Overexpression of HMGA1 Figures as a Potential Prognostic Factor in Endometrioid Endometrial Carcinoma (EEC)
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Samples
2.2. RNA Extraction, Reverse Transcription and RT-qPCR
2.3. Immunohistochemistry
2.4. Analyses of HMGA1 and HMGA2 Expression Data Deposited in the Cancer Genome Atlas (TCGA)
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
3. Results
3.1. Clinicopathological Features
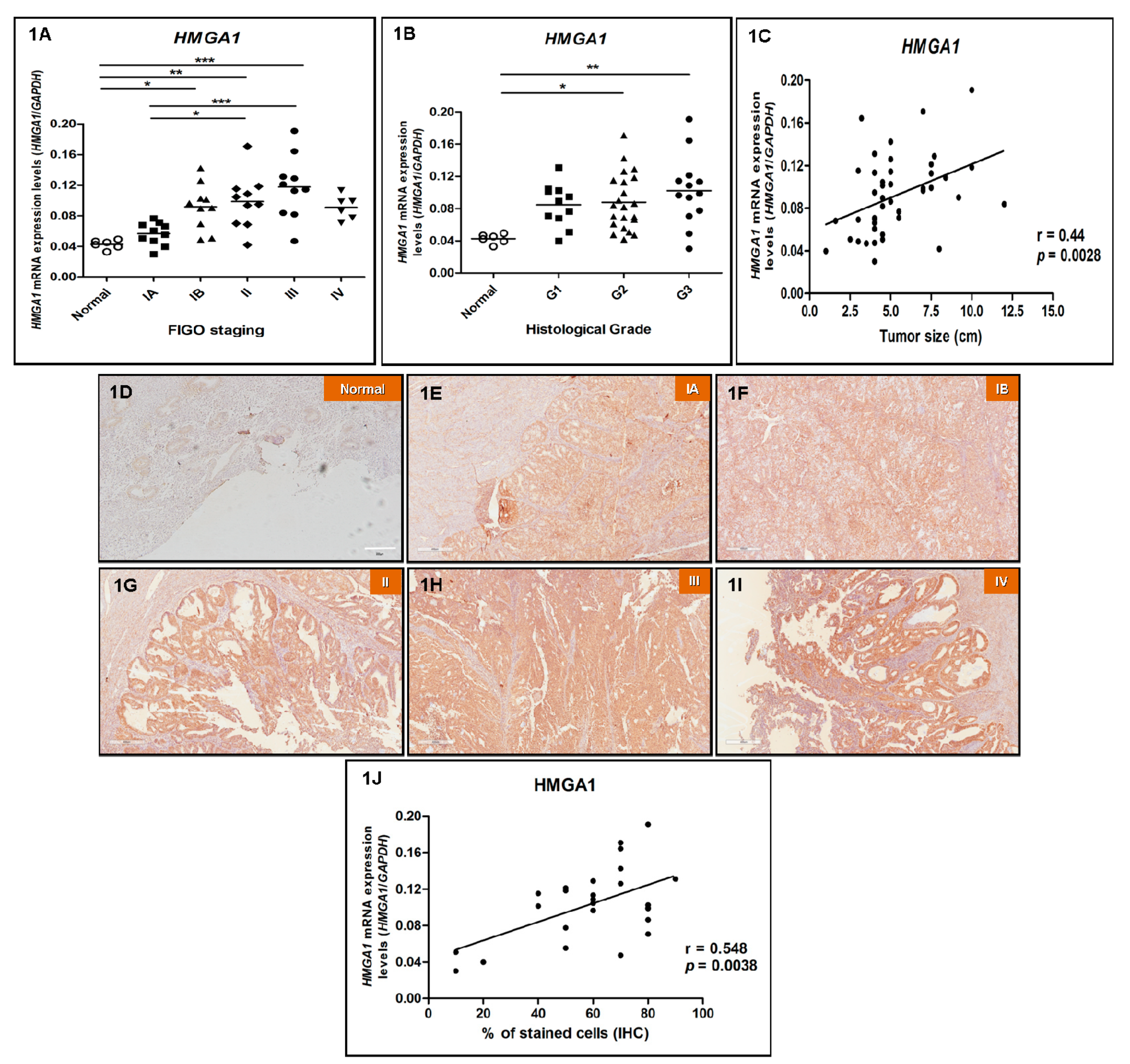
3.2. HMGA1 is Overexpressed in EEC and Its Expression Positively Correlates with Increasing Tumor Staging, Grade and Size
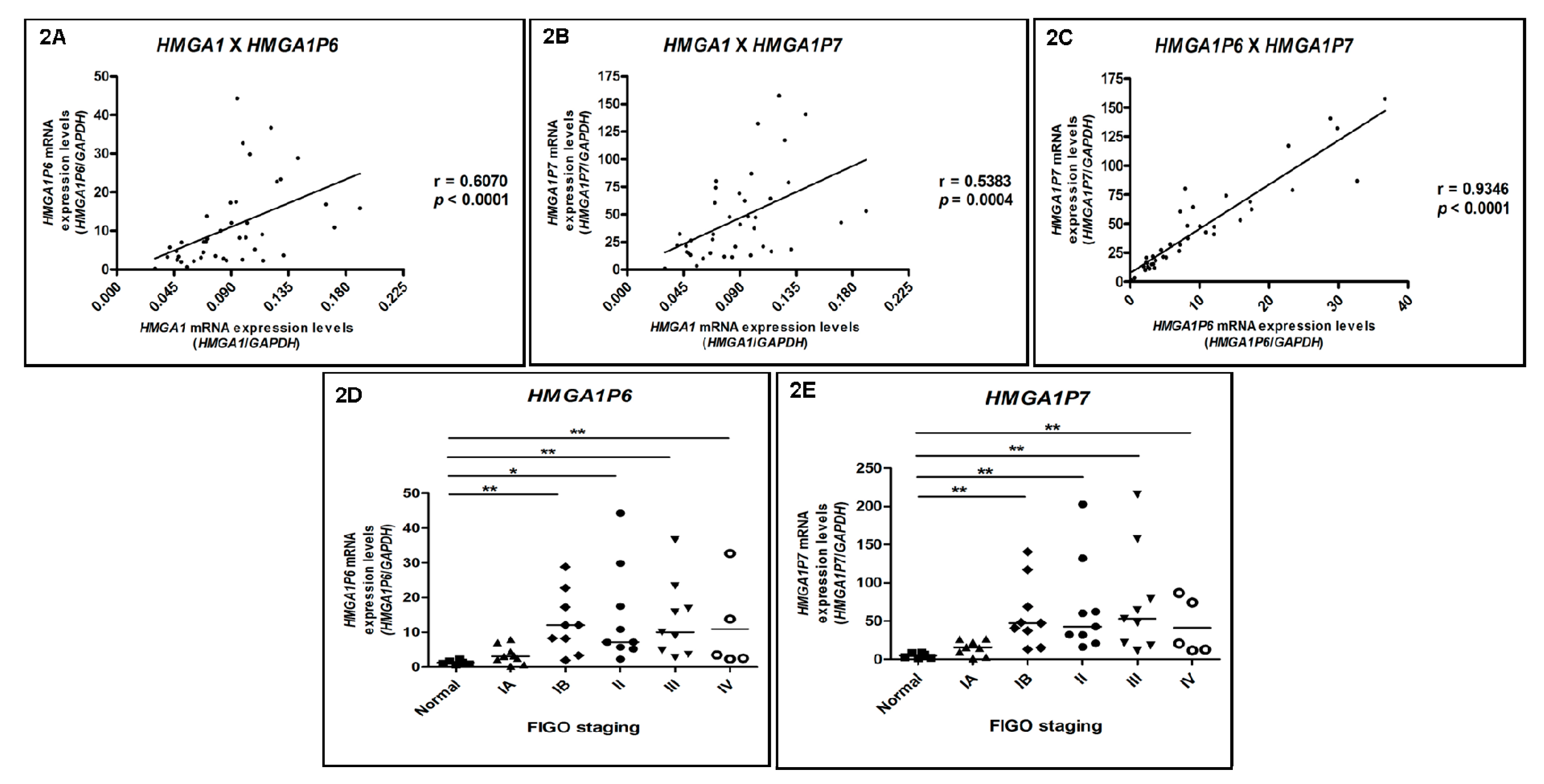
3.3. HMGA1P6 and HMGA1P7 Expression Follows the Same Pattern Observed for HMGA1 Expression
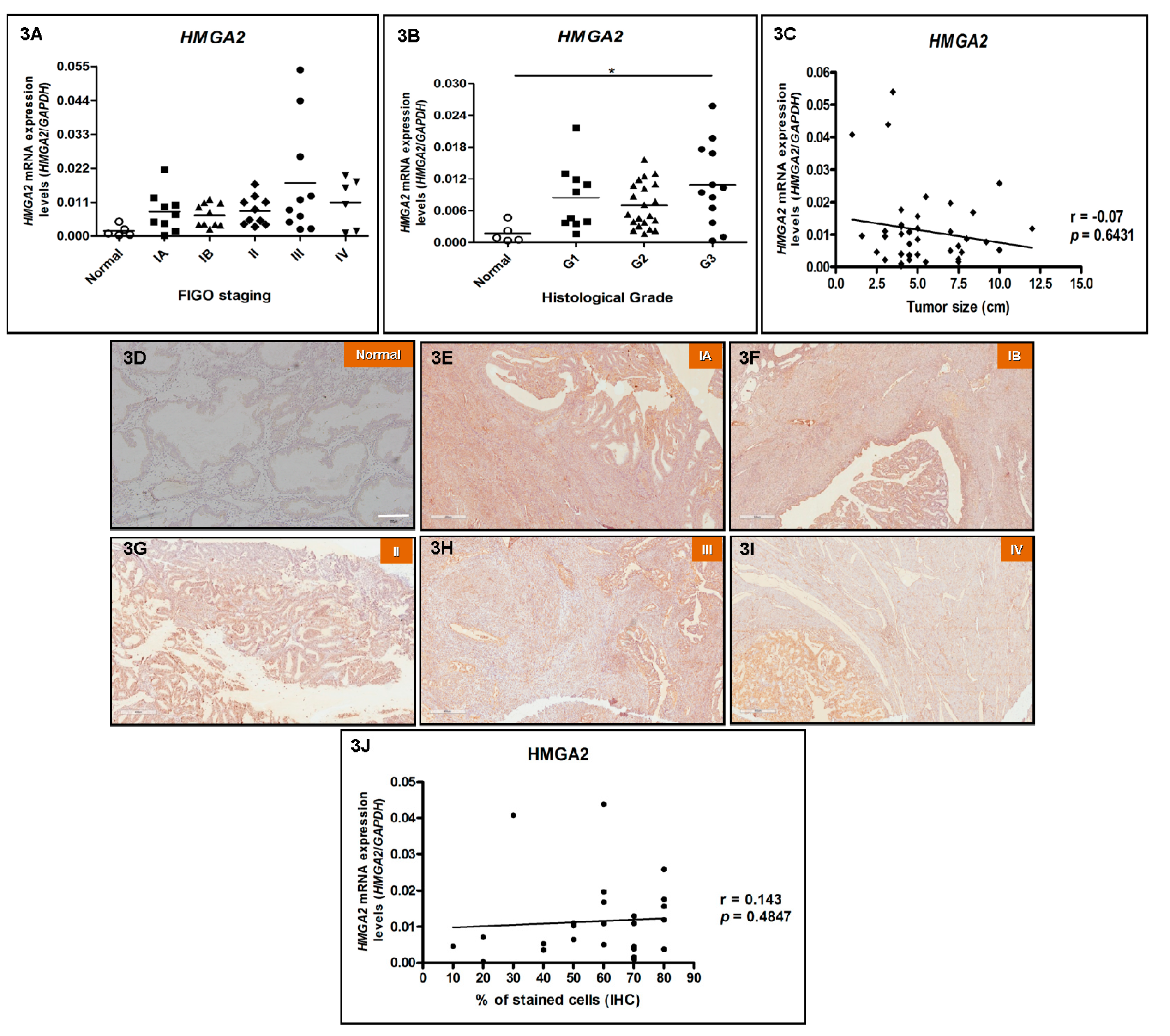
3.4. HMGA2 Expression Does Not Correlate with Increasing Tumor Staging and Tumor Size in EEC
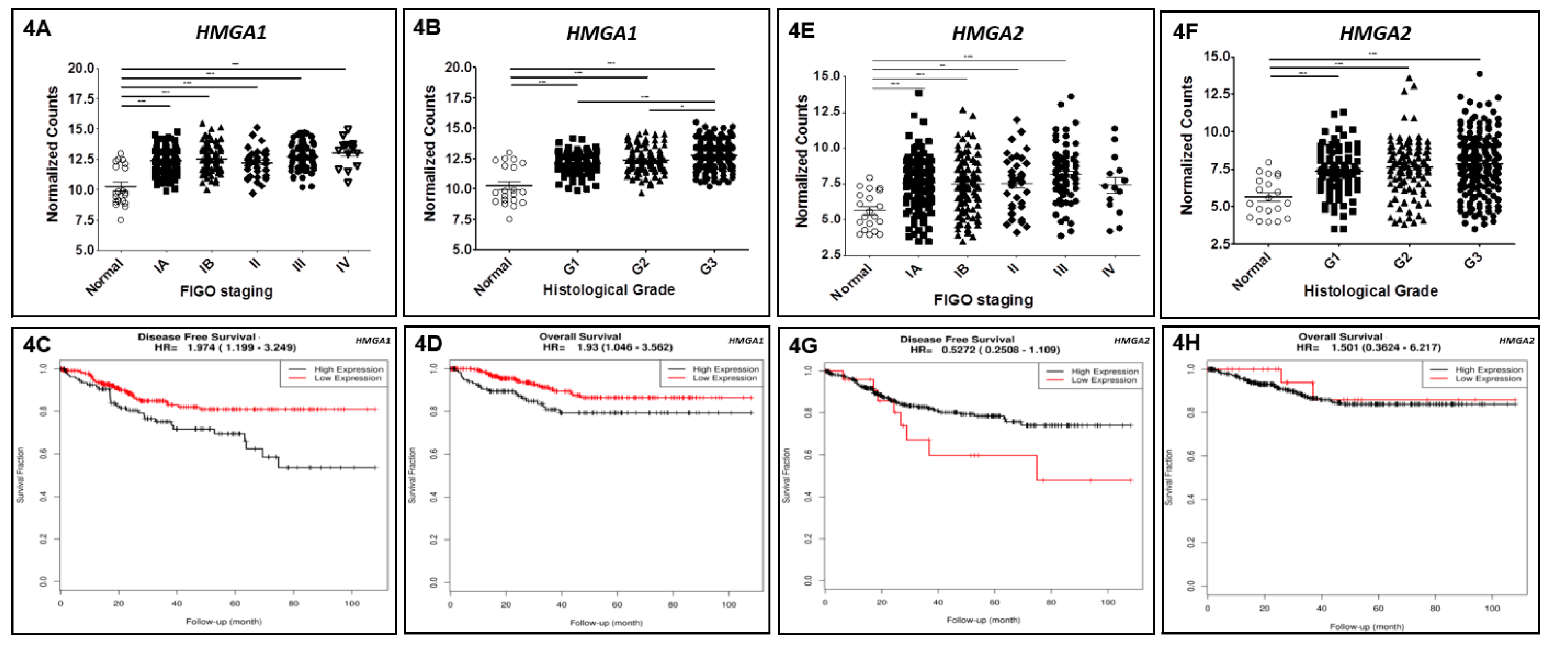
3.5. Reanalysis of TCGA Data Confirms HMGA1 Upregulation in EEC and Reveals Its Impact on Patients’ Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, S.; Lyytinen, H.K.; Dyba, T.; Ylikorkala, O.; Pukkala, E. Endometrial cancer associated with various forms of postmenopausal hormone therapy: A case control study. Int. J. Cancer 2011, 128, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A.; et al. Obesity, inflammatory markers and endometrial cancer risk: A prospective case-control study. Endocr. Relat. Cancer 2010, 17, 1007–1019. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial Cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Uharcek, P. Prognostic factors in endometrial carcinoma. J. Obstet. Gynecol. Res. 2008, 34, 776–783. [Google Scholar] [CrossRef]
- Dizon, D.S. Treatment options for advanced endometrial carcinoma. Gynecol. Oncol. 2010, 117, 373–381. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Felix, A.S.; Yang, H.P.; Bell, D.W.; Sherman, M.E. Epidemiology of Endometrial Carcinoma: Etiologic importance of hormonal and metabolic influences. Adv. Exp. Med. Biol. 2017, 943, 3–46. [Google Scholar] [CrossRef]
- Silva, J.L.; Paulino, E.; Dias, M.F.; Melo, A.C. Endometrial cancer: Redefining the molecular-targeted approach. Cancer Chemother. Pharmacol. 2015, 76, 1–11. [Google Scholar] [CrossRef]
- Sgarra, R.; Pegoraro, S.; Ros, G.; Penzo, C.; Chiefari, E.; Foti, D.; Brunetti, A.; Manfioletti, G. High Mobility Group A (HMGA) proteins: Molecular instigators of breast cancer onset and progression. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Resar, L.M. The high mobility group A1 gene: Transforming inflammatory signals into cancer? Cancer Res. 2010, 70, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.K.; Mali, P.; Huang, X.; Ye, Z.; Dowey, S.N.; Resar, L.M.S.; Zou, C.; Zhang, Y.A.; Tong, J.; Cheng, L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011, 21, 518–529. [Google Scholar] [CrossRef]
- Karp, J.E.; Smith, B.D.; Resar, L.S.; Greer, J.M.; Blackford, A.; Zhao, M.; Moton-Nelson, D.; Alino, K.; Levis, M.J.; Gore, S.D.; et al. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood 2011, 117, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Joseph, B.; Hillion, J.; Segal, J.; Karp, J.E.; Resar, L.M. Flavopiridol induces BCL-2 expression and represses oncogenic transcription factors in leukemic blasts from adults with refractory acute myeloid leukemia. Leuk. Lymphoma 2011, 52, 1999–2006. [Google Scholar] [CrossRef]
- Schuldenfrei, A.; Belton, A.; Kowalski, J.; Talbot, C.C., Jr.; Di Cello, F.; Poh, W.; Tsai, H.L.; Shah, S.N.; Huso, T.H.; Huso, D.L.; et al. HMGA1 drives stem cell, inflammatory pathway, and cell cycle progression genes during lymphoid tumorigenesis. BMC Genomics 2011, 12, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Belton, A.; Gabrovsky, A.; Bae, Y.K.; Reeves, R.; Iacobuzio-Donahue, C.; Huso, D.L.; Resar, L.M.S. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS ONE 2012, 7, e30034. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Resar, L.M. High mobility group A1 and cancer: Potential biomarker and therapeutic target. Histol. Histopathol. 2012, 27, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Sumter, T.F.; Xian, L.; Huso, M.; Koo, M.; Chang, Y.T.; Almasri, T.N.; Chia, L.; Inglis, C.; Reid, D.; Resar, L.M. The high mobility group A1 (HMGA1) transcriptome in cancer and development. Curr. Mol. Med. 2016, 16, 353–393. [Google Scholar] [CrossRef]
- Fusco, A.; Fedele, M. Roles of HMGA proteins in cancer. Nat. Rev. Cancer 2007, 7, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Fusco, A. HMGA and cancer. Biochim. Biophys. Acta 2010, 1799, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; DiCello, F.; Hillion, J.; Ronnett, B.M.; Elbahloul, O.; Ashfaq, R.; Dhara, S.; Prochownik, E.; Tworkoski, K.; Reeves, R.; et al. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer Res. 2007, 67, 3998–4004. [Google Scholar] [CrossRef]
- Hillion, J.; Roy, S.; Heydarian, M.; Cope, L.; Xian, L.; Koo, M.; Luo, L.Z.; Kellyn, K.; Ronnett, B.M.; Huso, T. The High Mobility Group A1 (HMGA1) gene is highly overexpressed in human uterine serous carcinomas and carcinosarcomas and drives Matrix Metalloproteinase-2 (MMP-2) in a subset of tumors. Gynecol. Oncol. 2016, 141, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, X.; Zhang, W.; Wei, Y.; Li, Y.; Zhang, Q.; Dong, R.; Kwon, J.S.; Liu, Z.; Zheng, W.; et al. Overexpression and oncogenic function of HMGA2 in endometrial serous carcinogenesis. Am. J. Cancer Res. 2016, 6, 249–259. [Google Scholar]
- Esposito, F.; De Martino, M.; Petti, M.G.; Forzati, F.; Tornincasa, M.; Federico, A.; Arra, C.; Pierantoni, G.M.; Fusco, A. HMGA1 pseudogenes as candidate proto-oncogenic competitive endogenous RNAs. Oncotarget 2014, 5, 8341–8354. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; De Martino, M.; D’Angelo, D.; Mussnich, P.; Raverot, G.; Jaffrain-Rea, M.L.; Fraggetta, F.; Trouillas, J.; Fusco, A. HMGA1-pseudogene expression is induced in human pituitary tumors. Cell Cycle 2015, 14, 1471–1475. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palumbo, J.A.; Meireles Da Costa, N.; Esposito, F.; De Martino, M.; D’Angelo, D.; de Sousa, V.P.; Martins, I.; Nasciutti, L.E.; Fusco, A.; Ribeiro Pinto, L.F. HMGA2 overexpression plays a critical role in the progression of esophageal squamous carcinoma. Oncotarget 2016, 7, 25872–25874. [Google Scholar] [CrossRef]
- Palumbo, A., Jr.; De Martino, M.; Esposito, F.; Fraggetta, F.; Neto, P.N.; Valverde Fernandes, P.; Santos, I.C.; Dias, F.L.; Nasciutti, L.E.; Meireles Da Costa, N.; et al. HMGA2, but not HMGA1, are overexpressed in human larynx carcinomas. Histopathology 2018, 72, 1102–1114. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2012, 141, 52–67. [Google Scholar] [CrossRef]
- Liau, S.S.; Jazag, A.; Whang, E.E. HMGA1 is a determinant of cellular invasiveness and in vivo metastatic potential in pancreatic adenocarcinoma. Cancer Res. 2006, 66, 11613–11622. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Guo, Z.; Ma, X.; Song, Y.; Guo, Y. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch. Gynecol. Obstet. 2017, 295, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Watanabe, T.; Sugiyama, M.; Uchimura, H.; Chiappetta, G.; Fusco, A.; Atomi, Y. Determination of high mobility group I(Y) expression level in colorectal neoplasias: A potential diagnostic marker. Cancer Res. 1999, 59, 1169–1174. [Google Scholar] [PubMed]
- Chiappetta, G.; Manfioletti, G.; Pentimalli, F.; Abe, N.; Di Bonito, M.; Vento, M.T.; Giuliano, A.; Fedele, M.; Viglietto, G.; Santoro, M.; et al. High mobility group HMGI(Y) protein expression in human colorectal hyperplastic and neoplastic diseases. Int. J. Cancer 2001, 91, 147–151. [Google Scholar] [CrossRef]
- Piscuoglio, S.; Zlobec, I.; Pallante, P.; Sepe, R.; Esposito, F.; Zimmermann, A.; Diamantis, I.; Terracciano, L.; Fusco, A.; Karamitopoulou, E. HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology 2012, 60, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Chen, F.; Liu, J. Elevated expression of HMGA1 correlates with the malignant status and prognosis of non-small cell lung cancer. Tumour Biol. 2015, 36, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Liau, S.S.; Rocha, F.; Matros, E.; Redston, M.; Whang, E. High mobility group AT-hook 1 (HMGA1) is an independent prognostic factor and novel therapeutic target in pancreatic adenocarcinoma. Cancer 2008, 113, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, G.; Botti, G.; Monaco, M.; Pasquinelli, R.; Pentimalli, F.; Di Bonito, M.; D’Aiuto, G.; Fedele, M.; Iuliano, R.; Palmieri, E.A.; et al. HMGA1 protein overexpression in human breast carcinomas: Correlation with ErbB2 expression. Clin. Cancer Res. 2004, 10, 7637–7644. [Google Scholar] [CrossRef]
- Romero-Pérez, L.; Castilla, M.Á.; López-García, M.Á.; Díaz-Martín, J.; Biscuola, M.; Ramiro-Fuentes, S.; Oliva, E.; Matias-Guiu, X.; Prat, J.; Cano, A.; et al. Molecular events in endometrial carcinosarcomas and the role of high mobility group AT-hook 2 in endometrial carcinogenesis. Hum. Pathol. 2013, 44, 244–254. [Google Scholar] [CrossRef]
- Ma, J.; Li, D.; Kong, F.F.; Yang, D.; Yang, H.; Ma, X.X. miR-302a-5p/367-3p-HMGA2 axis regulates malignant processes during endometrial cancer development. J. Exp. Clin. Cancer Res. 2018, 37, 19. [Google Scholar] [CrossRef]
- Montserrat, N.; Mozos, A.; Llobet, D.; Dolcet, X.; Pons, C.; de Herreros, A.G.; Matias-Guiu, X.; Prat, J. Epithelial to mesenchymal transition in early stage endometrioid endometrial carcinoma. Hum. Pathol. 2012, 43, 632–643. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Xu, C.; Zhou, Z.; Zhu, Z.; You, T. HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer Lett. 2014, 355, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Palumbo Júnior, A.; Meireles Da Costa, N.; Esposito, F.; Fusco, A.; Ribeiro Pinto, L.F. High Mobility Group A proteins in esophageal carcinomas. Cell Cycle 2016, 15, 2410–2413. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Characteristics | Patients (%) | Controls (%) | p |
|---|---|---|---|
| Median age (years) | 64 | 39.5 | 0.003 |
| Variation | 42–83 | 35–72 | |
| Hypertension | 0.07 | ||
| Yes | 29 (63.0) | 1 (16.7) | |
| No | 17 (37.0) | 5 (83.3) | |
| Diabetes | 0.57 | ||
| Yes | 9 (19.6) | 0 (0) | |
| No | 37 (80.4) | 6 (100) | |
| Heart diseases | 1.00 | ||
| Yes | 4 (8.7) | 0 (0) | |
| No | 42 (91.3) | 6 (100) | |
| Obesity or Overweight | 0.63 | ||
| Yes | 35 (76.1) | 4 (66.7) | |
| No | 11 (23.9) | 2 (33.3) | |
| Nulliparity | 1.00 | ||
| Yes | 6 (13.0) | 0 (0) | |
| No | 36 (78.3) | 6 (100) | |
| N/A | 4 (8.7) | ||
| Contraceptive use | 0.33 | ||
| Yes | 11 (23.9) | 3 (50) | |
| No | 32 (69.6) | 3 (50) | |
| N/A | 3 (6.5) | - | |
| Exogenous Estrogen Therapy | 0.16 | ||
| Yes | 0 (0.0) | 1 (16.7) | |
| No | 42 (91.3) | 5 (83.3) | |
| N/A | 4 (8.7) | - | |
| Menopause | |||
| Yes | 38 (82.6) | 3 (50.0) | 0.06 |
| No | 6 (13.0) | 3 (50.0) | |
| N/A | 2 (4.4) | - | |
| FIGO Stage | |||
| IA | 10 (21.7) | ||
| IB | 10 (21.7) | ||
| II | 10 (21.7) | ||
| III | 10 (21.7) | ||
| IV | 6 (13.0) | ||
| Histological Grade | |||
| Well differentiated (G1) | 11 (23.9) | ||
| Moderate differentiated (G2) | 22(47.8) | ||
| Poorly differentiated (G3) | 13 (28.3) | ||
| Lymphovascular Infiltration | |||
| Yes | 7 (15.2) | ||
| No | 29 (63.0) | ||
| N/A | 10 (21.8) | - | |
| Myometrial Invasion | |||
| <50% | 22 (47.8) | ||
| ≥50% | 24 (52.2) | ||
| Recurrence | |||
| Yes | 13 (28.3) | ||
| No | 33 (71.7) |
| Overall Survival | ||||
| Category | HR | 95% CI | p Value | |
| Univariate Analysis | ||||
| Age | <median vs. ≥median | 1.872 | 0.9818–3.571 | 0.0569 |
| Histological Grade | G3 vs. G2 vs. G1 | 2.381 | 1.457–3.892 | 0.00054 |
| FIGO Stage | Late vs. Early | 3.713 | 2.011–6.853 | 0.0000274 |
| HMGA1 | High vs. Low | 1.93 | 1.046–3.562 | 0.03 |
| HMGA2 | High vs. Low | 1.501 | 0.3624–6.217 | 0.67 |
| Multivariate Analysis | ||||
| Age | <median vs. ≥median | 2.03 | 1.0417–3.940 | 0.03749 |
| Histological Grade | G3 vs. G2 vs. G1 | 2.013 | 1.2120–3.334 | 0.00683 |
| FIGO Stage | Late vs. Early | 3.33 | 1.7751–6.259 | 0.00018 |
| HMGA1 | High vs. Low | 1,315 | 0.7192–2.558 | 0.34638 |
| Disease-Free Survival | ||||
| Category | HR | 95% CI | p Value | |
| Univariate Analysis | ||||
| Age | <median vs. ≥median | 1.628 | 0.9766–2.715 | 0.0616 |
| Histological Grade | G3 vs. G2 vs. G1 | 1,453 | 1.054–2.003 | 0.0224 |
| FIGO Stage | Late vs. Early | 1.922 | 1.128–3.275 | 0.0163 |
| HMGA1 | High vs. Low | 1.974 | 1.199–3.249 | 0.00749 |
| HMGA2 | High vs. Low | 0.5272 | 0.2508–1.109 | 0.889 |
| Multivariate Analysis | ||||
| Age | <median vs. ≥median | 1.5992 | 0.9458–2.704 | 0.0798 |
| Histological Grade | G3 vs. G2 vs. G1 | 1.2960 | 0.9298–1.806 | 0.1259 |
| FIGO Stage | Late vs. Early | 1.8945 | 1.0702–3.196 | 0.0276 |
| HMGA1 | High vs. Low | 1.7849 | 1.0465–3.044 | 0.0334 |
| HMGA1 | HMGA2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Early Stages | Late Stages | Early Stages | Late Stages | |||||
| rho | padj | rho | padj | rho | padj | rho | padj | |
| MMP2 | 0.13 | 0.6 | 0.12 | 1 | 0.21 | 0.008 | 0.33 | 0.07 |
| MMP9 | 0.17 | 0.06 | 0.38 | <0.01 | 0.12 | 0.8 | 0.003 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo Júnior, A.; de Sousa, V.P.L.; Esposito, F.; De Martino, M.; Forzati, F.; Moreira, F.C.d.B.; Simão, T.d.A.; Nasciutti, L.E.; Fusco, A.; Ribeiro Pinto, L.F.; et al. Overexpression of HMGA1 Figures as a Potential Prognostic Factor in Endometrioid Endometrial Carcinoma (EEC). Genes 2019, 10, 372. https://doi.org/10.3390/genes10050372
Palumbo Júnior A, de Sousa VPL, Esposito F, De Martino M, Forzati F, Moreira FCdB, Simão TdA, Nasciutti LE, Fusco A, Ribeiro Pinto LF, et al. Overexpression of HMGA1 Figures as a Potential Prognostic Factor in Endometrioid Endometrial Carcinoma (EEC). Genes. 2019; 10(5):372. https://doi.org/10.3390/genes10050372
Chicago/Turabian StylePalumbo Júnior, Antonio, Vanessa Paiva Leite de Sousa, Francesco Esposito, Marco De Martino, Floriana Forzati, Fábio Carvalho de Barros Moreira, Tatiana de Almeida Simão, Luiz Eurico Nasciutti, Alfredo Fusco, Luis Felipe Ribeiro Pinto, and et al. 2019. "Overexpression of HMGA1 Figures as a Potential Prognostic Factor in Endometrioid Endometrial Carcinoma (EEC)" Genes 10, no. 5: 372. https://doi.org/10.3390/genes10050372
APA StylePalumbo Júnior, A., de Sousa, V. P. L., Esposito, F., De Martino, M., Forzati, F., Moreira, F. C. d. B., Simão, T. d. A., Nasciutti, L. E., Fusco, A., Ribeiro Pinto, L. F., Bessa Pereira Chaves, C., & Meireles Da Costa, N. (2019). Overexpression of HMGA1 Figures as a Potential Prognostic Factor in Endometrioid Endometrial Carcinoma (EEC). Genes, 10(5), 372. https://doi.org/10.3390/genes10050372






