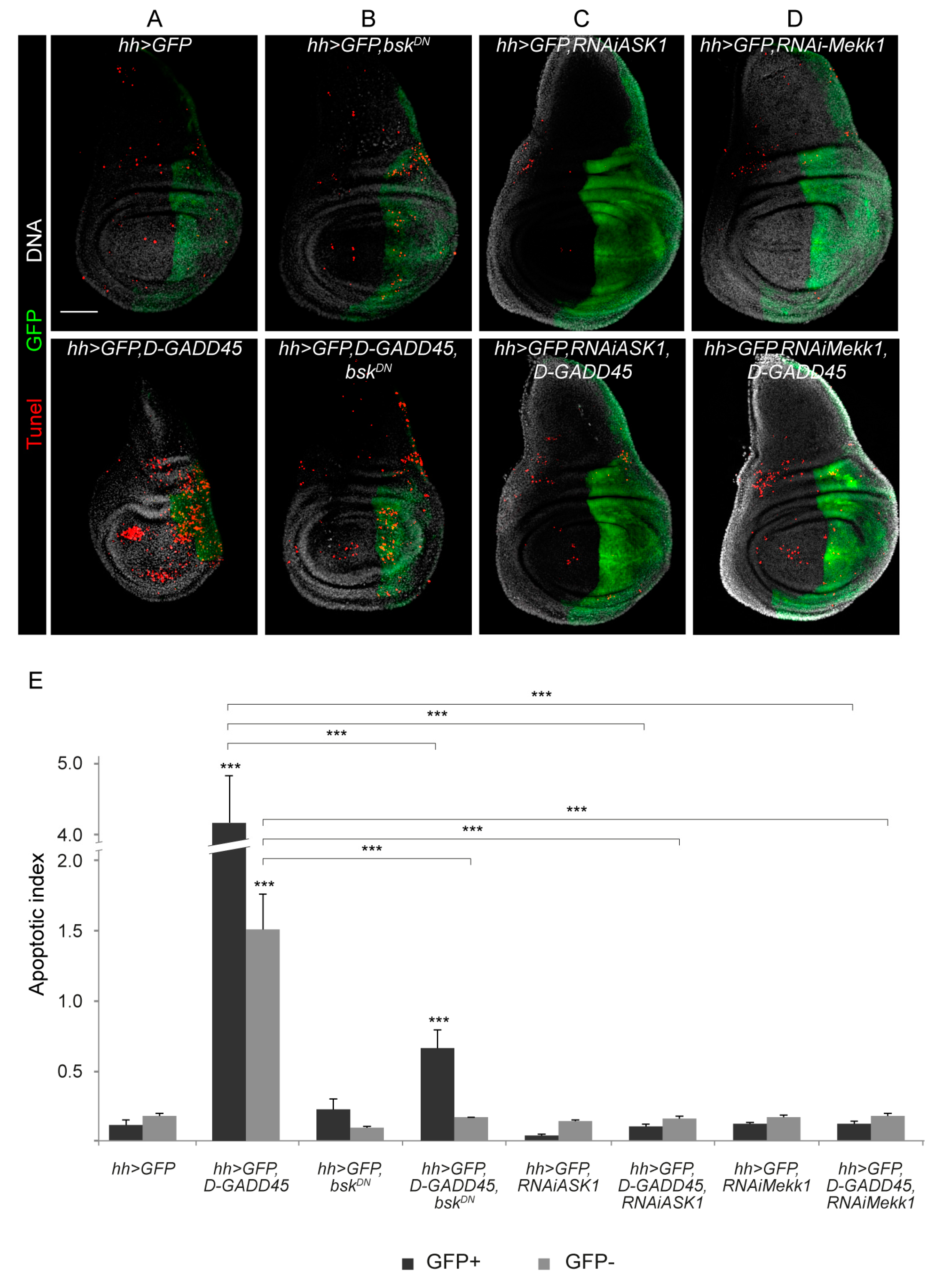
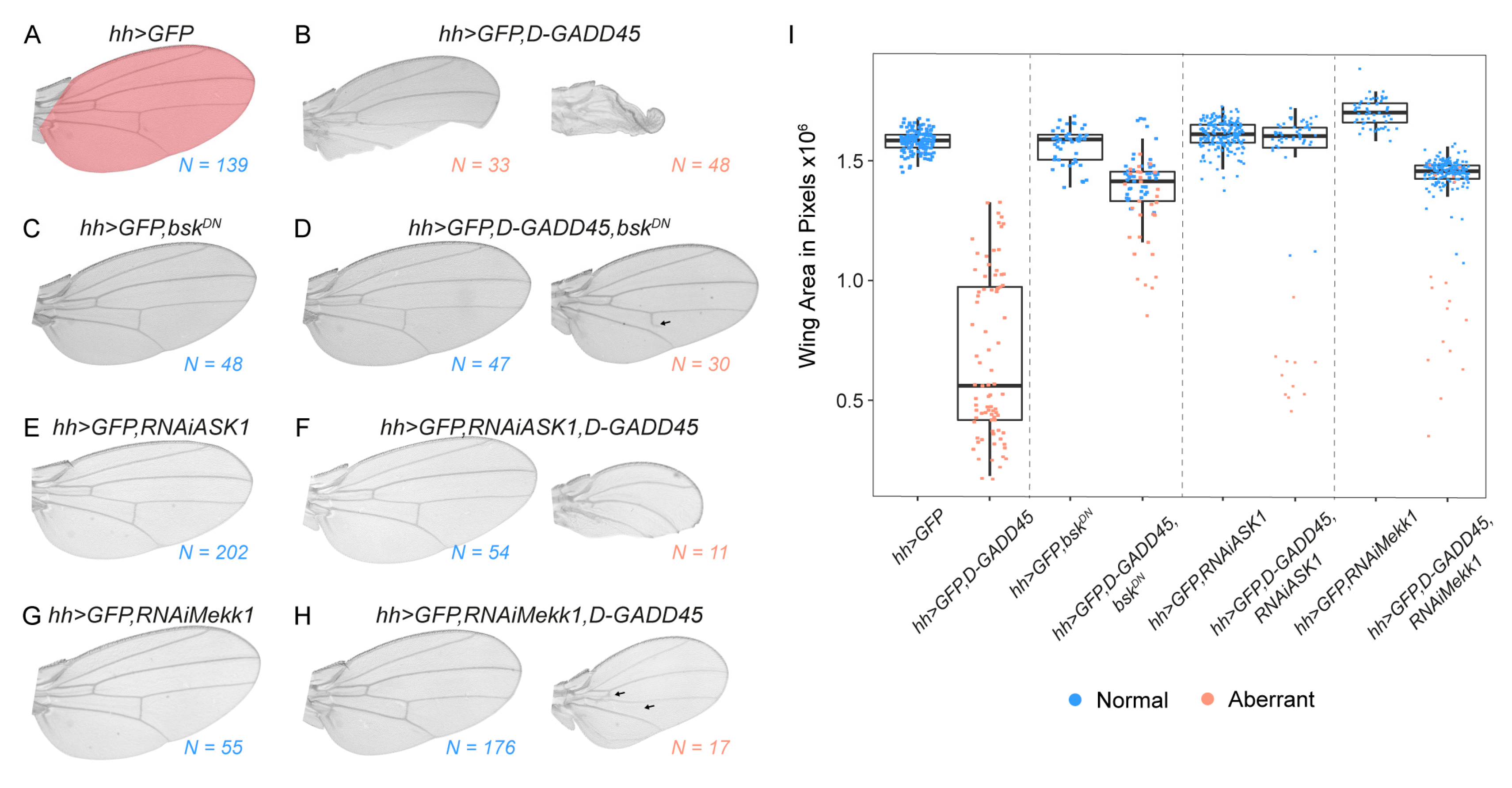
3.1. Sustained Activation of D-GADD45 Induces JNK-Dependent Apoptosis
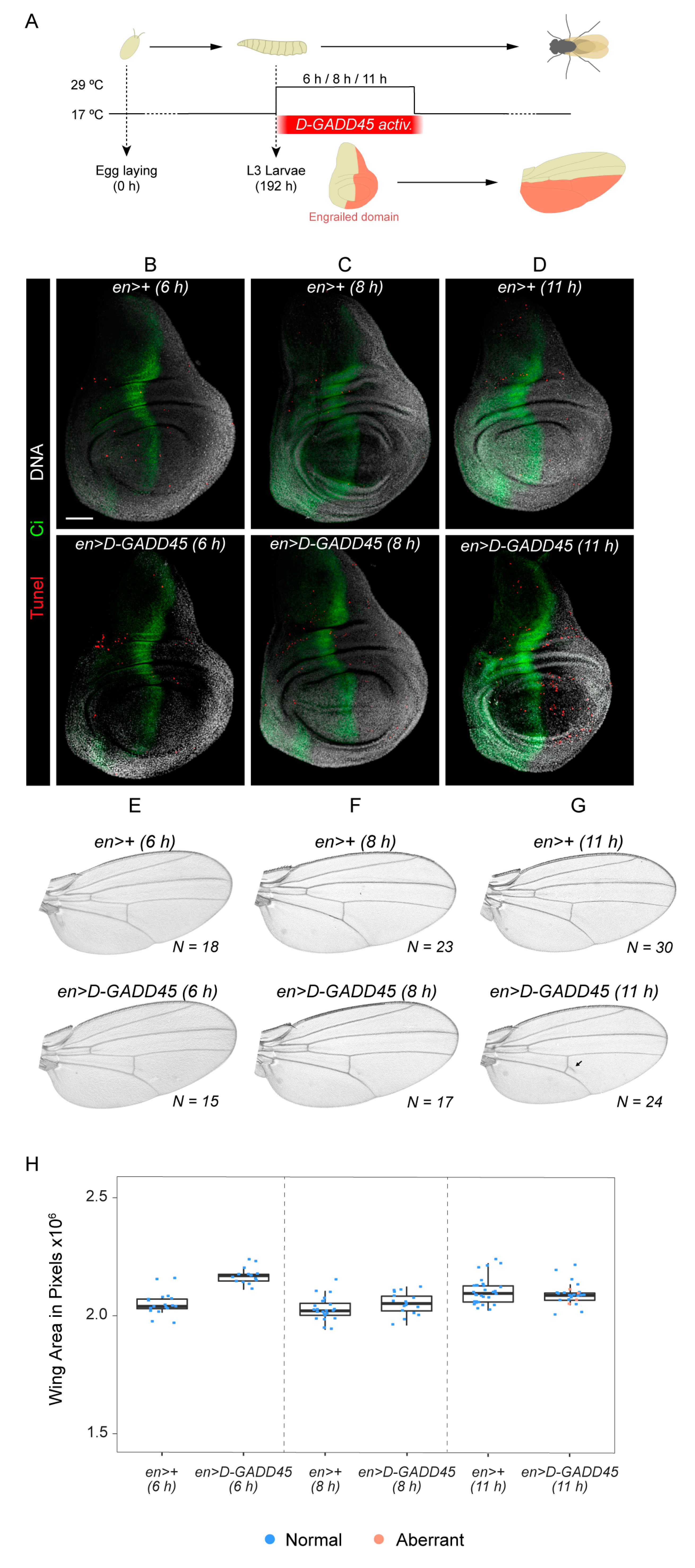
3.2. Increased Expression of D-GADD45 Decreases Cell Proliferation Independently of the JNK Signaling Pathway
3.3. D-GADD45 is Required for Regeneration of Wing Imaginal Discs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fornace, A.J.; Alamo, I.; Hollander, M.C. DNA damage-inducible transcripts in mammalian cells. Proc. Natl. Acad. Sci. USA 1988, 85, 8800–8804. [Google Scholar] [CrossRef] [PubMed]
- Fornace, A.J.; Nebert, D.W.; Hollander, M.C.; Luethy, J.D.; Papathanasiou, M.; Papathanasiou, M.; Fargnoli, J.; Holbrook, N.J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 1989, 9, 4196–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Lord, K.A.; Alamo, I.; Hollander, M.C.; Carrier, F.; Ron, D.; Kohn, K.W.; Hoffman, B.; Liebermann, D.A.; Fornace, A.J. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 1994, 14, 2361–2371. [Google Scholar] [CrossRef]
- Carrier, F.; Georgel, P.T.; Pourquier, P.; Blake, M.; Kontny, H.U.; Antinore, M.J.; Gariboldi, M.; Myers, T.G.; Weinstein, J.N.; Pommier, Y.; et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol. Cell. Biol. 1999, 19, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, D.A.; Hoffman, B. Gadd45 in stress signaling. J. Mol. Signal. 2008, 3, 1–8. [Google Scholar] [CrossRef]
- Tamura, R.E.; de Vasconcellos, J.F.; Sarkard, D.; Libermann, T.A.; Fisher, P.B.; Zerbinia, L.F. GADD45 proteins: central players in tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Elsaeidi, F.; Goldman, D. Injury-dependent muller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. J. Neurosci. 2012, 32, 1096–1109. [Google Scholar] [CrossRef]
- Hirose, K.; Shimoda, N.; Kikuchi, Y. Transient reduction of 5-methylcytosine and 5-hydroxymethylcytosine is associated with active DNA demethylation during regeneration of zebrafish fin. Epigenetics 2013, 8, 899–906. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, X.; Hou, Q.; Shi, S.; Wang, L.; Chen, P.; Zhu, X.; Zeng, C.; Qin, W.; Zhou, W.; et al. GADD45B mediates podocyte injury in zebrafish by activating the ROS-GADD45B-p38 pathway. Cell Death Dis. 2016, 7, e2068. [Google Scholar] [CrossRef]
- Niehrs, C.; Schäfer, A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol. 2012, 22, 220–227. [Google Scholar] [CrossRef]
- Salvador, J.M.; Brown-Clay, J.D.; Fornace, A.J., Jr. Gadd45 in Stress Signaling, Cell Cycle Control., and Apoptosis; Springer: New York, NY, USA, 2013; ISBN 9781461482888. [Google Scholar]
- Takekawa, M.; Saito, H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 1998, 95, 521–530. [Google Scholar] [CrossRef]
- De Smaele, E.; Zazzeroni, F.; Papa, S.; Nguyen, D.U.; Jin, R.; Jones, J.; Cong, R.; Franzoso, G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 2001, 414, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Zazzeroni, F.; Bubici, C.; Jayawardena, S.; Alvarez, K.; Matsuda, S.; Nguyen, D.U.; Pham, C.G.; Nelsbach, A.H.; Melis, T.; et al. Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 2004, 6, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Monti, S.M.; Vitale, R.M.; Bubici, C.; Jayawardena, S.; Alvarez, K.; De Smaele, E.; Dathan, N.; Ruvo, M.; Pedone, C.; et al. Insights into the Structural Basis of the GADD45β-mediated Inactivation of the JNK Kinase, MKK7/JNKK2. J. Biol. Chem. 2007, 282, 19029–19041. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Z.; Takekawa, M.; Ge, Q.; Saito, H. Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol. Cell. Biol. 2007, 27, 2765–2776. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 2016, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Tornatore, L.; Marasco, D.; Dathan, N.; Vitale, R.M.; Benedetti, E.; Papa, S.; Franzoso, G.; Ruvo, M.; Monti, S.M. Gadd45β forms a homodimeric complex that binds tightly to MKK7. J. Mol. Biol. 2008, 378, 97–111. [Google Scholar] [CrossRef]
- Ueda, T.; Kohama, Y.; Kuge, A.; Kido, E.; Sakurai, H. GADD45 family proteins suppress JNK signaling by targeting MKK7. Arch. Biochem. Biophys. 2017, 635, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, L.; Huang, C. Gadd45 proteins as critical signal transducers linking NF-κB to MAPK cascades. Curr. Cancer Drug Targets 2009, 9, 915–930. [Google Scholar] [CrossRef]
- Peretz, G.; Bakhrat, A.; Abdu, U. Expression of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-Jun N-terminal kinase pathway. Genetics 2007, 177, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Stramer, B.; Winfield, M.; Shaw, T.; Millard, T.H.; Woolner, S.; Martin, P. Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 2008, 9, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Plyusnina, E.N.; Shaposhnikov, M.V.; Moskalev, A.A. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology 2011, 12, 211–226. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Smit-McBride, Z.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Tacutu, R.; Fraifeld, V.E. Gadd45 proteins: Relevance to aging, longevity and age-related pathologies. Ageing Res. Rev. 2012, 11, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Ruiz-Romero, M.; Beltran, S.; Bosch, M.; Punset, A.; Serras, F.; Corominas, M. Gene expression following induction of regeneration in Drosophila wing imaginal discs. Expression profile of regenerating wing discs. BMC Dev. Biol. 2010, 10, 94. [Google Scholar] [CrossRef]
- Vizcaya-Molina, E.; Klein, C.C.; Serras, F.; Mishra, R.K.; Guigó, R.; Corominas, M. Damage-responsive elements in Drosophila regeneration. Genome Res. 2018, 28, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Serras, F.; Martín-Blanco, E.; Baguñà, J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 2005, 280, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Bergantinos, C.; Corominas, M.; Serras, F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 2010, 137, 1169–1179. [Google Scholar] [CrossRef]
- Santabárbara-Ruiz, P.; López-Santillán, M.; Martínez-Rodríguez, I.; Binagui-Casas, A.; Pérez, L.; Milán, M.; Corominas, M.; Serras, F. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bohmann, D. A versatile φC31 based reporter system for measuring AP-1 and NRF2 signaling in Drosophila and in tissue culture. PLoS ONE 2012, 7, e34063. [Google Scholar] [CrossRef]
- Repiso, A.; Bergantinos, C.; Serras, F. Cell fate respecification and cell division orientation drive intercalary regeneration in Drosophila wing discs. Development 2013, 140, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 289–295. [Google Scholar]
- Lin, A. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. BioEssays 2003, 25, 17–24. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Zhan, Q.; Coursen, J.D.; Khan, M.A.; Kontny, H.U.; Yu, L.; Hollander, M.C.; O’Connor, P.M.; Fornace, A.J.; Harris, C.C. GADD45 induction of a G2/M cell cycle checkpoint. Proc. Natl. Acad. Sci. USA 1999, 96, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Hara, T.; Hirano, T.; Hibi, M.; Hirano, T.; Miyajima, A. A novel oncostatin m-inducible gene OIG37 forms a gene family with MyD118 and GADD45 and negatively regulates cell growth. J. Biol. Chem. 1999, 274, 24766–24772. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Antinore, M.J.; Lung, F.-D.T.; Dong, X.; Zhao, H.; Fan, F.; Colchagie, A.B.; Blanck, P.; Roller, P.P.; Fornace, A.J.; et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45 -mediated growth suppression. J. Biol. Chem. 2000, 275, 16602–16608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bae, I.; Krishnaraju, K.; Azam, N.; Fan, W.; Smith, K.; Hoffman, B.; Liebermann, D.A. CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene 1999, 18, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Tong, T.; Fan, W.; Fan, F.; Antinore, M.J.; Zhu, X.; Mazzacurati, L.; Li, X.; Petrik, K.L.; Rajasekaran, B.; et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene 2002, 21, 8696–8704. [Google Scholar] [CrossRef]
- Zhu, N.; Shao, Y.; Xu, L.; Yu, L.; Sun, L. Gadd45-α and Gadd45-γ utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol. Biol. Rep. 2009, 36, 2075–2085. [Google Scholar] [CrossRef]
- Cosolo, A.; Jaiswal, J.; Csordás, G.; Grass, I.; Uhlirova, M.; Classen, A.-K. JNK-dependent cell cycle stalling in G2 promotes survival and senescence-like phenotypes in tissue stress. Elife 2019, 8, e41036. [Google Scholar] [CrossRef] [PubMed]
- Willsey, H.R.; Zheng, X.; Pastor-Pareja, J.; Willsey, A.J.; Beachy, P.A.; Xu, T. Localized JNK signaling regulates organ size during development. Elife 2016, 5, e11491. [Google Scholar] [CrossRef]
- Mattila, J.; Omelyanchuk, L.; Kyttälä, S.; Turunen, H.; Nokkala, S. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int. J. Dev. Biol. 2005, 49, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Santabárbara-Ruiz, P.; Esteban-Collado, J.; Pérez, L.; Viola, G.; Abril, J.F.; Milán, M.; Corominas, M.; Serras, F. Ask1 and Akt act synergistically to promote ROS-dependent regeneration in Drosophila. PLoS Genet. 2019, 15, e1007926. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.O.; Moore, K.A.; Chapin, A.; Hollien, J.; Metzstein, M.M. Degradation of Gadd45 mRNA by nonsense-mediated decay is essential for viability. Elife 2016, 5, e12876. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilleri-Robles, C.; Serras, F.; Corominas, M. Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila. Genes 2019, 10, 378. https://doi.org/10.3390/genes10050378
Camilleri-Robles C, Serras F, Corominas M. Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila. Genes. 2019; 10(5):378. https://doi.org/10.3390/genes10050378
Chicago/Turabian StyleCamilleri-Robles, Carlos, Florenci Serras, and Montserrat Corominas. 2019. "Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila" Genes 10, no. 5: 378. https://doi.org/10.3390/genes10050378
APA StyleCamilleri-Robles, C., Serras, F., & Corominas, M. (2019). Role of D-GADD45 in JNK-Dependent Apoptosis and Regeneration in Drosophila. Genes, 10(5), 378. https://doi.org/10.3390/genes10050378




