A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Approximately 2 μL of MWCNT (60 ppm) is first dropped on the interdigitated electrode by using a microjet.
- (2)
- The solvent is evaporated, and the MWCNT film is furnished for 6 h at 70 °C.
- (3)
- Approximately 2 μL solution of the selected polymers is dropped on the MWCNT layer by using a microjet.
- (4)
- The solvent is evaporated completely, and the selected polymer film is furnished for 24 h at 60 °C.
- (5)
- After performing the aforementioned casting steps, the resistance of each sensor is confirmed to limit the value within 1–50 kΩ.
3. Gas-Sensing System Design
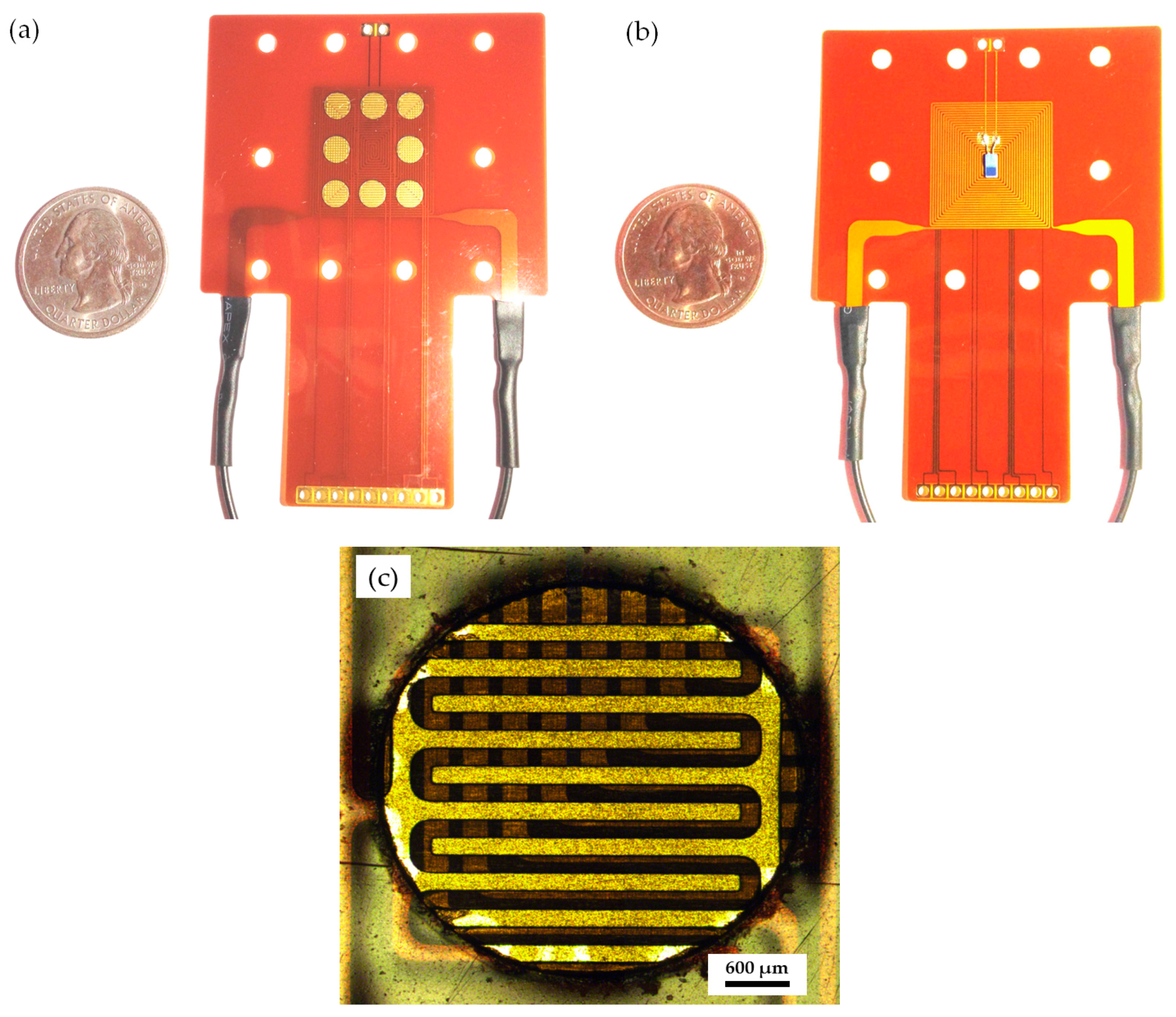
3.1. Flexible Gas Sensor Array
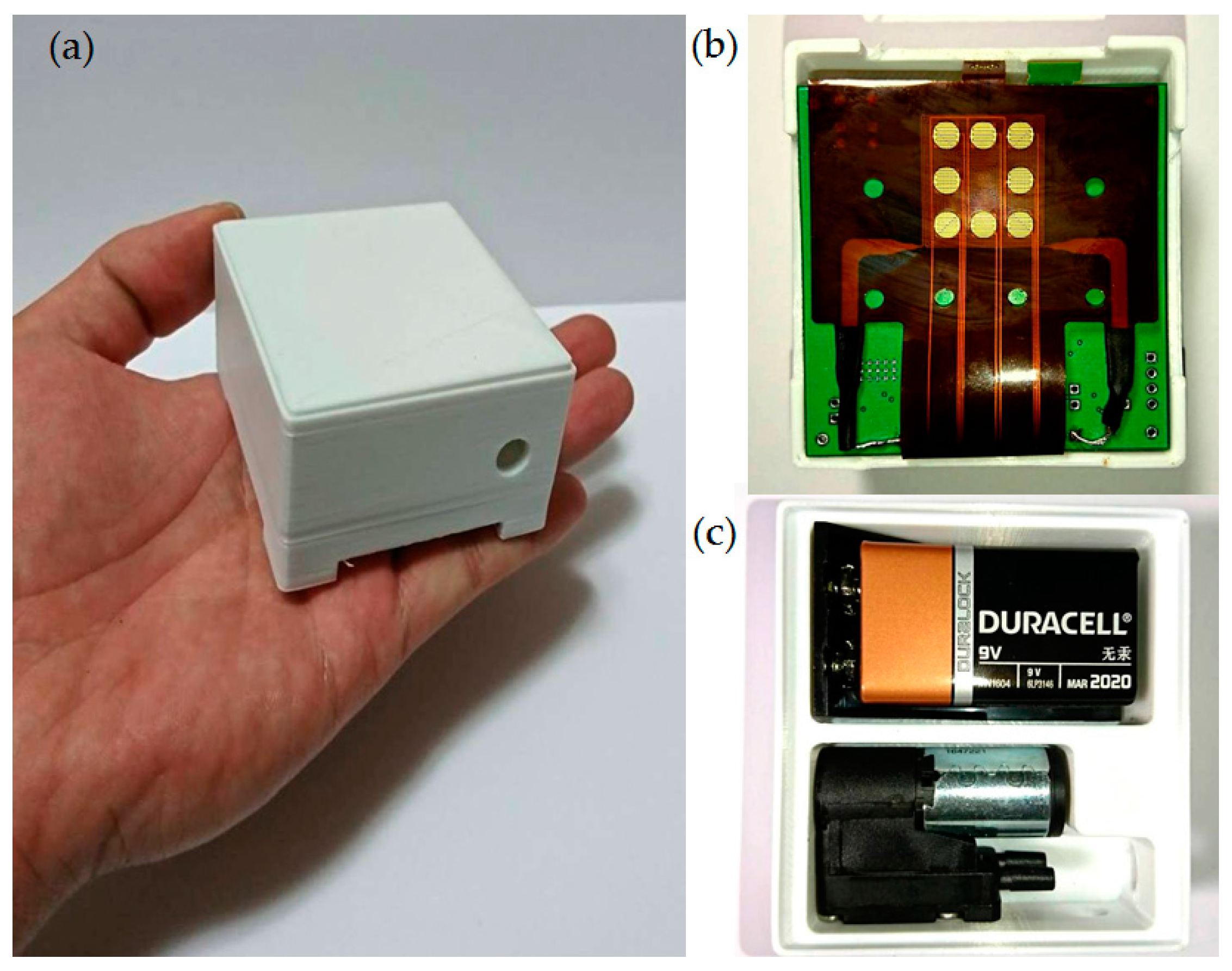
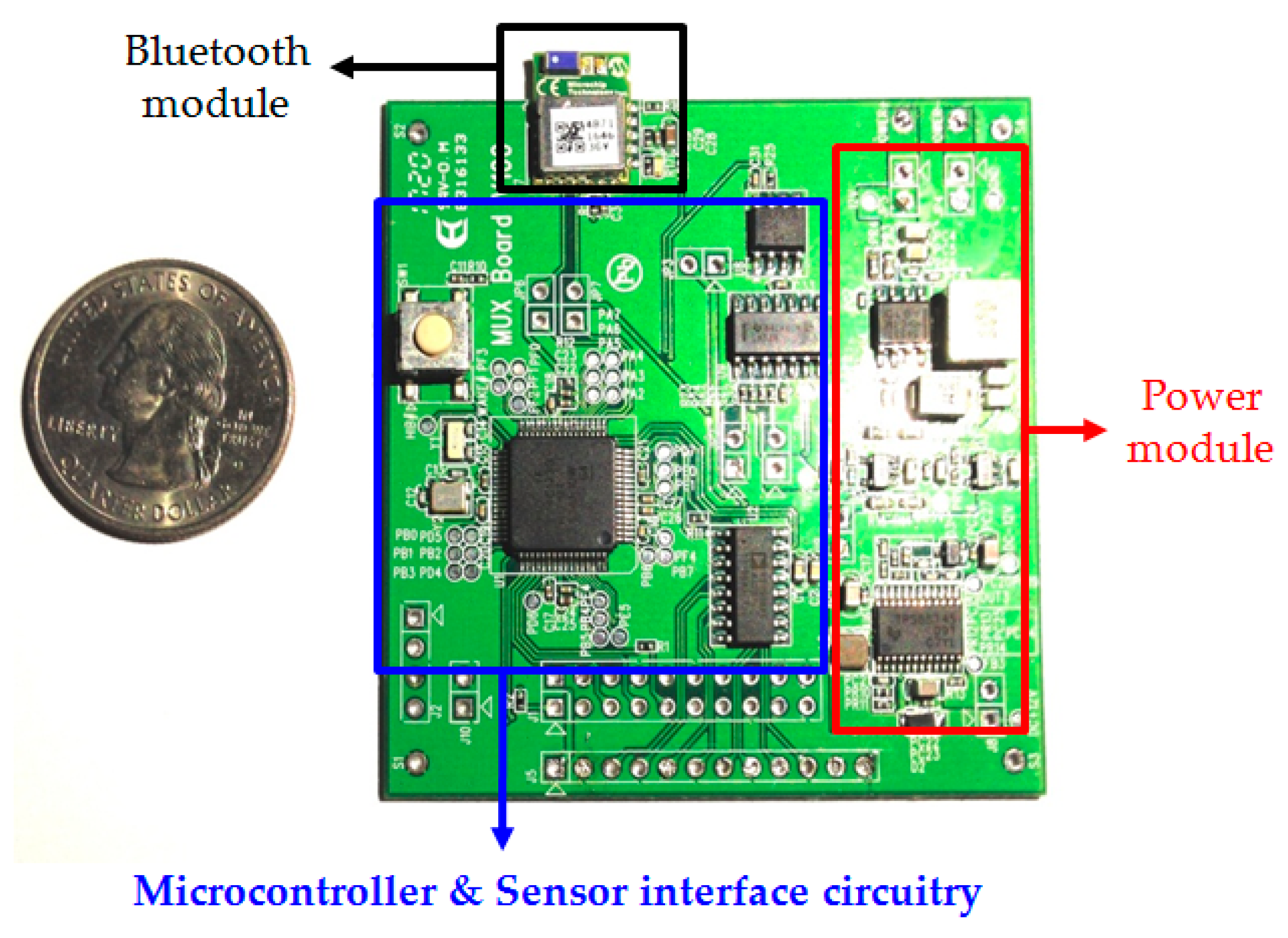
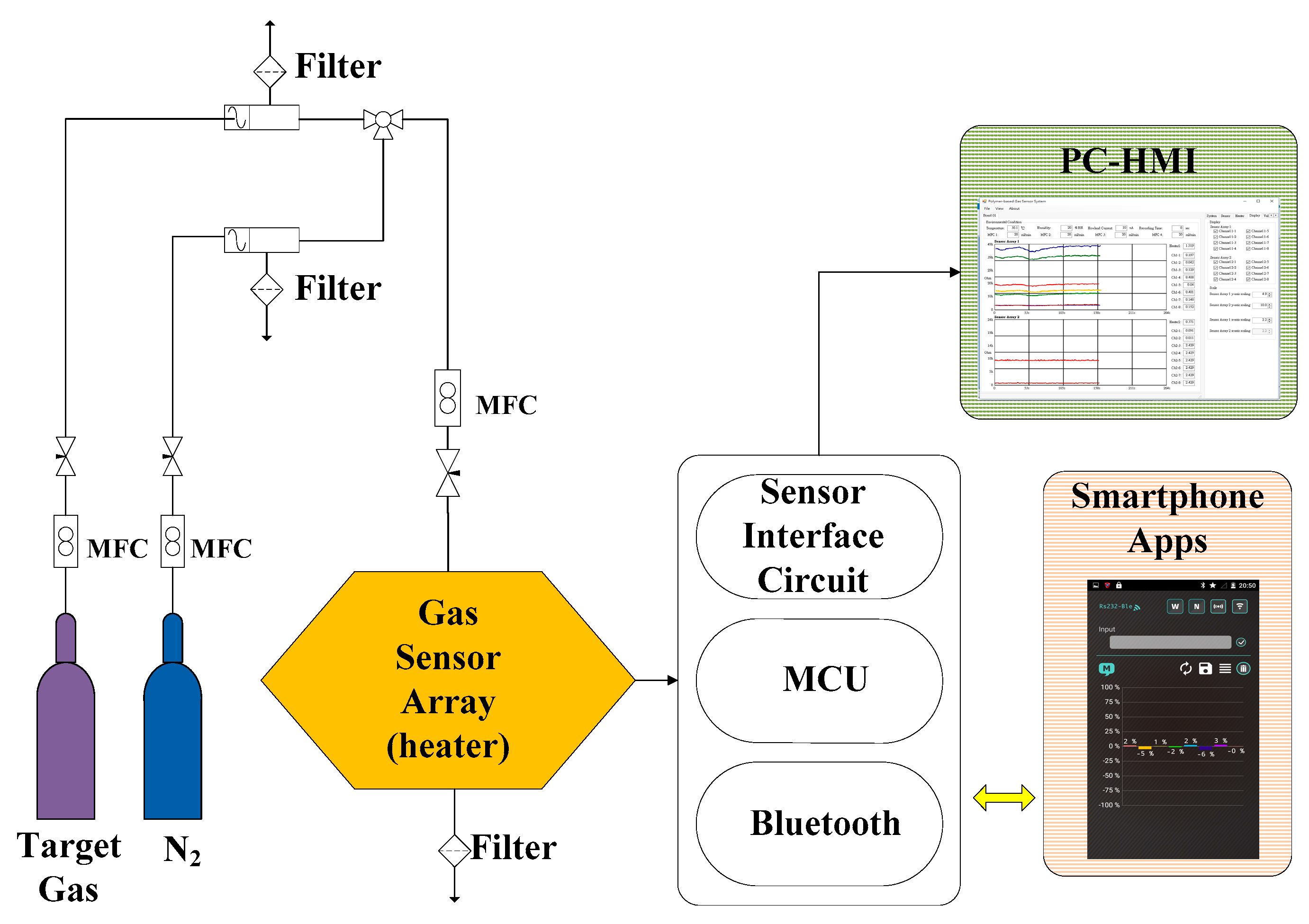
3.2. Gas-Sensing System Architecture
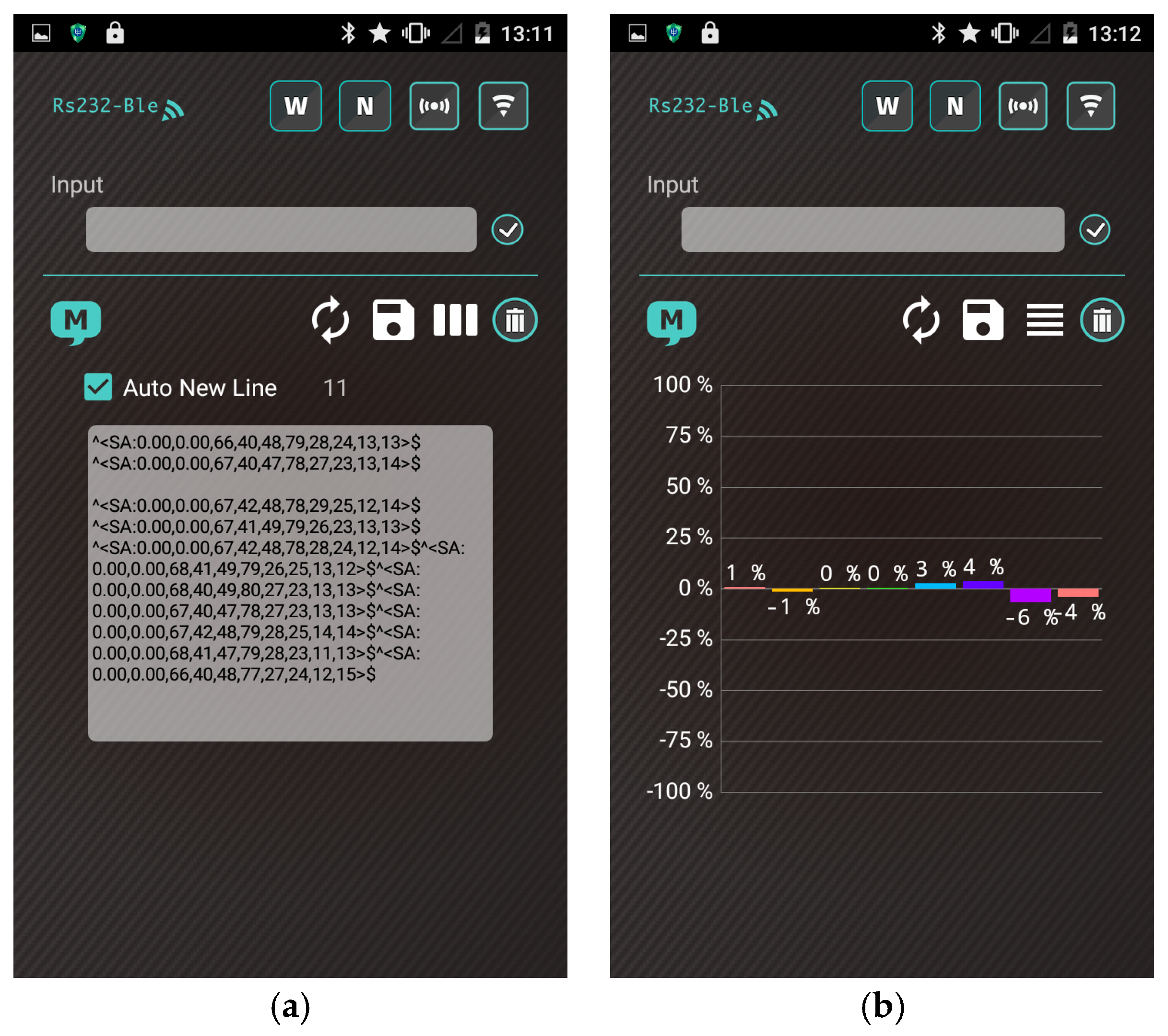
3.3. Smartphone Application
- (1)
- The user turns on the gas-sensing system, and the application establishes a secure Bluetooth connection with the gas-sensing system.
- (2)
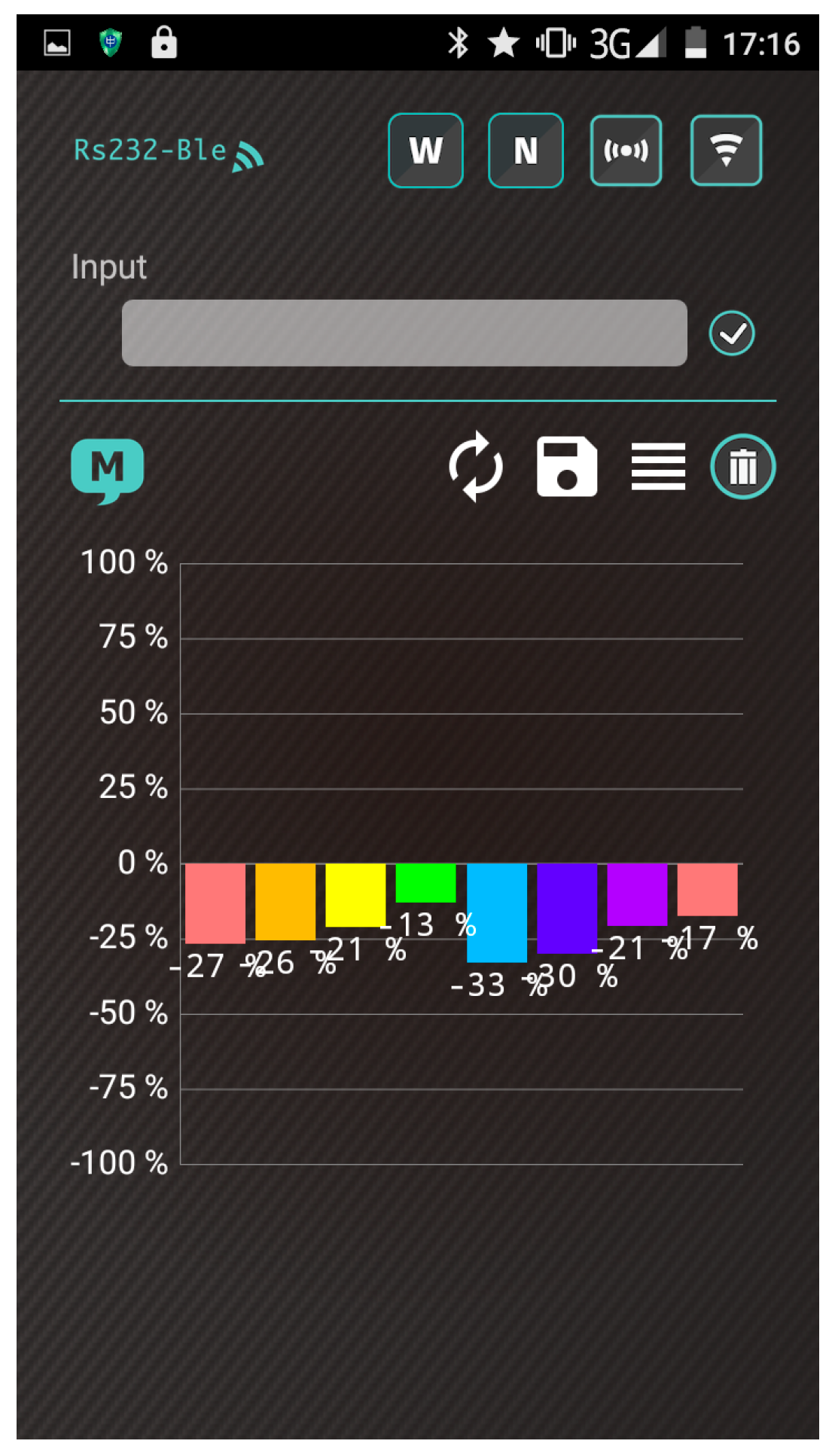
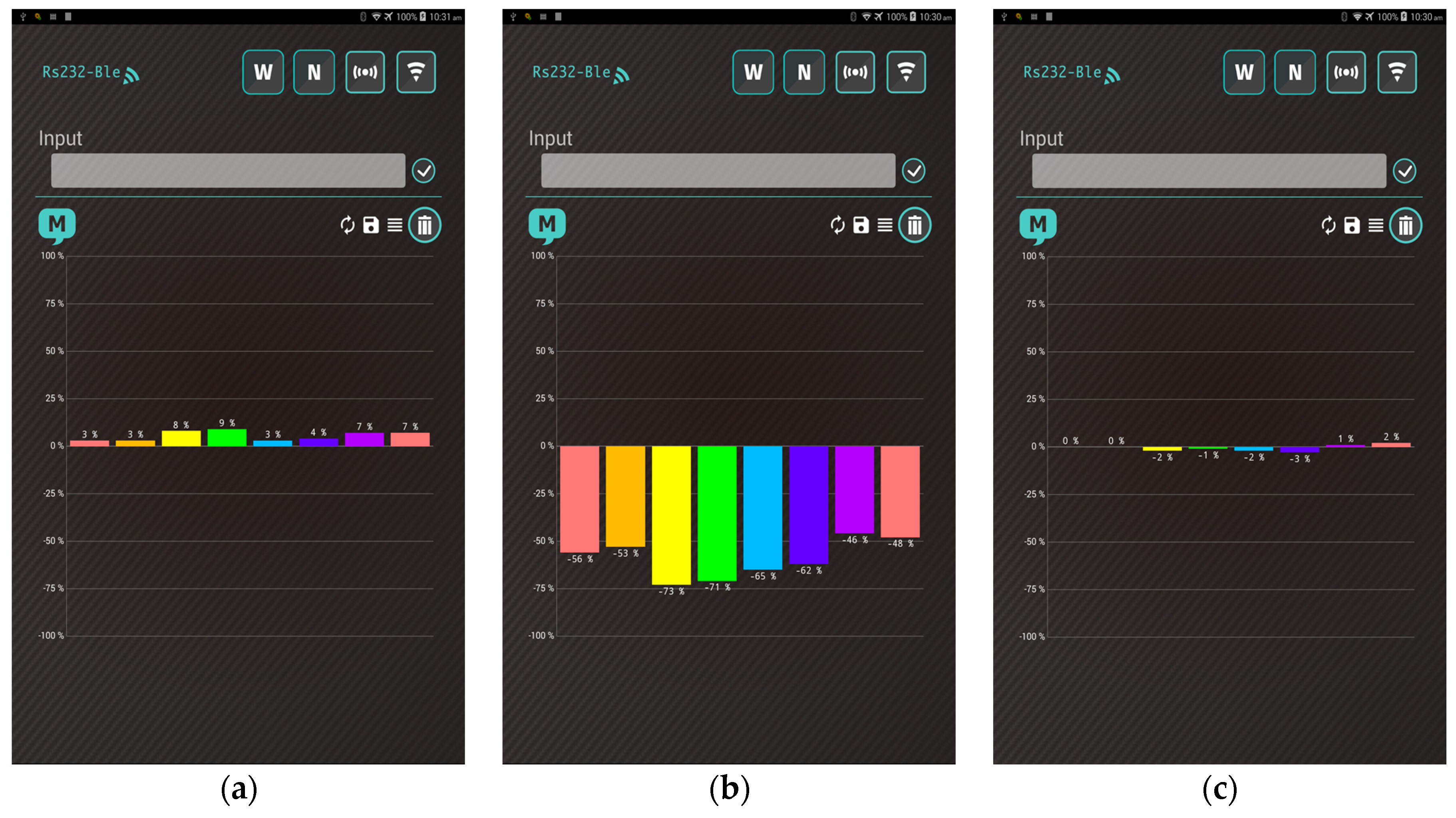
- The heater remains stable at the operating temperature (30 s). Subsequently, the app receives a stream of sensor data under the normal condition in real time from the gas-sensing system, as shown in Figure 7a.
- (3)
- A real-time bar chart graph of the normalized values with respect to the selected polymers for sensing activities is constructed and displayed in the app, as shown in Figure 7b. The sensor response data are refreshed at intervals of 1 s. Table 2 presents the polymer/MWCNT composite sensors and the relative bar chart channel numbers (from left to right).
- (4)
- The app continuously logs sensor response for 10 min. After the subjects complete the task, the app clears the data and resets to step (2) for a new cycle.
- (5)
- These data and graphs are stored on the device and uploaded to cloud servers online.
4. Results and Discussion
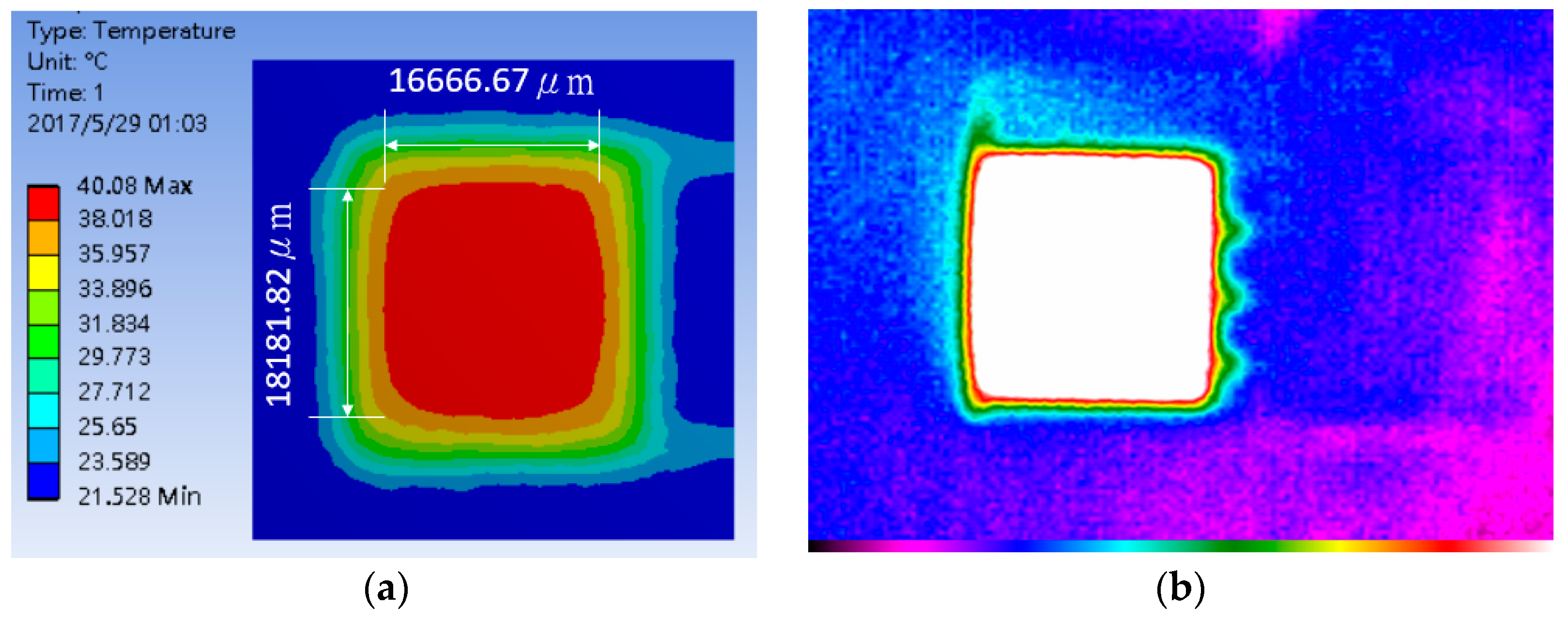
4.1. Heater Performance
4.2. Thermal Stability
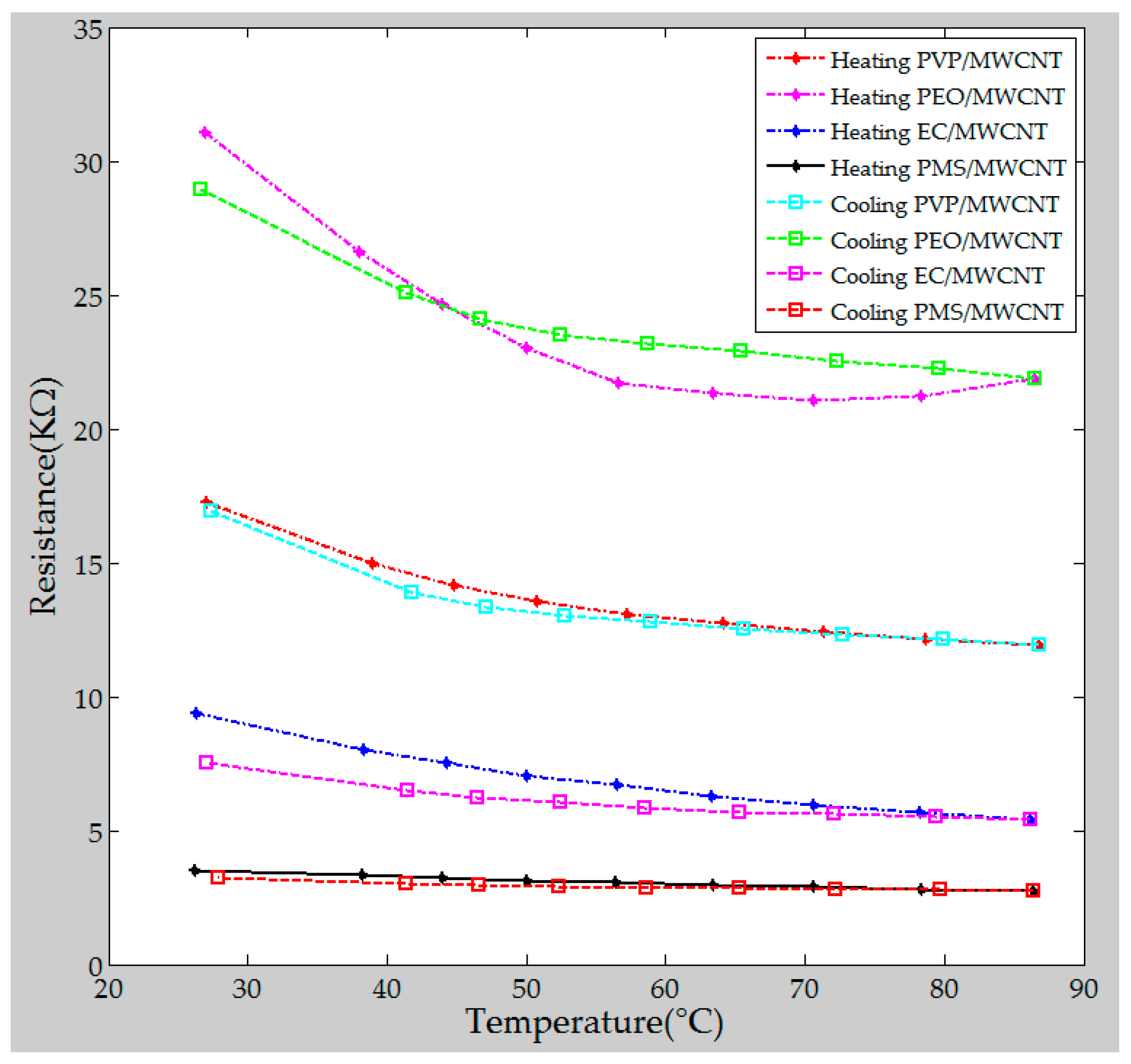
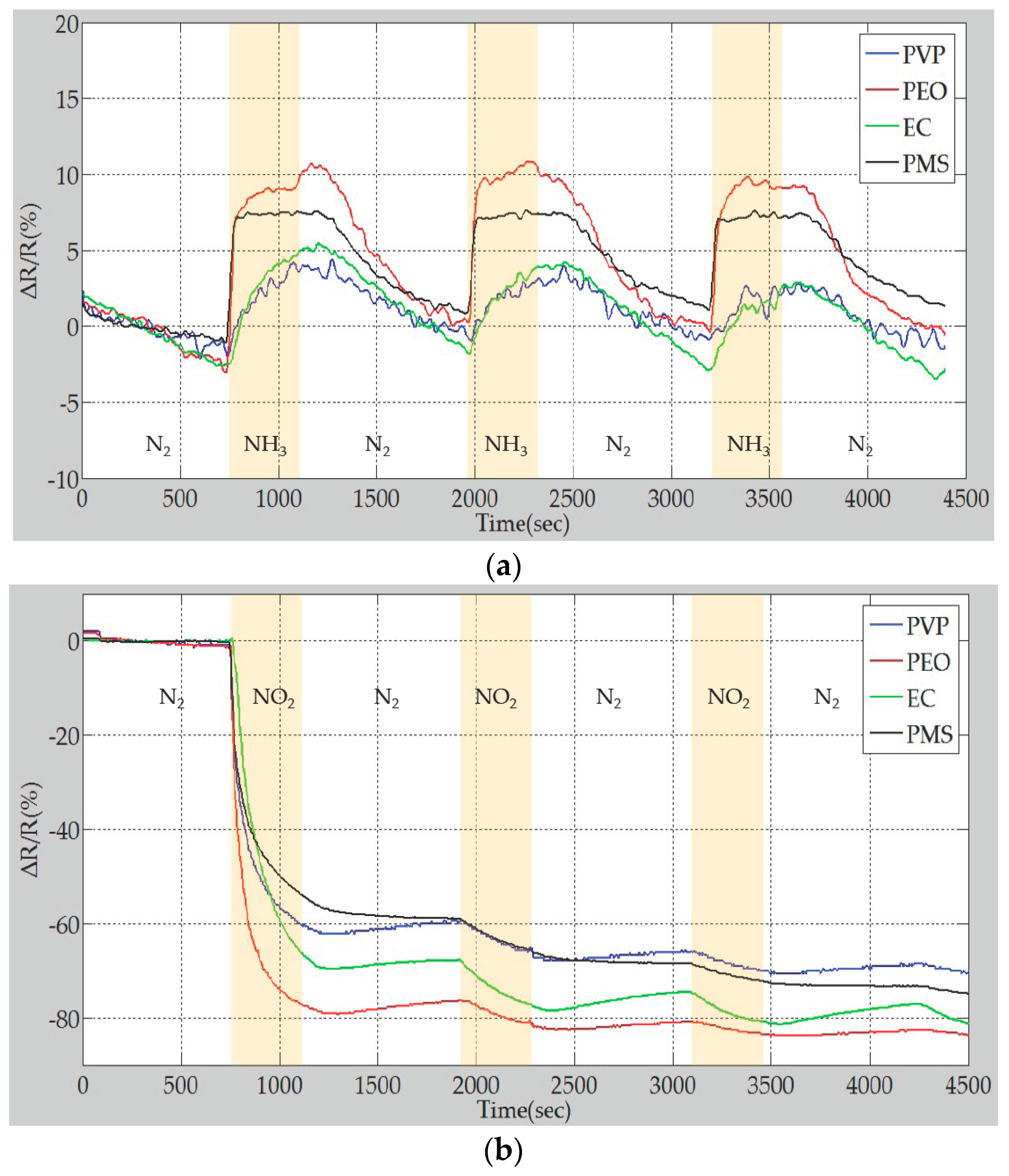
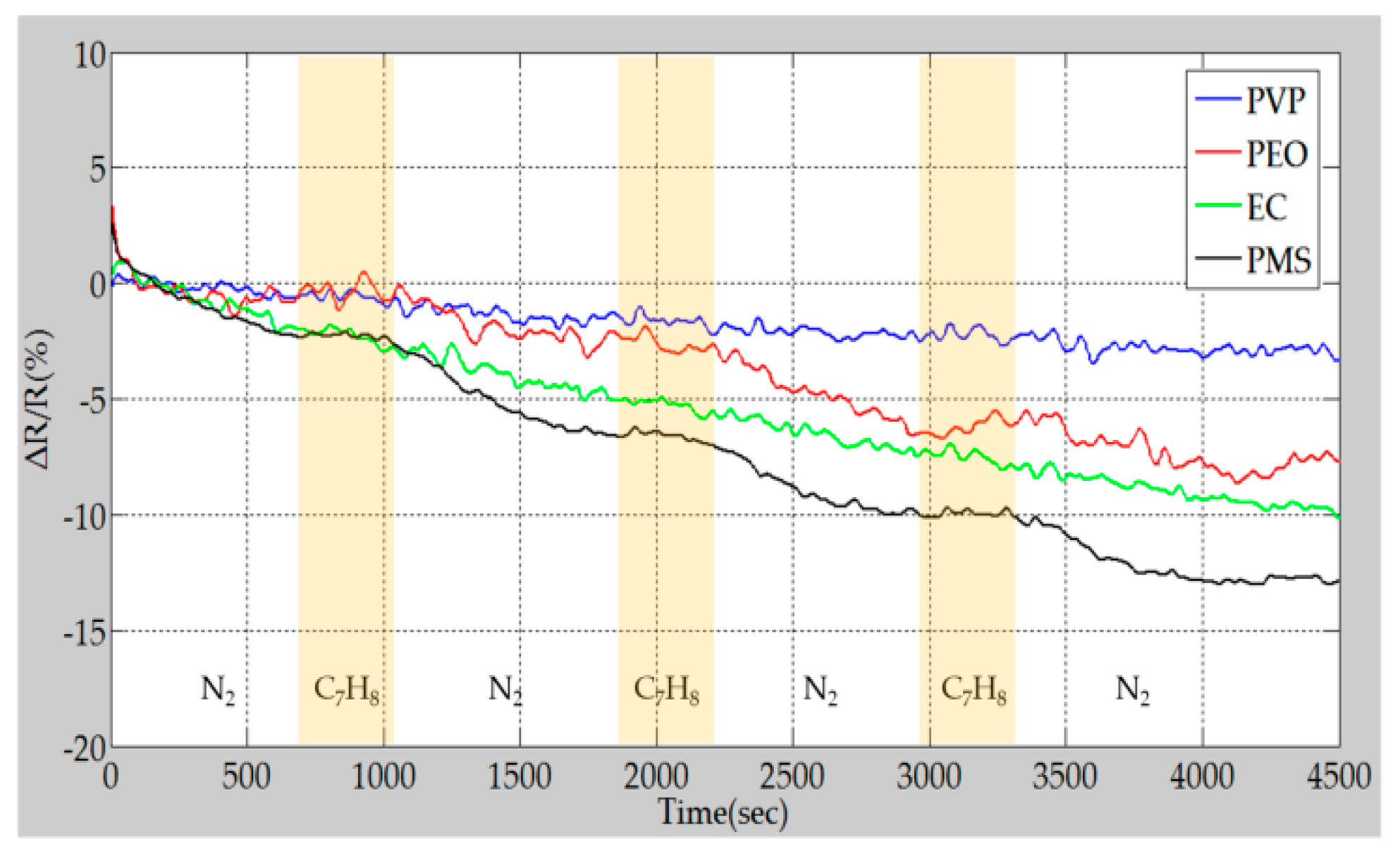
4.3. Sensing Performance
4.4. Smartphone App Communication
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osseiran, N.; Chriscaden, K. WHO Releases Country Estimates on Air Pollution Exposure and Health Impact. Available online: http://www.who.int/mediacentre/news/releases/2016/air-pollution-estimates/en/ (accessed on 23 July 2017).
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Gugamsetty, B.; Wei, H.; Liu, C.N.; Awasthi, A.; Hsu, S.C.; Tsai, C.J.; Roam, G.D.; Wu, Y.C.; Chen, C.F. Source characterization and apportionment of PM10, PM2.5 and PM0.1 by using positive matrix factorization. Aerosol Air Qual. Res. 2012, 12, 476–491. [Google Scholar] [CrossRef]
- Tucker, W.G. An overview of PM 2.5 sources and control strategies. Fuel Process Technol. 2000, 65, 379–392. [Google Scholar] [CrossRef]
- Wolkoff, P. Volatile organic compounds—Sources, measurements, emissions, and the impact on indoor air quality. Indoor Air 1995, 5, 1–73. [Google Scholar] [CrossRef]
- Wallace, L.H. exposure to volatile organic pollutants: Implications for indoor air studies. Annu. Rev. Energy Environ. 2001, 26, 269–301. [Google Scholar] [CrossRef]
- Tsow, F.; Forzani, E.; Rai, A.; Wang, R.; Tsui, R.; Mastroianni, S.; Knobbe, C.; Tao, N.; Gandolfi, A.J. A wearable and wireless sensor system for real-time monitoring of toxic environmental volatile organic compounds. IEEE Sens. J. 2009, 9, 1734–1740. [Google Scholar] [CrossRef]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Lin, M.N.; Feng, C.T.; Yang, K.L.; Lo, Y.S. Measurement of toxic volatile organic compounds in indoor air of semiconductor foundries using multisorbent adsorption/thermal desorption coupled with gas chromatographyemass spectrometry. J. Chromatogr. A 2003, 996, 225–231. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Yu, J.Z. Determination of airborne carbonyls: Comparison of a thermal desorption/GC method with the standard DNPH/HPLC method. Environ. Sci. Technol. 2004, 38, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Helmig, D.; Pollock, W.; Greenberg, J.; Zimmerman, P. Gas chromatography mass spectrometry analysis of volatile organic trace gases at Mauna Loa Observatory, Hawaii. J. Geophys. Res. 1996, 101, 14697–14710. [Google Scholar] [CrossRef]
- Lanucara, F.; Holman, S.W.; Gray, C.J.; Eyers, C.E. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 2014, 6, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Catalan, L.J.J.; Liang, V.; Jia, C.Q. Comparison of various detection limit estimates for volatile sulphur compounds by gas chromatography with pulsed flame photometric detection. J. Chromatogr. A 2006, 1136, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liou, N.; Sun, E. Applications of open-path Fourier transform infrared for identification of volatile organic compound pollution sources and characterization of source emission behaviors. J. Air Waste Manag. Assoc. 2008, 58, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Sys. Man Cybern. C 2010, 40, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Mu, X.; Yang, Y.; Mason, A.J. Low power multimode electrochemical gas sensor array system for wearable health and safety monitoring. IEEE Sens. J. 2014, 14, 3391–3399. [Google Scholar] [CrossRef]
- Alizadeh, T.; Zeynali, S. Electronic nose based on the polymer coated SAW sensors array for the warfare agent simulants classification. Sens. Actuators B 2008, 129, 412–423. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Tomar, M.; Sharma, M.U.; Gupta, V. Effect of metal oxide sensing layers on the distinct detection of ammonia using surface acoustic wave (SAW) sensors. Sens. Actuators B 2013, 187, 563–573. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Bruckenstein, S. A novel quartz crystal microbalance gas sensor based on porous film coatings. A high sensitivity porous poly(methylmethacrylate) water vapor sensor. Anal. Chim. Acta 2013, 785, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Hannon, A.; Lu, Y.; Li, J.; Meyyappan, M. A sensor array for the detection and discrimination of methane and other environmental pollutant gases. Sensors 2016, 16, 1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Hou, Z.; Xu, D.; Wei, L.; Kong, E.S.W.; Zhang, Y. Flexible gas sensors with assembled carbon nanotube thin films for DMMP vapor detection. Sens. Actuators B 2010, 150, 708–714. [Google Scholar] [CrossRef]
- Ishihara, S.; Azzarelli, J.M.; Krikorian, M.; Swager, T.M. Ultratrace detection of toxic chemicals: Triggered disassembly of supramolecular nanotube wrappers. J. Am. Chem. Soc. 2016, 138, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.K.; Philip, B.; Witchurch, A.; Varadan, V.K.; Reddy, C.C. A compact wireless gas sensor using a carbon nanotube/PMMA thin film chemiresistor. Smart Mater. Struct. 2004, 13, 1045–1049. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A novel wearable electronic nose for healthcare based on flexible printed chemical sensor array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Kim, J. Multi-walled carbon nanotubes-cellulose paper for a chemical vapor sensor. Sens. Actuators B 2010, 150, 308–313. [Google Scholar] [CrossRef]
- Mangu, R.; Rajaputra, S.; Singh, V.P. MWCNT–polymer composites as highly sensitive and selective room temperature gas sensors. Nanotechnology 2011, 22, 215502. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chiou, J.C.; Wang, Y.P.; Wang, L.C. Flexible polymer/multi-walled carbon nanotube composite sensor array equipped with microheater for gas sensing. IEEE 2016. [Google Scholar] [CrossRef]
- Chiou, J.C.; Wu, C.C.; Huang, Y.C.; Chang, S.C.; Lin, T.M. Effects of operating temperature on droplet casting of flexible polymer/multi-walled carbon nanotube composite gas sensors. Sensors 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Vermesh, O.; Grecu, M.; Javey, A.; Wang, Q.; Dai, H. Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection. Nano Lett. 2003, 3, 347–351. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar]
- Krco, S. Bluetooth Based Wireless Sensor Networks—Implementation Issues and Solutions. Invited paper. In Proceedings of the 10th Telecommunications Forum Telfor 2002, Belgrade, Serbia, 26–28 November 2002. [Google Scholar]
- Leopold, M.; Dydensborg, M.B.; Bonnet, P. Bluetooth and sensor networks: A reality check. In Proceedings of the 1st International Conference on Embedded Networked Sensor Systems, Los Angeles, CA, USA, 5–7 November 2003; pp. 103–113. [Google Scholar]
- Zhang, Y.; Xiao, H. Bluetooth-based sensor networks for remotely monitoring the physiological signals of a patient. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 1040–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, J.R.; Lewis, A.L.; Vick, T.A.; Stratford, P.W. Swelling of zwitterionic polymer films characterized by spectroscopic ellipsometry. Macromolecules 2001, 34, 8768–8776. [Google Scholar] [CrossRef]
- Toomey, R.; Freidank, D.; Ruhe, J. Swelling behavior of thin, surface-attached polymer networks. Macromolecules 2004, 37, 882–887. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Wang, L.; Zhu, D. Adsorption of Hydroxyl- and Amino-Substituted Aromatics to Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 6862–6868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Y.D.; Xie, G.Z.; Du, X.S.; Tai, H.L. Gas sensors based on multiple-walled carbon nanotubes-polyethylene oxide films for toluene vapor detection. Sens. Actuators B. 2014, 191, 24–30. [Google Scholar] [CrossRef]
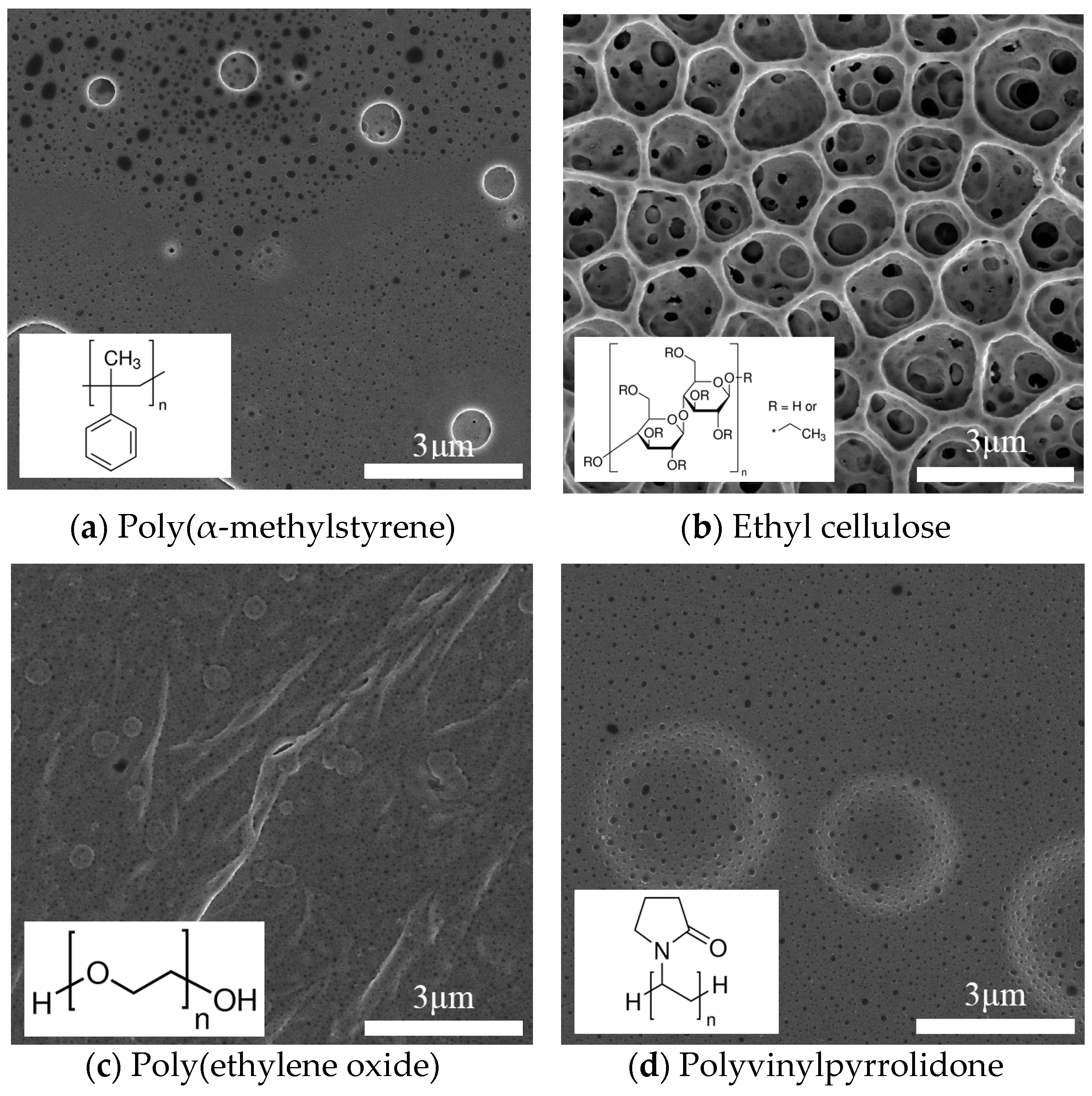


| Sensor Name | Sensing Film | Element Number |
|---|---|---|
| PVP1 | 1 wt % PVP/MWCNT | 3,1 |
| PVP2 | 1 wt % PVP/MWCNT | 2,1 |
| PEO1 | 1 wt % PEO/MWCNT | 1,1 |
| PEO2 | 1 wt % PEO/MWCNT | 3,2 |
| EC1 | 1 wt % EC/MWCNT | 1,2 |
| EC2 | 1 wt % EC/MWCNT | 3,3 |
| PMS1 | 1 wt % PMS/MWCNT | 2,3 |
| PMS2 | 1 wt % PMS/MWCNT | 1,3 |
| * | Resistance Temperature Detector (RTD) | 2,2 |
| Channel No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Sensor Name | PVP1 | PVP2 | PEO1 | PEO2 | EC1 | EC2 | PMS1 | PMS2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, J.-C.; Wu, C.-C. A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films. Polymers 2017, 9, 457. https://doi.org/10.3390/polym9090457
Chiou J-C, Wu C-C. A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films. Polymers. 2017; 9(9):457. https://doi.org/10.3390/polym9090457
Chicago/Turabian StyleChiou, Jin-Chern, and Chin-Cheng Wu. 2017. "A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films" Polymers 9, no. 9: 457. https://doi.org/10.3390/polym9090457
APA StyleChiou, J.-C., & Wu, C.-C. (2017). A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films. Polymers, 9(9), 457. https://doi.org/10.3390/polym9090457




