An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites
Abstract
:1. Introduction
2. A brief synopsis of host hybridization
5. Ecological and evolutionary significance of parasite hybrids.
6. Concluding remarks and future directions
References and Notes
- Barton, N.H. The role of hybridization in evolution. Mol. Ecol. 2001, 10, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.S.; Moulia, C.; Newcombe, G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu. Rev. Ecol. Syst. 1999, 30, 565–591. [Google Scholar] [CrossRef]
- Wolinska, J.; Lively, C.M.; Spaak, P. Parasites in hybridizing communities: the Red Queen again? Trends Parasitol. 2008, 24, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B.; Giraud, T.; Hood, M.E. Maximized virulence in a sterilizing pathogen: the anther-smut fungus and its co-evolved hosts. J. Evolution. Biol. 2008, 21, 1544–1554. [Google Scholar] [CrossRef]
- Brant, S.V.; Ortí, G. Evidence for gene flow in parasitic nematodes between two host species of shrews. Mol. Ecol. 2003, 12, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Slotman, M.; Parmakelis, A.; Marshall, J.C.; Awono-Ambene, P.H.; Antonion-Nkondjo, C.; Simard, F.; Caccone, A.; Powell, J.R. Patterns of selection in anti-malarial immune genes in malaria vectors: evidence for adaptive evolution in LRIM1 in Anopheles arabiensis. PloS One 2007, 2, e793. [Google Scholar] [CrossRef] [PubMed]
- Parmakelis, A.; Slotman, M.A.; Marshall, J.C.; Awono-Ambene, P.H.; Antonio-Nkondjio, C.; Simard, F.; Caccone, A.; Powell, J.R. The molecular evolution of four anti-malarial immune genes in the Anopheles gambiae species complex. BMC Evolution. Biol. 2008, 8, 79. [Google Scholar] [CrossRef]
- Huang, J.; Mullapudi, N.; Sicheritz-Ponten, T.; Kissinger, J.C. A first glimpse into the pattern and scale of gene transfer in the Apicomplexa. Int. J. Parasitol. 2004, 34, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.W.; McConkey, G.A.; Westhead, D.R. Prediction of horizontal gene transfers in eukaryotes: approaches and challenges. Biochem. Soc. Trans. 2009, 37, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Striepen, B.; Pruijssers, A.J.P.; Huang, J.; Li, C.; Gubbels, M.; Umejiego, N.N.; Hedstrom, L.; Kissinger, J.C. Gene transfer in the evolution of parasite nucleotide biosynthesis. P. Natl. Acad. Sci U. S. A. 2004, 101, 3154–3159. [Google Scholar] [CrossRef]
- Brindley, P.J.; Mitreva, M.; Ghedin, E.; Lustigman, S. Helminth genomics: the implications for human health. PLoS Neglect. Trop. D. 2009, 3, e538. [Google Scholar] [CrossRef] [Green Version]
- Wasmuth, J.; Daub, J.; Peregrín-Alvarez, J.M.; Finney, C.A.M.; Parkinson, J. The origins of apicomplexan sequence innovation. Genome Res. 2010, 19, 1202–1213. [Google Scholar] [CrossRef]
- Fenn, K.; Conlon, C.; Jones, M.; Quail, M.A.; Holroyd, N.E.; Parkhill, J.; Blaxter, M. Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2006, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Bandi, C.; McCall, J.W.; Genchi, C.; Corona, S.; Venco, L.; Sacchi, L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 1999, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Hoerauf, A.; Nissen-Pahle, K.; Schmetz, C.; Henkle-Duhrsen, K.; Blaxter, M.L.; Büttner, D.W.; Gallin, M.Y.; Al-Qaoud, K.M.; Lucius, R.; Fleischer, B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999, 103, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; Vincze, T.; Ingram, J.; Moran, L.; Lapidus, A.; Omelchenko, M.; Kyrpides, N.; Ghedin, E.; Wang, S.; Goltsman, E.; Joukov, V.; Ostrovskaya, O.; Tsukerman, K.; Mazur, M.; Comb, D.; Koonin, E.; Slatko, B. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning Hotopp, J.C.; Clark, M.E.; Oliveira, D.C.S.G.; Foster, J.M.; Fischer, P.; Muñoz Torres, M.C.; Giebel, J.D.; Kumar, N.; Ishmael, N.; Wang, S.; Ingram, J.; Nen, R.V.; Shepard, J.; Tomkins, J.; Richards, S. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 2007, 317, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.N.; Mitreva, M.; Heinz, M.; Martin, J.; Brattig, N.W.; Weil, G.J.; Fischer, P.U. Wolbachia sequences in the chromosomal genome of Onchocercia flexuosa indicate past Wolbachia endosymbiosis. Am. J. Trop. Med. Hyg. 2008, 29 (S), 127. [Google Scholar] [PubMed]
- McNulty, S.N.; Foster, J.M.; Mitreva, M.; Dunning-Hotopp, J.C.; Martin, J.; Fischer, K.; Wu, B.; Davis, P.J.; Kumar, S.; Brattig, N.W.; Slatko, B.E.; Weil, G.J.; Fischer, P.U. Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS One. In press. [PubMed]
- Wasmuth, J.; Daub, J.; Peregrín-Alvarez, J.M.; Finney, C.A.M.; Parkinson, J. The origins of apicomplexan sequence innovation. Genome. Res. 2009, 19, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Fast, N.M.; Kissinger, J.C.; Roos, D.S; Keeling, P.J. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol. Biol. Evol. 2001, 18, 418–426. [Google Scholar] [PubMed]
- Hannaert, V.; Saavedra, E.; Duffieux, F.; Szikora, J.; Rigden, D.J.; Michels, P.A.M.; Opperdoes, F.R. Plant-like traits associated with metabolism of Trypanosoma parasites. P. Natl. Acad. Sci. U. S. A. 2003, 100, 1067–1071. [Google Scholar] [CrossRef]
- Striepen, B.; White, M.W.; Li, C.; Guerini, M.N.; Banoo-Malik, S.; Logsdon Jr., J.M.; Liu, C.; Abrahamsen, M.S. Genetic complementation in apicomplexan parasites. P. Natl. Acad. Sci. U. S. A. 2002, 99, 6304–6309. [Google Scholar] [CrossRef]
- Nixon, J.E.J.; Wang, A.; Field, J.; Morrison, H.G.; McArthur, A.G.; Sogin, M.L.; Loftus, B.J.; Samuelson, J. Evidence for lateral transfer of genes encoding ferrdoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 2002, 1, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.O.; Sjögren, Å.M.; Davis, L.A.M.; Embley, T.M.; Roger, A.J. Phylogenetic analyses of diplomonad genes reveal frequent lateral gene transfers affecting eukaryotes. Curr. Biol. 2003, 13, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, M.J.; Lupas, A.; Italia, M.J.; Koretke, K.K.; Volker, C.; Brown, J.R. Phylogenetic analyses do not support horizontal gene transfers from bacteria to vertebrates. Nature 2001, 411, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Sicheritz-Ponten, T.; Andersson, S.G. A phylogenomic approach to microbial evolution. Nucleic Acids Res. 2001, 29, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L. Natural hybridization and the evolution of domesticated, pest and disease organisms. Mol. Ecol. 2004, 13, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, K.; Noda, Y.; Shibahara, T.; Itagaki, I. Morphological comparisons and hypotheses on the origin of polyploids in parthenogenetic Fasciola sp. J. Parasitol. 2000, 86, 724–729. [Google Scholar] [PubMed]
- Thanh, G.T.N.; Nguyen, V.D.; Vercruysse, J.; Dorny, P.; Thanh, H.L. Genotypic characterization and species identification of Fasciola spp. with implications regarding the isolates infecting goats in Vietnam. Exp. Parasitol. 2009, 123, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, D.; Southgate, V.R.; Vercruysse, J.; Moore, P.J. Observations on natural and experimental interactions between Schistosoma bovis and S. curassoni from West Africa. Acta Trop. 1990, 47, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, G.; Malmberg, M. Gyrodactylus in salmon and rainbow trout. In Parasites and diseases in natural waters and aquaculture in Nordic countries; Stenmark, A., Malmberg, G., Eds.; Proc. Aoo-Tax-Symp: Stockholm, Sweden, 1986. [Google Scholar]
- Mo, T.A. Variation of opisthaptoral hard parts of Gyrodactylus salaris Malmberg, 1957 (Monogenea: Gyrodactylidae) on rainbow trout Oncorhynchus mykiss (Walbaum, 1792) in a fish farm, with comments on the spreading of the parasite in south-eastern Norway. Syst. Parasitol. 1991, 20, 1–9. [Google Scholar] [CrossRef]
- Wright, C.A.; Ross, G.C. Hybrids between Schistosoma haematobium and Smattheei and their identification by isoelectric focusing of enzymes. T. Roy. Soc. Trop. Med. H. 1980, 74, 326–332. [Google Scholar] [CrossRef]
- Okamoto, M.; Nakao, M.; Blair, D.; Anantaphruti, M.T.; Waikagul, J.; Ito, A. Evidence of hybridization between Taenia saginata and Taenia asiatica. Parasitol. Int. 2010, 59, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; Loker, E.S. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074. [Google Scholar] [CrossRef] [PubMed]
- Criscione, C.D.; Anderson, J.D.; Sudimack, D.; Peng, W.; Jha, B.; Williams-Blangero, S.; Anderson, T.J.C. Disentangling hybridization and host colonization in parasitic roundworms of humans and pigs. Proc. R. Soc. B. 2007, 274, 2669–2677. [Google Scholar] [CrossRef]
- Anderson, E.C.; Thompson, E.A. A model-based method for identifying species hybrids using multilocus genetic data. Genetics 2002, 160, 1217–1229. [Google Scholar] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [PubMed]
- Corander, J.; Marttinen, P. Bayesian identification of admixture events using multilocus molecular markers. Mol. Ecol. 2006, 15, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Southgate, V.R.; Jourdane, J.; Tchuenté, L.A.T. Recent studies on the reproductive biology of the schistosomes and their relevance to speciation in the Digenea. Int. J. Parasitol. 1998, 28, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, M.L.; Blouin, M.S.; Criscione, C.D. Applying evolutionary genetics to schistosome epidemiology. Infect. Genet. Evol. 2010, 10, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; DeJong, R.J.; Lwambo, J.S.; Mungai, B.N.; Mkoji, G.M.; Loker, E.S. First report of a natural hybrid between Schistosoma mansoni and S. rodhaini. J. Parasitol. 2003, 89, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.J.; Evans, N.A. Parthenogenesis and asexual multiplication among parasitic platyhelminths. Parasitology 1983, 86, 121–160. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, K.; Akahane, H.; Habe, S.; Moriyama, N. The geographical distribution of common liver fluke (the genus Fasciola) with normal and abnormal spermatogenesis. J. Vet. Med. Sci. 1982, 44, 223–231. [Google Scholar]
- Itagaki, T.; Sakaguchi, K.; Terasaki, K.; Sasaki, O.; Yoshihara, S.; Truong, V.D. Occurrence of spermic diploid and aspermic triploid forms of Fasciola in Vietnam and their molecular characterization based on nuclear and mitochondrial DNA. Parasitol. Int. 2009, 58, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Im, K.; Yong, T. Phylogenetic relationships of ribosomal ITS2 and mitochondrial CO1 among diploid and triploid Paragonimus westermani isolates. Korean J. Parasitol. 2003, 41, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Blair, D. Paragonimus and the genus Paragonimus. Adv. Parasit. 1999, 42, 113–222. [Google Scholar]
- D’Souza, T.G.; Storhas, M.; Schulenburg, H.; Beukeboom, L.W.; Michiels, N.K. Occasional sex in an ‘asexual’ polyploidy hermaphrodite. Proc. R. Soc. B. 2004, 271, 1001–1007. [Google Scholar] [CrossRef]
- Tibayrenc, M.; Ayala, F.J. The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol. 2002, 18, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Victoir, K.; De Doncker, S.; Cabrera, L.; Alvarez, E.; Arevalo, J.; Llanos-Cuentas, A.; Le Ray, D.; Dujardin, J.C. Direct identification of Leishmania species in biopsies from patients with American tegumentary leishmaniasis. T. Roy. Soc. Trop. Med. Hyg. 2003, 97, 80–87. [Google Scholar] [CrossRef]
- Ravel, C.; Cortes, S.; Pralong, F.; Morio, F.; Dedet, J.P.; Campino, L. First report of genetic hybrids between two very divergent Leishmania species: Leishmania infantum and Leishmania major. Int. J. Parasitol. 2006, 36, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Nolder, D.; Roncal, N.; Davies, C.R.; Llanos-Cuentas, A.; Miles, M.A. Multiple hybrid genotypes of Leishmania (Viannia) in a focus of mucocutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2007, 76, 573–578. [Google Scholar] [PubMed]
- Miles, M.A.; Llewellyn, M.S.; Lewis, M.D.; Yeo, M.; Baleela, R.; Fitzpatrick, S.; Gaunt, M.W.; Mauricio, I.L. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology 2009, 136, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.A.; Ayala, F.J. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. P. Natl. Acad. Sci. U. S. A. 2001, 98, 7396–7401. [Google Scholar] [CrossRef]
- Sturm, N.R.; Vargas, N.S.; Westenberger, S.J.; Zingales, B.; Campbell, D.A. Evidence for multiple hybrid groups in Trypanosoma cruzi. Int. J. Parasitol. 2003, 33, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Westenberger, S.J.; Barabé, C.; Campbell, D.A.; Sturm, N.R. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics 2005, 171, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, M.W.; Yeo, M.; Frame, I.A.; Stothard, J.R.; Carrasco, H.J.; Taylor, M.C.; Mena, S.S.; Veazey, P.; Miles, G.A.J.; Acosta, N.; de Arias, A.R.; Miles, M.A. Mechanism of genetic exchange in American trypanosomes. Nature 2003, 421, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W. Sex and evolution in trypanosomes. Int. J. Parasitol. 2001, 31, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Whittington, H. Genetic exchange in Trypanosoma brucei: selection of hybrid trypanosomes by introduction of genes conferring drug resistance. Mol. Biochem. Parasit. 1993, 60, 19–26. [Google Scholar] [CrossRef]
- Gibson, W.; Peacock, L.; Ferris, V.; Williams, K.; Bailey, M. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei . Parasite. Vector. 2008, 1, 4. [Google Scholar] [CrossRef]
- Lasek-Nesselquist, E.; Welch, D.M.; Thompson, R.C.A.; Steuart, R.F.; Sogin, M.L. Genetic exchange within and between assemblages of Giardia duodenalis. J. Eukaryot. Microbiol. 2009, 56, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.A.; Hopkins, R.M.; Homan, W.L. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today 2000, 16, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L. Humans and associated lineages. In Evolution through genetic exchange; Arnold, M.L., Ed.; Oxford University Press Inc.: New York, U. S. A., 2006. [Google Scholar]
- Torrico, M.C.; de Doncker, S.; Arevalo, J.; Le Ray, D.; Dujardin, J.C. In vitro promastigote fitness of putative Leishmania (Viannia) braziliensis/Leishmania (Viannia) peruviana hybrids. Acta Trop. 1999, 72, 99–110. [Google Scholar] [CrossRef] [PubMed]
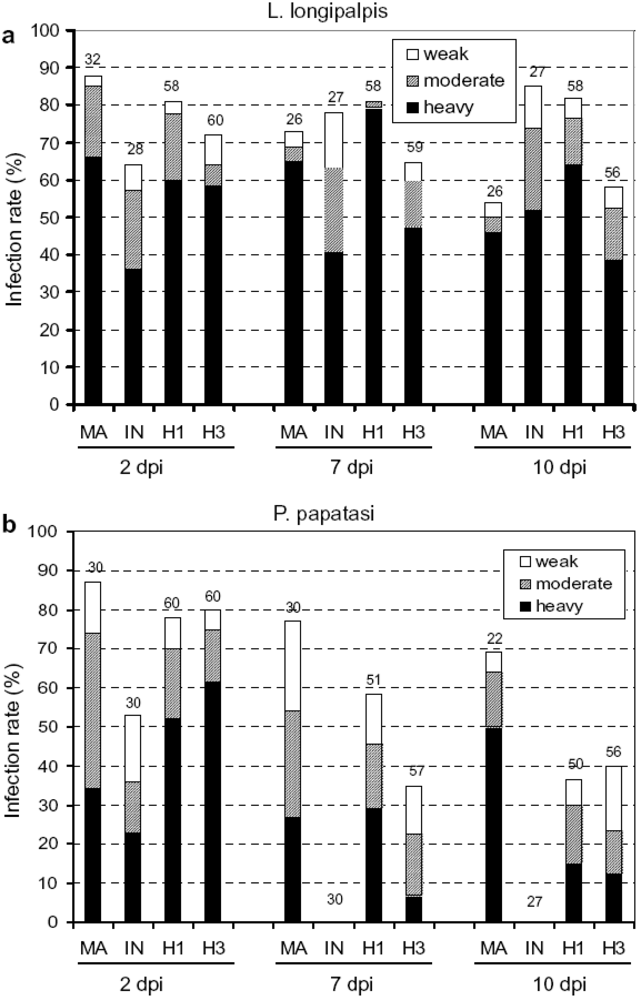
- Volf, P.; Benkova, I.; Myskova, J.; Sadlova, J.; Campino, L.; Ravel, C. Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int. J. Parasitol. 2007, 37, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Kamhawi, S. Phlebotominae sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Dybdahl, M.F.; Jokela, J.; Delph, L.F.; Koskella, B.; Lively, C.M. Hybrid fitness in a locally adapted parasite. Am. Nat. 2008, 172, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Théron, A. Hybrids between Schistosoma mansoni and S. rodhaini: characterization by cercarial emergence rhythms. Parasitology 1989, 99, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Trouvé, S.; Renaud, F.; Durand, P.; Jourdane, J. Experimental evidence of hybrid breakdown between genetically distinct populations of Echinostoma caproni. Parasitology 1998, 117, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Tchuenté, L.A.T.; Jourdane, J.; Southgate, V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Southgate, V.R.; Rollinson, D.; Ross, G.C.; Knowles, R.J. Mating behavior in mixed infections of Schistosoma haematobium and S. intercalatum. J. Nat. His. 1982, 16, 491–496. [Google Scholar] [CrossRef]
- Webster, B.L.; Southgate, V.R. Mating interactions of Schistosoma haematobium and S. intercalatum with their hybrid offspring. Parasitology 2003, 123, 327–338. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Lumme, J. Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea: Gyrodactylidae). Evolution 2002, 56, 2445–2458. [Google Scholar] [PubMed]
- Kuusela, J.; Ziętara, M.S.; Lumme, J. Hybrid origin of Baltic salmon-specific parasite Gyrodactylus salaris: a model for speciation by host switch for hemiclonal organisms. Mol. Ecol. 2007, 16, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, M.S.; Lumme, J. Comparison of molecular phylogeny and morphological systematic in fish parasite genus Gyrodactylus Nordmann, 1832 (Monogenea, Gyrodactylidae). Zoologica Poloniae 2004, 49, 5–28. [Google Scholar]
- Poulin, R. Evolutionary Ecology of Parasites, 2nd ed.; Princeton University Press: Princeton, New Jersey, U. S. A., 2007. [Google Scholar]
- Criscione, C.D.; Blouin, M.S. Life cycles shape parasite evolution: comparative population genetics of salmon trematodes. Evolution 2004, 58, 198–102. [Google Scholar] [PubMed]
- Detwiler, J.T.; Bos, D.H.; Minchella, D.J. Revealing the secret lives of cryptic species: examining the phylogenetic relationships of echinostome parasites in North America. Mol. Phylogenet. Evol. 2010, 55, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Locke, S.A.; McLaughlin, J.D.; Dayanandan, S.; Marcogliese, D.J. Diversity and specificity in Diplostomum spp. metacercariae in freshwater fishes revealed by cytochrome c oxidase I and internal transcribed spacer sequences. Int. J. Parasitol. 2010, 40, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Haas, B.J.; LoVerde, P.T.; Wilson, R.A.; Dillon, G.P.; Cerqueira, G.C.; Mashiyama, S.T.; Al-Lzikani, B.; Andrade, L.F.; Ashton, P.D.; et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009, 460, 352–U65. [Google Scholar] [CrossRef] [PubMed]
- Criscione, C.D.; Valentim, C.L.L.; Hirai, H.; LoVerde, P.T.; Anderson, T.J.C. Genomic linkage map of the human blood fluke Schistosoma mansoni. Genome Biol. 2009, 10, R71. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, A.; Tweedie, A.; McLellan, S.; Taylor, S.; Hall, N.; Berriman, M.; El-Sayed, N.M.; Hope, M.; Turner, C.M.R.; Tait, A. The genetic map and comparative analysis with the physical map of Trypanosoma brucei. Nucleic Acids Res. 2005, 33, 6688–6693. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Tait, A.; Sweeney, L.; Tweedie, A.; Morrison, L.; Turner, C.M.R.; MacLeod, A. Genetic analysis of the human infective trypanosome, Trypanosoma brucei gambiense: chromosomal segregation, crossing over and the construction of a genetic map . Genome Biol. 2008, 9, R103. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.D.; Yuan, K.; Zhou, X.M.; Hu, M.; El-Osta, Y.G.A.; Gasser, R.B. Molecular epidemiological investigation of Ascaris genotypes in China based on single-strand conformation polymorphism analysis of ribosomal DNA. Electrophoresis 2003, 24, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.C. The dangers of using single locus markers in parasite epidemiology: Ascaris as a case study. Trends Parasitol. 2001, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Vrijenhoek, R.C. Genetic differentiation among larval nematodes infecting fishes. J. Parasitol. 1978, 64, 790–798. [Google Scholar] [CrossRef]
- Chilton, N.B.; Beveridge, I.; Hoste, H.; Gasser, R.B. Evidence for hybridization between Paramacropostrongylus iugalis and P. typicus (Nematoda: Strongyloidea) in grey kangaroos, Macropus fuliginosus and M. giganteus, in a zone of sympatry in eastern Australia. Int. J. Parasitol. 1997, 27, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bullini, L.; Nascetti, G.; Ciafrè, S.; Rumore, F.; Biocca, E. Ricerche cariologiche ed su Parascaris univalens e Parascaris equorum. Accademia Nazionale dei Lincei, Rendiconti Classe Scienze Fisiche Matamatiche e Naturali, serie VIII 1978, 65, 151–156. [Google Scholar]
- Paggi, L.; Nascetti, G.; Cianchi, R.; Orecchia, P.; Mattiucci, S.; D’Amelio, S.; Berland, B.; Brattey, J.; Smith, J.W.; Bullini, L. Genetic evidence for three species within Pseudoterranova decipiens (Nematoda, Ascaridida, Ascaridoidea) in the North Atlantic and Norwegian and Barents Sea. Int. J. Parasitol. 1991, 21, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, M.S.; Kuusela, J.; Lumme, J. Escape from an evolutionary dead end: a triploid clone of Gyrodactylus salaris is able to revert to sex and switch host (Platyhelminthes, Monogenea, Gyrodactylidae). Hereditas 2006, 143, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, T.; Tsutsumi, K. Triploid form of Fasciola in Japan: genetic relationships between Fasciola hepatica and Fasciola gigantica determined by ITS-2 sequence of nuclear rDNA. Int. J. Parasitol. 1998, 28, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Q.; Dong, S.J.; Nie, K.; Wang, C.R.; Song, H.Q.; Li, A.X.; Huang, W.Y.; Zhu, X.Q. Sequence analysis of the first internal transcribed spacer of rDNA supports the existence of the intermediate Fasciola between Fhepatica and F. gigantica in mainland China. Parasitol. Res. 2007, 101, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Agatsuma, T.; Arakawa, Y.; Iwagami, M.; Honzako, Y.; Cahyaningsih, U.; Kang, S.Y.; Hong, S.J. Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitol. Int. 2000, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Van De, N.; Agatsuma, T; Nguyen, T.G.T.; Nguyen, Q.D.; Mcmanus, D.P.; Blair, D. Human fascioliasis and the presence of hybrid/introgressed forms of Fasciola hepatica and Fasciola gigantica in Vietnam. Int. J. Parasitol. 2008, 38, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Ichinomiya, M.; Ohtori, M.; Ichikawa, M.; Shibahara, T.; Itagaki, T. Molecular characterization of Fasciola hepatica, Fasciola gigantica, and aspermic Fasciola sp. in China based on nuclear and mitochondrial DNA. Parasitol. Res. 2009, 105, 809–815. [Google Scholar] [CrossRef] [PubMed]
- van Herwerden, L.; Blair, D.; Agatsuma, T. Genetic diversity in parthenogenetic triploid Paragonimus westermani. Int. J. Parasitol. 1999, 29, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.A.; Ahn, J.S.; Kim, S.H.; Rhyu, M.G.; Kong, Y.; Cho, S.Y. PwRn1, a novel Ty3/gypsy-like retrotransposon of Paragonimus westermani: molecular characters and its differentially preserved mobile potential according to host chromosomal polyploidy. BMC Genomics 2008, 9, 482. [Google Scholar] [CrossRef]
- Agatsuma, T.; Iwagami, M.; Sato, Y.; Iwashita, J.; Hong, S.J.; Kang, S.Y.; Ho, L.Y.; Su, K.E.; Kawashima, K.; Abe, T. The origin of the triploid in Paragonimus westermani on the basis of variable regions in the mitochondrial DNA. J. Helminthol. 2003, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kruger, F.J.; Evans, A.C. Do all human urinary infections with Schistosoma mattheei represent hybridization between Schistosoma haematobium and Schistosoma mattheei? J. Helminthol. 1990, 64, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.G. Hybridization experiments on five species of African schistosomes. J. Helminthol. 1970, 44, 253–314. [Google Scholar] [PubMed]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, P.L. Hybridization of Schistosoma mansoni and S. rodhaini. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 3–4. [Google Scholar] [CrossRef]
- Badaraco, J.L.; Ayala, F.J.; Bart, J.M.; Gottstein, B.; Haag, K.L. Using mitochondrial and nuclear markers to evaluate the degree of genetic cohesion among Echinococcus populations. Exp. Parasitol. 2008, 119, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Belli, A.A.; Miles, M.A.; Kelly, J.M. A putative Leishmania panamensis/Leishmania braziliensis hybrid is a causative agent of human cutaneous leishmaniasis in Nicaragua. Parasitology 1994, 109, 435–442. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.T.; Cupolillo, E.; Volpini, A.C.; Almeida, R.; Romero, G.A.S. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Trop. Med. Int. Health 2006, 11, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Kuhls, K.; Chicharro, C.; Cañavate, C.; Cortes, S.; Campino, L.; Haralambous, C.; Soteriadou, K.; Pratlong, F.; Dedet, J.P.; Mauricio, I.; Miles, M.; Schaar, M.; Ochsenreither, S.; Radtke, O.A.; Schönian, G. Differentiation and gene flow among European populations of Leishmania infantum MON-1. PLoS Neglect. Trop. D. 2008, 2,, e261. [Google Scholar] [CrossRef]
- Seridi, N.; Amro, A.; Kuhls, K.; Belkaid, M.; Zidane, C.; Al-Jawabreh, A.; Schönian, G. Genetic polymorphism of Algerian Leishmania infantum strains revealed by multilocus microsatellite analysis. Microbes Infect. 2008, 10, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, I.L.; Yeo, M.; Baghaei, M.; Doto, D.; Pratlong, F.; Zemanova, E.; Dedet, J.P.; Lukes, J.; Miles, M.A. Towards multilocus sequence typing of the Leishmania donovani complex: resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD). Int. J. Parasitol. 2006, 36, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; De Doncker, S.; Coronado, X.; Barnabé, C.; Tibyarenc, M.; Solari, A.; Dujardin, J.C. Evolutionary history of Trypanosoma cruzi according to antigen genes. Parasitology 2008, 135, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Brisse, S.; Henriksson, J.; Barnabé, C.; Douzery, E.J.P.; Berkvens, D.; Serrano, M.; Carvalho, M.R.C.; Buck, G.A.; Dujardin, J.C.; Tibayrenc, M. Infect. Genet. Evol. 2003, 2, 173–183. [Google Scholar] [CrossRef]
- Pena, S.D.J.; Machado, C.R.; Macedo, A.M. Trypansoma cruzi: ancestral genomes and population structure. Mem. I. Oswaldo Cruz 2009, 104 (S1), 108–114. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Detwiler, J.T.; Criscione, C.D. An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites. Genes 2010, 1, 102-123. https://doi.org/10.3390/genes1010102
Detwiler JT, Criscione CD. An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites. Genes. 2010; 1(1):102-123. https://doi.org/10.3390/genes1010102
Chicago/Turabian StyleDetwiler, Jillian T., and Charles D. Criscione. 2010. "An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites" Genes 1, no. 1: 102-123. https://doi.org/10.3390/genes1010102
APA StyleDetwiler, J. T., & Criscione, C. D. (2010). An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites. Genes, 1(1), 102-123. https://doi.org/10.3390/genes1010102