Influence of the Molar Mass on Long-Chain Branching of Polypropylene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Thermal Analysis
2.3. Molar Mass Determination
2.4. Rheology
2.5. Extensional Rheology
2.6. Sample Preparation
3. Results and Discussion
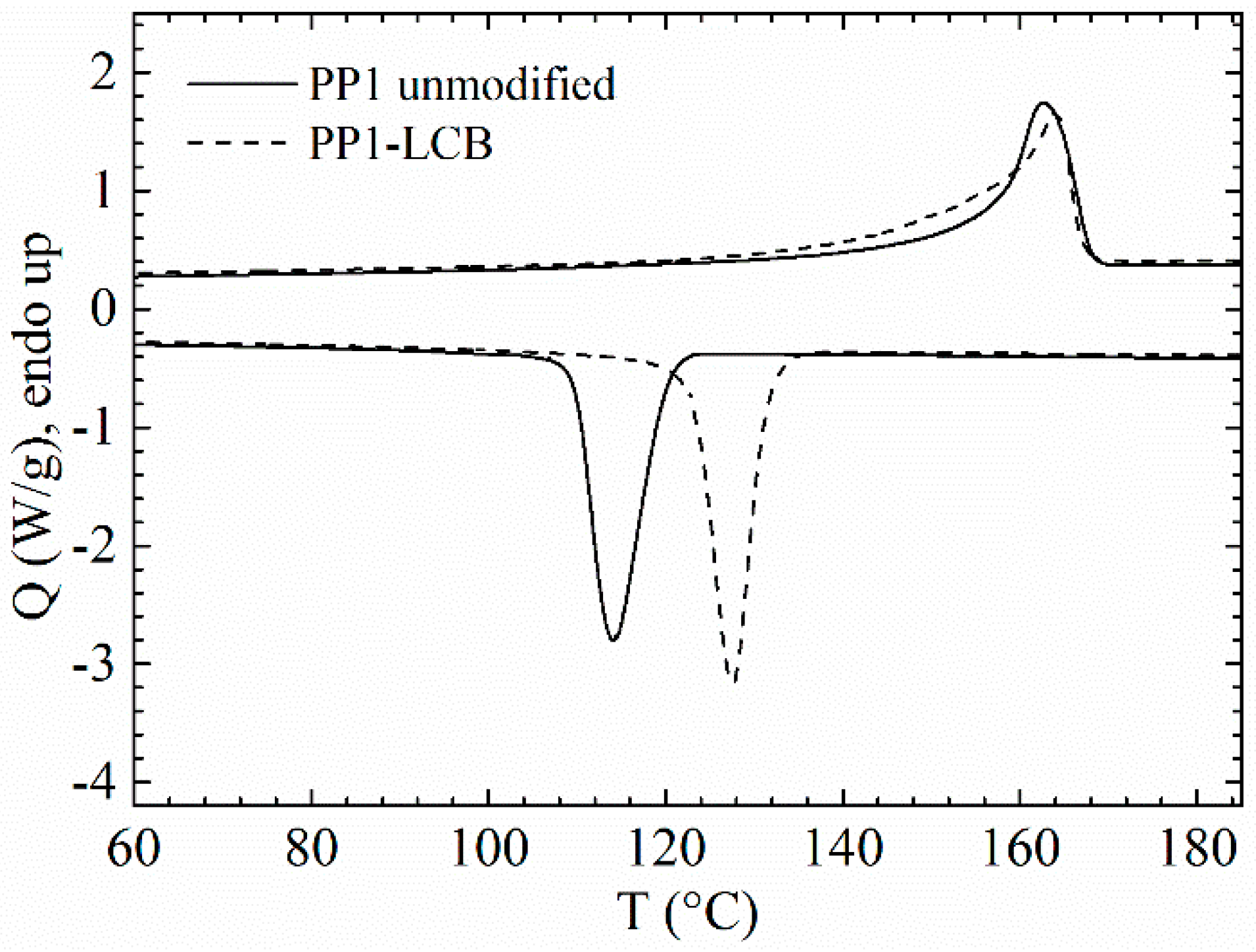
3.1. Thermal Analysis
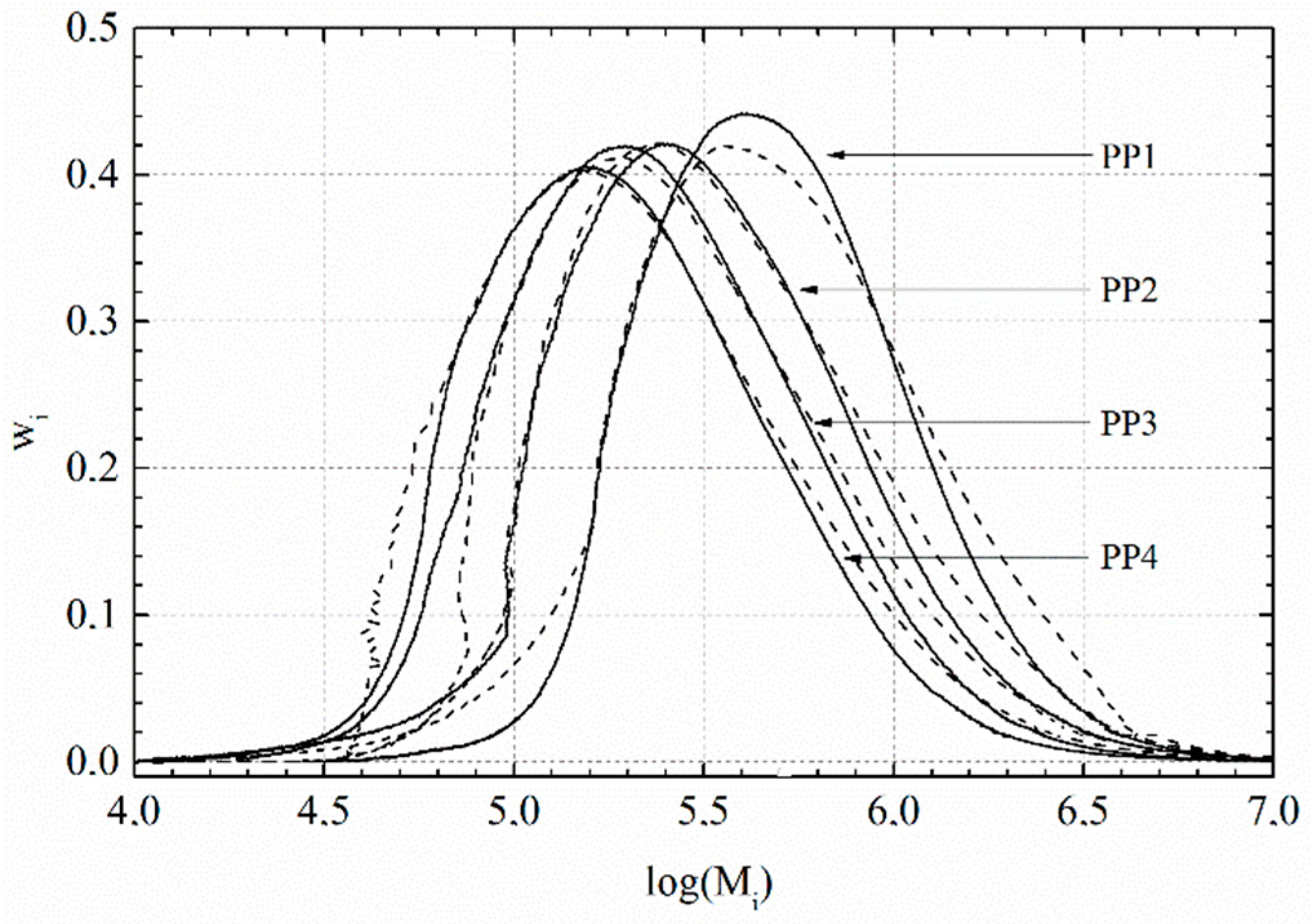
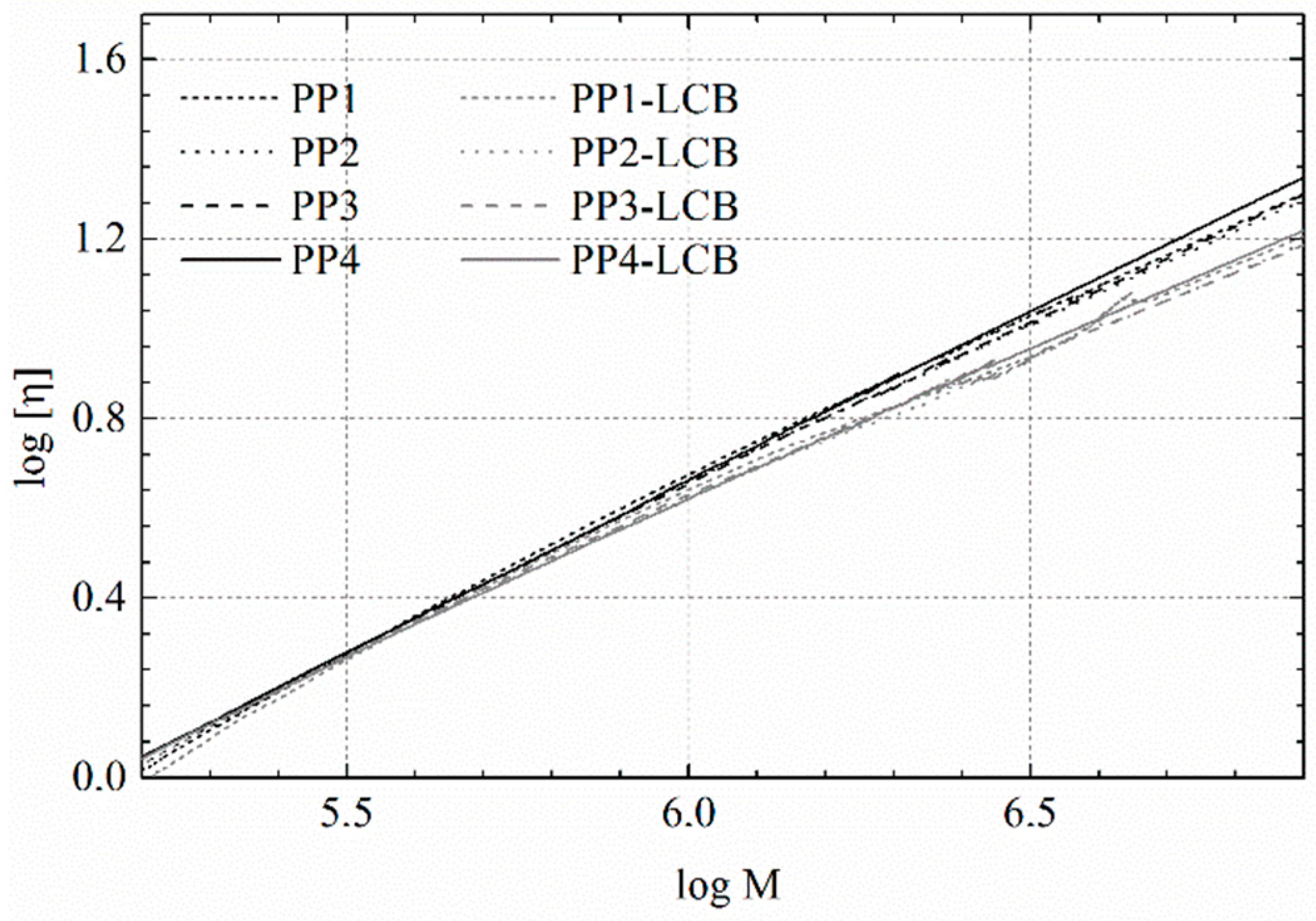
3.2. MMD and LCB of the PP Samples
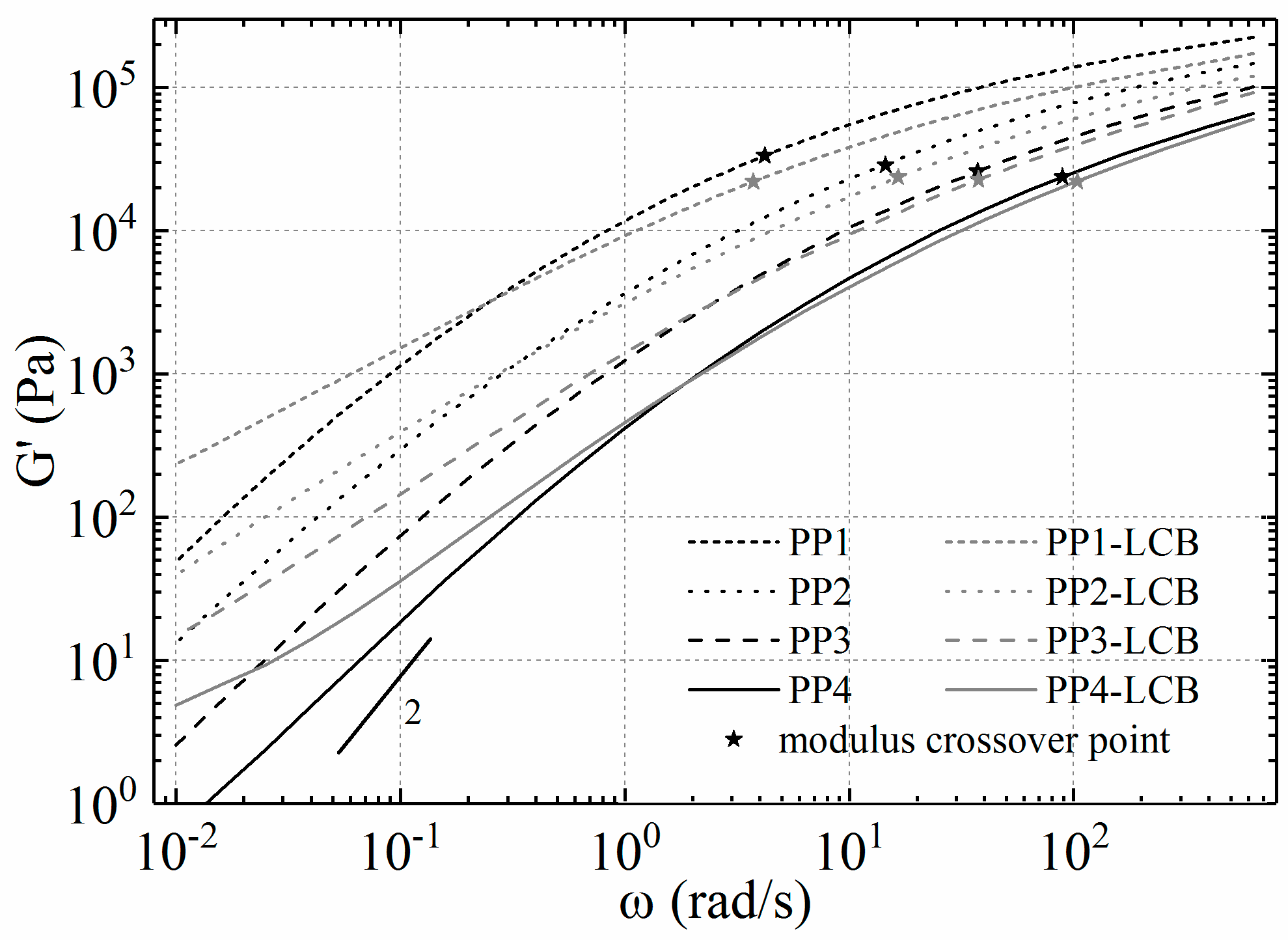
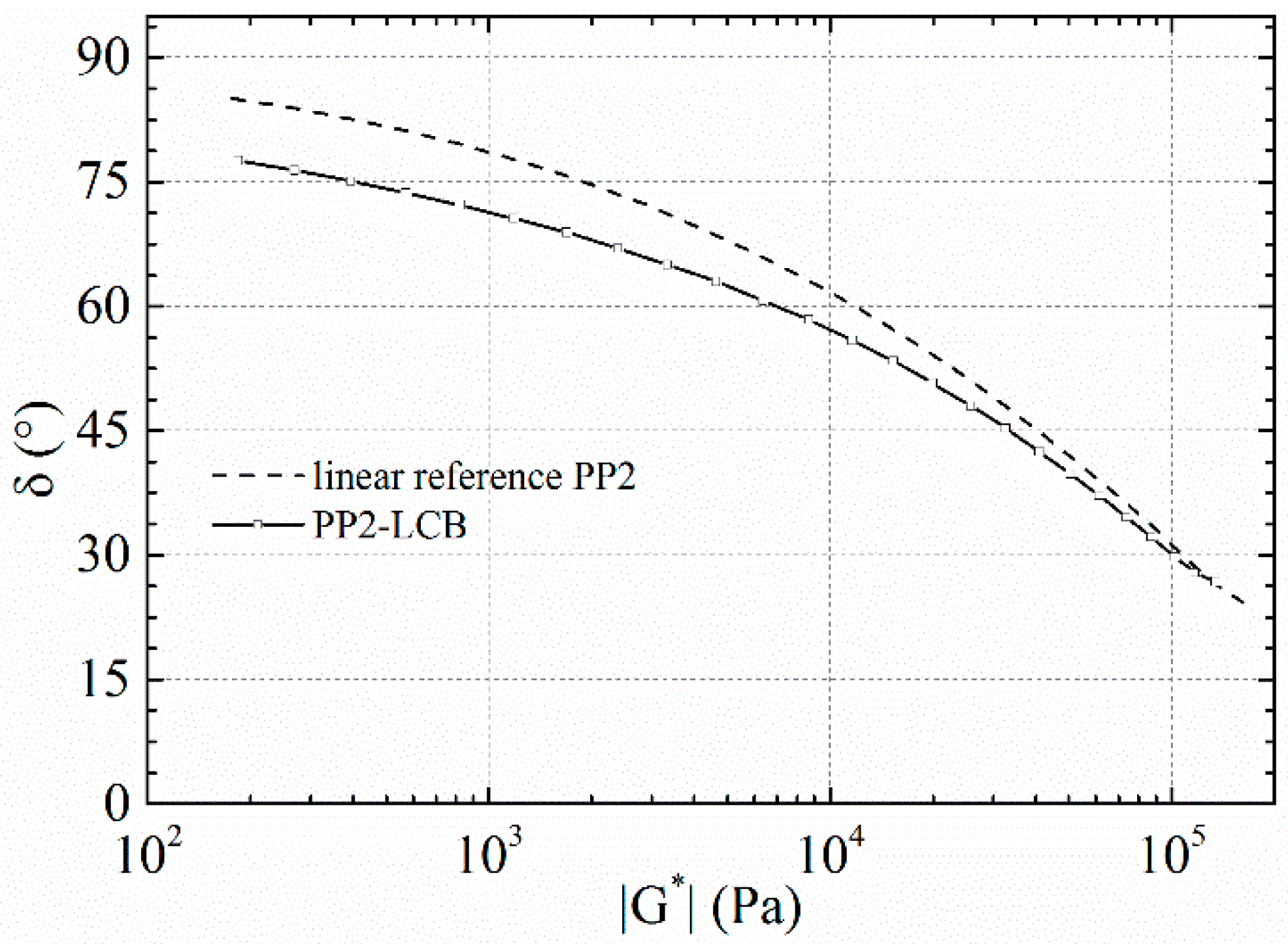
3.3. Dynamic Rheology
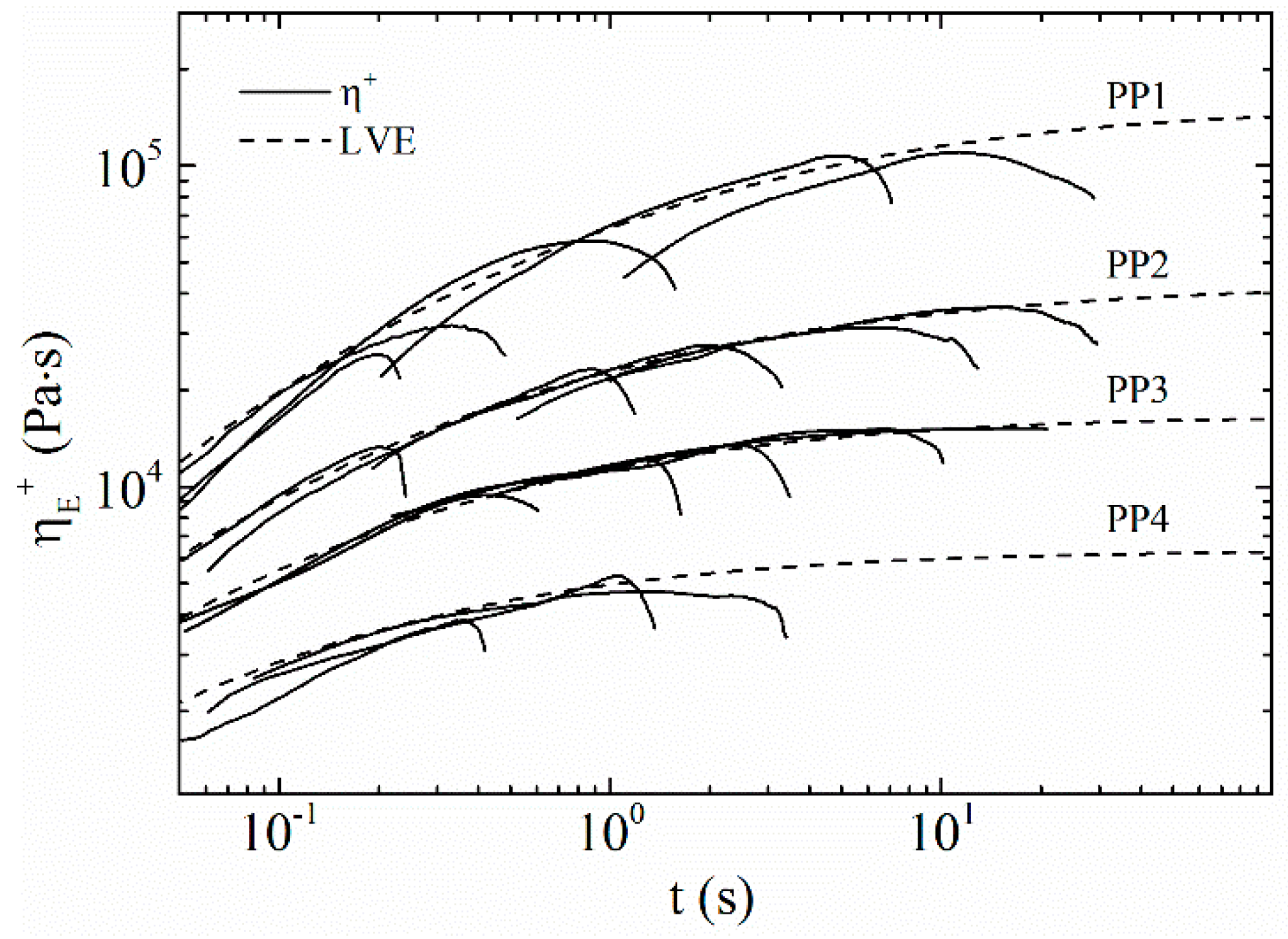
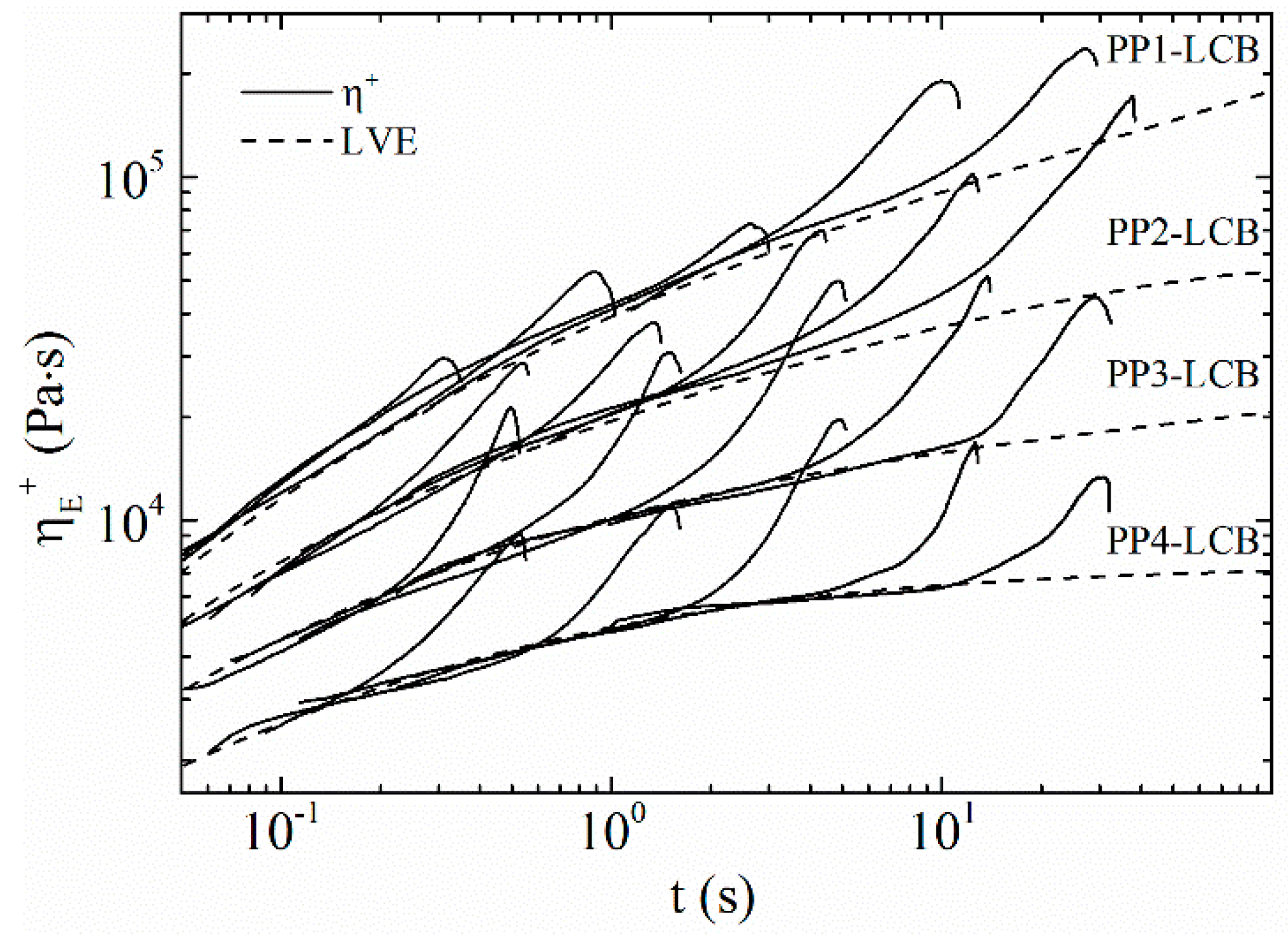
3.4. Extensional Rheology
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Pasquini, N.; Addeo, A. Polypropylene Handbook; Hanser: Cincinnati, OH, USA, 2005. [Google Scholar]
- Plastics Europe. Plastics—The Facts; Plastics Europe—Association of Plastics Manufacturers: Brussels, Belgium, 2015. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (psw): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, F.; Karlsson, S. Quality concepts for the improved use of recycled polymeric materials: A review. Macromol. Mater. Eng. 2008, 293, 274–297. [Google Scholar] [CrossRef]
- Jolly, R.; Rhin, C. Recycling of materials in industry the recycling of lead-acid batteries: Production of lead and polypropylene. Resour. Conserv. Recycl. 1994, 10, 137–143. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (pp) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; Rocha, M.C.G. Rheological properties of polypropylene during multiple extrusion. Polym. Test. 2005, 24, 86–93. [Google Scholar] [CrossRef]
- González-González, V.A.; Neira-Velázquez, G.; Angulo-Sánchez, J.L. Polypropylene chain scissions and molecular weight changes in multiple extrusion. Polym. Degrad. Stab. 1998, 60, 33–42. [Google Scholar] [CrossRef]
- Garcia, P.S.; Scuracchio, C.H.; Cruz, S.A. Effect of residual contaminants and of different types of extrusion processes on the rheological properties of the post-consumer polypropylene. Polym. Test. 2013, 32, 1237–1243. [Google Scholar] [CrossRef]
- Brandrup, J. Recycling and Recovery Of Plastics; Hanser Publisher: München Wien, Austria, 1996. [Google Scholar]
- Goodship, V. Introduction to Plastic Recycling, 2nd ed.; Smithers Rapra Technology Ltd.: Shropshire, UK, 2007. [Google Scholar]
- Siddique, R.; Khatib, J.; Kaur, I. Use of recycled plastic in concrete: A review. Waste Manag. 2008, 28, 1835–1852. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.H.; Kim, M.S. Application to refrigerator plastics by mechanical recycling from polypropylene in waste-appliances. Mater. Des. 2012, 34, 252–257. [Google Scholar] [CrossRef]
- Teh, J.W.; Rudin, A.; Keung, J.C. A review of polyethylene–polypropylene blends and their compatibilization. Adv. Polym. Technol. 1994, 13, 1–23. [Google Scholar] [CrossRef]
- Hettema, R.; Van Tol, J.; Janssen, L.P.B.M. In-situ reactive blending of polyethylene and polypropylene in co-rotating and counter-rotating extruders. Polym. Eng. Sci. 1999, 39, 1628–1641. [Google Scholar] [CrossRef]
- Hettema, R.; Pasman, J.; Janssen, L.P.B.M. Reactive extrusion of recycled bottle waste material. Polym. Eng. Sci. 2002, 42, 665–680. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. The effect of benzoyl peroxide and divinyl benzene on the properties of crosslinked recycled polyolefin blends. J. Macromol. Sci. Part B 2013, 1777–1785. [Google Scholar]
- Kim, B.K. Reactive extrusion of polyolefins and their blends. Korea Polym. J. 1996, 4, 215–226. [Google Scholar]
- Braun, D.; Richter, S.; Hellmann, G.P.; Rätzsch, M. Peroxy-initiated chain degradation, crosslinking, and grafting in pp–pe blends. J. Appl. Polym. Sci. 1998, 68, 2019–2028. [Google Scholar] [CrossRef]
- Borsig, E.; Fiedlerová, A.; Rychlá, L.; Lazár, M.; Rätzsch, M.; Haudel, G. Crosslinking of polypropylene–polyethylene blends by peroxide and the effect of pentaerythritol tetrallyl ether. J. Appl. Polym. Sci. 1989, 37, 467–478. [Google Scholar] [CrossRef]
- Chodak, I. Improving the properties of polyolefin waste by reactive processing. Polym. Plast. Technol. Eng. 2004, 43, 1769–1777. [Google Scholar] [CrossRef]
- Kruliš, Z.; Kokta, B.V.; Horák, Z.; Michálková, D.; Fortelný, I. Compatibilization as a procedure for recycling of commingled polyolefin waste. Macromol. Mater. Eng. 2001, 286, 156–160. [Google Scholar] [CrossRef]
- Hernández-Ortiz, J.C.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B.; D’Hooge, D.R.; Duchateau, J.N.E.; Remerie, K.; Toloza, C.; Vaz, A.L.; Schreurs, F. Modeling the reaction event history and microstructure of individual macrospecies in postpolymerization modification. AIChE J. 2017. [Google Scholar] [CrossRef]
- Passaglia, E.; Coiai, S.; Cicogna, F.; Ciardelli, F. Some recent advances in polyolefin functionalization. Polym. Int. 2014, 63, 12–21. [Google Scholar] [CrossRef]
- Parent, J.S.; Bodsworth, A.; Sengupta, S.S.; Kontopoulou, M.; Chaudhary, B.I.; Poche, D.; Cousteaux, S. Structure–rheology relationships of long-chain branched polypropylene: Comparative analysis of acrylic and allylic coagent chemistry. Polymer 2009, 50, 85–94. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Zhang, H.; Lin, W.; Wang, Y. Investigation on multifunctional monomer modified polypropylene and its foamability. J. Appl. Polym. Sci. 2013, 130, 1675–1681. [Google Scholar] [CrossRef]
- Zulli, F.; Andreozzi, L.; Passaglia, E.; Augier, S.; Giordano, M. Rheology of long-chain branched polypropylene copolymers. J. Appl. Polym. Sci. 2013, 127, 1423–1432. [Google Scholar] [CrossRef]
- Drooghaag, X.; Rousseaux, D.D.J.; Henry, G.R.P.; Sclavons, M.; Carlier, V.; Marchand-Brynaert, J. Mediated melt functionalization of polypropylene. Polym. Degrad. Stab. 2010, 95, 342–345. [Google Scholar] [CrossRef]
- Langston, J.A.; Colby, R.H.; Chung, T.C.M.; Shimizu, F.; Suzuki, T.; Aoki, M. Synthesis and characterization of long chain branched isotactic polypropylene via metallocene catalyst and t-reagent. Macromolecules 2007, 40, 2712–2720. [Google Scholar] [CrossRef]
- Li, S.; Xiao, M.; Wei, D.; Xiao, H.; Hu, F.; Zheng, A. The melt grafting preparation and rheological characterization of long chain branching polypropylene. Polymer 2009, 50, 6121–6128. [Google Scholar] [CrossRef]
- Diop, M.F.; Torkelson, J.M. Novel synthesis of branched polypropylene via solid-state shear pulverization. Polymer 2015, 60, 77–87. [Google Scholar] [CrossRef]
- El Mabrouk, K.; Parent, J.S.; Chaudhary, B.I.; Cong, R. Chemical modification of pp architecture: Strategies for introducing long-chain branching. Polymer 2009, 50, 5390–5397. [Google Scholar] [CrossRef]
- Wong, B.; Baker, W.E. Melt rheology of graft modified polypropylene. Polymer 1997, 38, 2781–2789. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, F.; Zhang, H. Isothermal and non-isothermal crystallization studies of long chain branched polypropylene containing poly (ethylene-co-octene) under quiescent and shear conditions. Polymers 2017. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Tsenoglou, C. Effect of long branches on the rheology of polypropylene. J. Rheol. 2004, 48, 895–914. [Google Scholar] [CrossRef]
- Auhl, D.; Stange, J.; Münstedt, H.; Krause, B.; Voigt, D.; Lederer, A.; Lappan, U.; Lunkwitz, K. Long-chain branched polypropylenes by electron beam irradiation and their rheological properties. Macromolecules 2004, 37, 9465–9472. [Google Scholar] [CrossRef]
- Stange, J.; Münstedt, H. Rheological properties and foaming behavior of polypropylenes with different molecular structures. J. Rheol. 2006, 50, 907–923. [Google Scholar] [CrossRef]
- Härth, M.; Kaschta, J.; Schubert, D.W. Shear and elongational flow properties of long-chain branched poly(ethylene terephthalates) and correlations to their molecular structure. Macromolecules 2014, 47, 4471–4478. [Google Scholar] [CrossRef]
- Nam, G.J.; Yoo, J.H.; Lee, J.W. Effect of long-chain branches of polypropylene on rheological properties and foam-extrusion performances. J. Appl. Polym. Sci. 2005, 96, 1793–1800. [Google Scholar] [CrossRef]
- Lagendijk, R.P.; Hogt, A.H.; Buijtenhuijs, A.; Gotsis, A.D. Peroxydicarbonate modification of polypropylene and extensional flow properties. Polymer 2001, 42, 10035–10043. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Hogt, A.H. The effect of long chain branching on the processability of polypropylene in thermoforming. Polym. Eng. Sci. 2004, 44, 973–982. [Google Scholar] [CrossRef]
- Buback, M.; Frauendorf, H.; Janssen, O.; Vana, P. Electrospray ionization mass spectrometric study of end-groups in peroxydicarbonate-initiated radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6071–6081. [Google Scholar] [CrossRef]
- Hogt, A.H.; Spijkerman, G.K. Extrusion Process for Enhancing the Melt Strength of Polypropylene. Patent WO1999027007 A1, 3 June 1999. [Google Scholar]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.M. Upcycling of polypropylene—The influence of polyethylene impurities. Polym. Eng. Sci. 2017. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.M. Long chain branching as an innovative up-cycling process of polypropylene post-consumer waste—possibilities and limitations. Waste Manag. 2017. submitted. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.H.; De Paoli, M.-A. Polypropylene compounding with recycled material i Statistical response surface analysis. Polym. Degrad. Stab. 2001, 71, 293–298. [Google Scholar] [CrossRef]
- Valenza, A.; La Mantia, F.P. Recycling of polymer waste: Part II—Stress degraded polypropylene. Polym. Degrad. Stab. 1988, 20, 63–73. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: Berlin, Germany, 2007; Volume 1076. [Google Scholar]
- DIN. Plastics—Dynamic Scanning Calorimetry (DSC)—Determination of the Oxidation Induction Time (OIT); BEUTH Verlag GmbH: Berlin, Germany, 2013; Volume 11357-6. [Google Scholar]
- Pfaendner, R. Nitroxyl radicals and nitroxylethers beyond stabilization: Radical generators for efficient polymer modification. C. R. Chim. 2006, 9, 1338–1344. [Google Scholar] [CrossRef]
- Wang, X.; Tzoganakis, C.; Rempel, G.L. Chemical modification of polypropylene with peroxide/pentaerythritol triacrylate by reactive extrusion. J. Appl. Polym. Sci. 1996, 61, 1395–1404. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. Crystallization behaviors of linear and long chain branched polypropylene. J. Appl. Polym. Sci. 2007, 104, 3592–3600. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Rheological and thermal properties of blends of a long-chain branched polypropylene and different linear polypropylenes. Chem. Eng. Sci. 2009, 64, 4719–4731. [Google Scholar] [CrossRef]
- Zimm, B.H.; Stockmayer, W.H. The dimensions of chain molecules containing branches and rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Zimm, B.H.; Kilb, R.W. Dynamics of branched polymer molecules in dilute solution. J. Polym. Sci. 1959, 37, 19–42. [Google Scholar] [CrossRef]
- Lecacheux, D.; Lesec, J.; Quivoron, C. High-temperature coupling of high-speed gpc with continuous viscometry. I. Long-chain branching in polyethylene. J. Appl. Polym. Sci. 1982, 27, 4867–4877. [Google Scholar] [CrossRef]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (sec) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2011, 399, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Provder, T. Chromatography of Polymers; American Chemical Society: Washington, DC, USA, 1993; Volume 521, p. 356. [Google Scholar]
- Berry, G. Thermodynamic and conformational properties of polystyrene. III. Dilute solution studies on branched polymers. J. Polym. Sci. Part B Polym. Phys. 1971, 9, 687–715. [Google Scholar] [CrossRef]
- Roovers, J.; Toporowski, P.; Martin, J. Synthesis and characterization of multiarm star polybutadienes. Macromolecules 1989, 22, 1897–1903. [Google Scholar] [CrossRef]
- Wood-Adams, P.M.; Dealy, J.M.; deGroot, A.W.; Redwine, O.D. Effect of molecular structure on the linear viscoelastic behavior of polyethylene. Macromolecules 2000, 33, 7489–7499. [Google Scholar] [CrossRef]
- Guapacha, J.; Vallés, E.M.; Quinzani, L.M.; Failla, M.D. Long-chain branched polypropylene obtained using an epoxy resin as crosslinking agent. Polym. Bull. 2016, 74, 1–22. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, W.; Zhao, S.; Xin, Z.; Shi, Y. Shear-induced β-form polypropylene in long chain branching isotactic polypropylene. Polym. Eng. Sci. 2016, 56, 240–247. [Google Scholar] [CrossRef]
- Litvinov, V.M.; Ries, M.E.; Baughman, T.W.; Henke, A.; Matloka, P.P. Chain entanglements in polyethylene melts. Why is it studied again? Macromolecules 2013, 46, 541–547. [Google Scholar] [CrossRef]
- Fleissner, M. Characterization of polymer molecular mass distribution from rheological measurements. Makromol. Chem. Macromol. Symp. 1992, 61, 324–341. [Google Scholar] [CrossRef]
- Trinkle, S.; Friedrich, C. Van gurp-palmen-plot: A way to characterize polydispersity of linear polymers. Rheol. Acta 2001, 40, 322–328. [Google Scholar] [CrossRef]
- Trinkle, S.; Walter, P.; Friedrich, C. Van gurp-palmen plot II—Classification of long chain branched polymers by their topology. Rheol. Acta 2002, 41, 103–113. [Google Scholar] [CrossRef]
- Tsenoglou, C.J.; Gotsis, A.D. Rheological characterization of long chain branching in a melt of evolving molecular architecture. Macromolecules 2001, 34, 4685–4687. [Google Scholar] [CrossRef]
- Jørgensen, J.K.; Stori, A.; Redford, K.; Ommundsen, E. Introduction of long-chain branches in linear polyethylene by light cross-linking with 1,3-benzenedisulfonyl azide. Polymer 2005, 46, 12256–12266. [Google Scholar] [CrossRef]
- Gabriel, C.; Münstedt, H. Strain hardening of various polyolefins in uniaxial elongational flow. J. Rheol. 2003, 47, 619–630. [Google Scholar] [CrossRef]
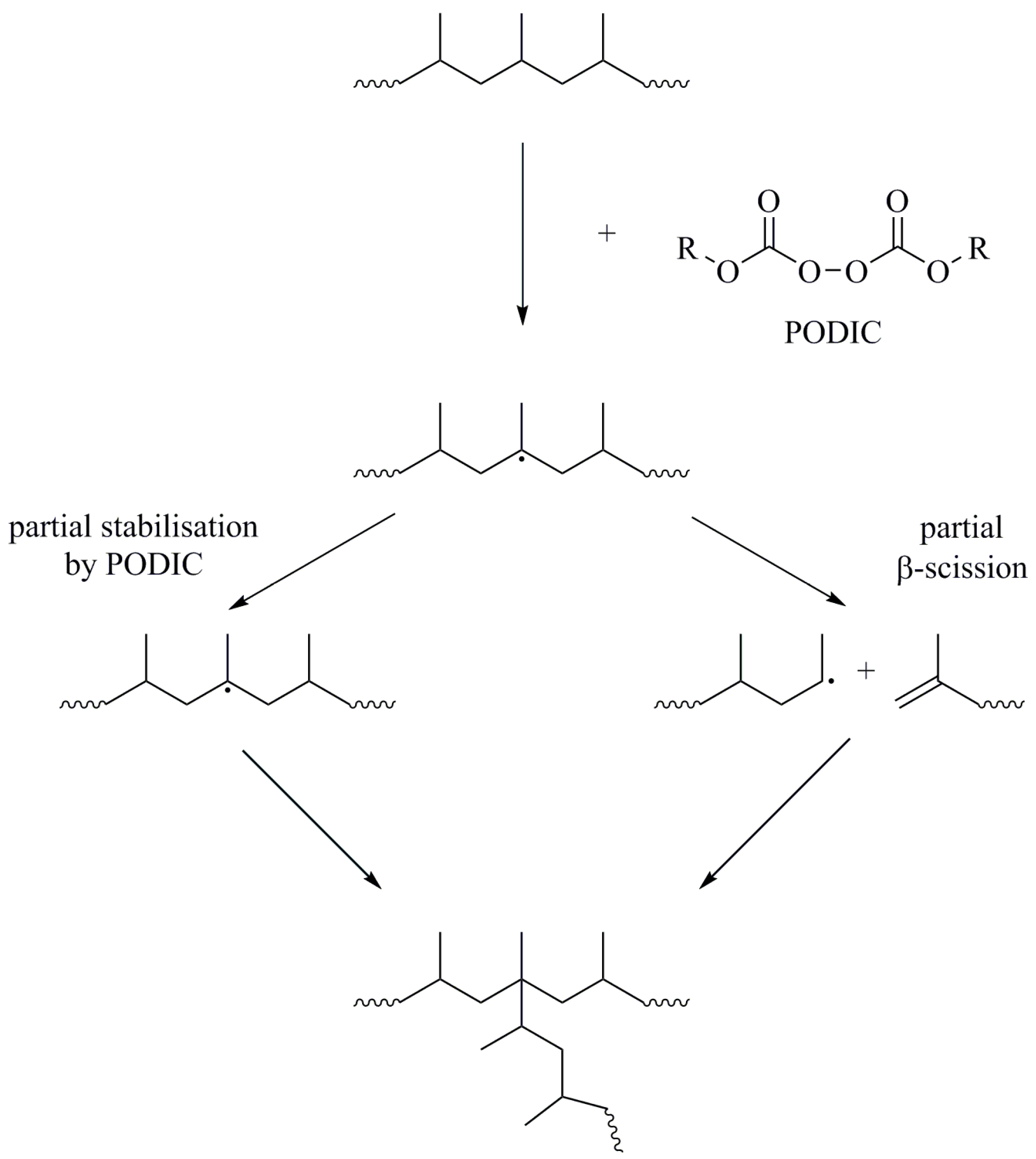
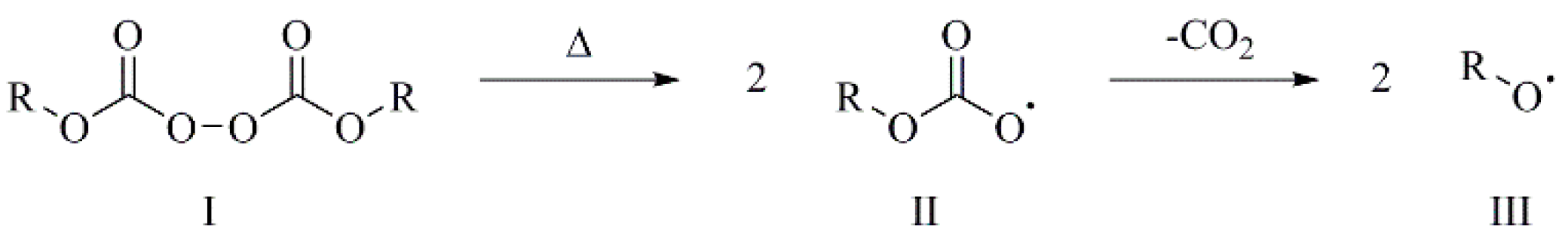
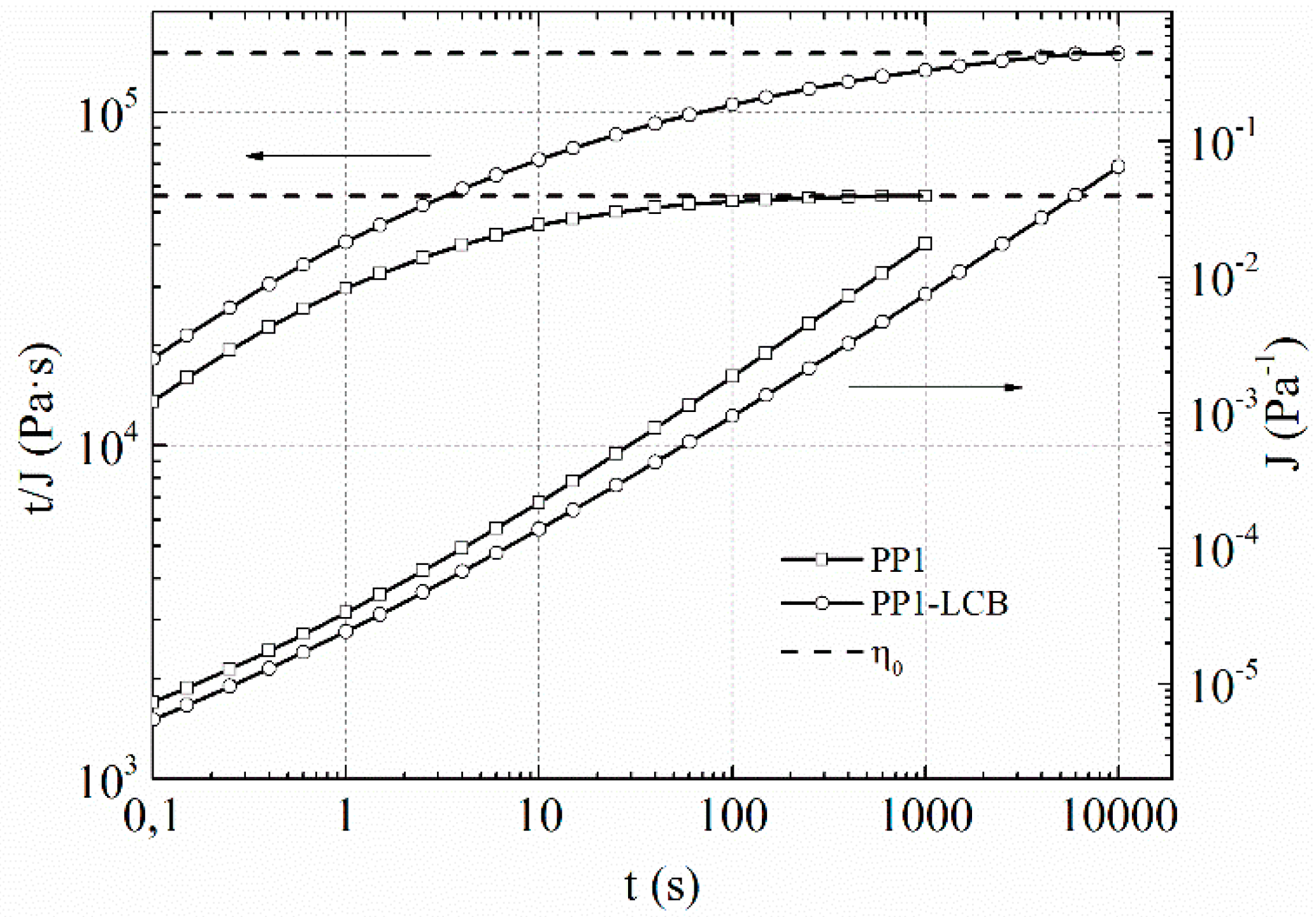
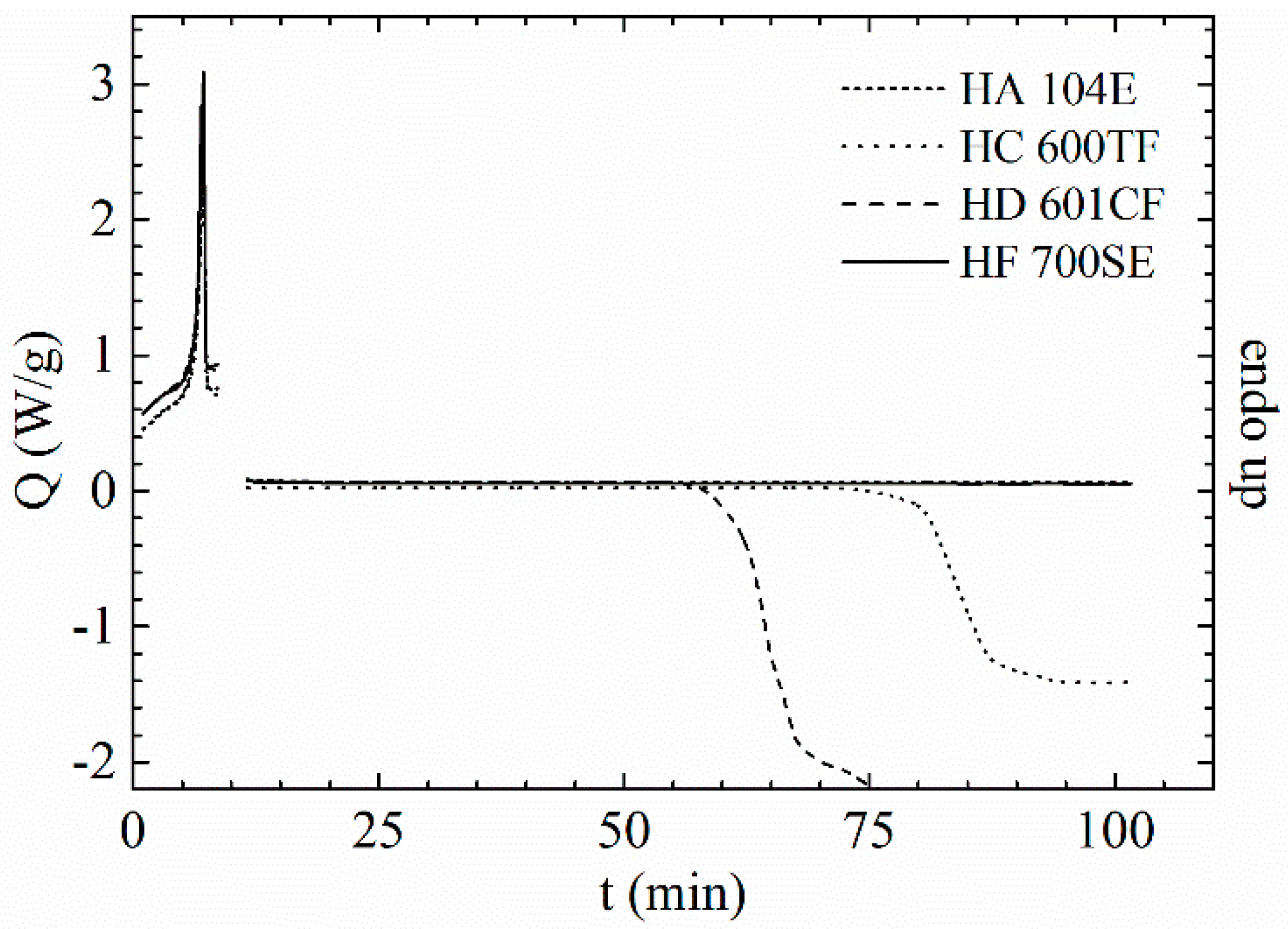
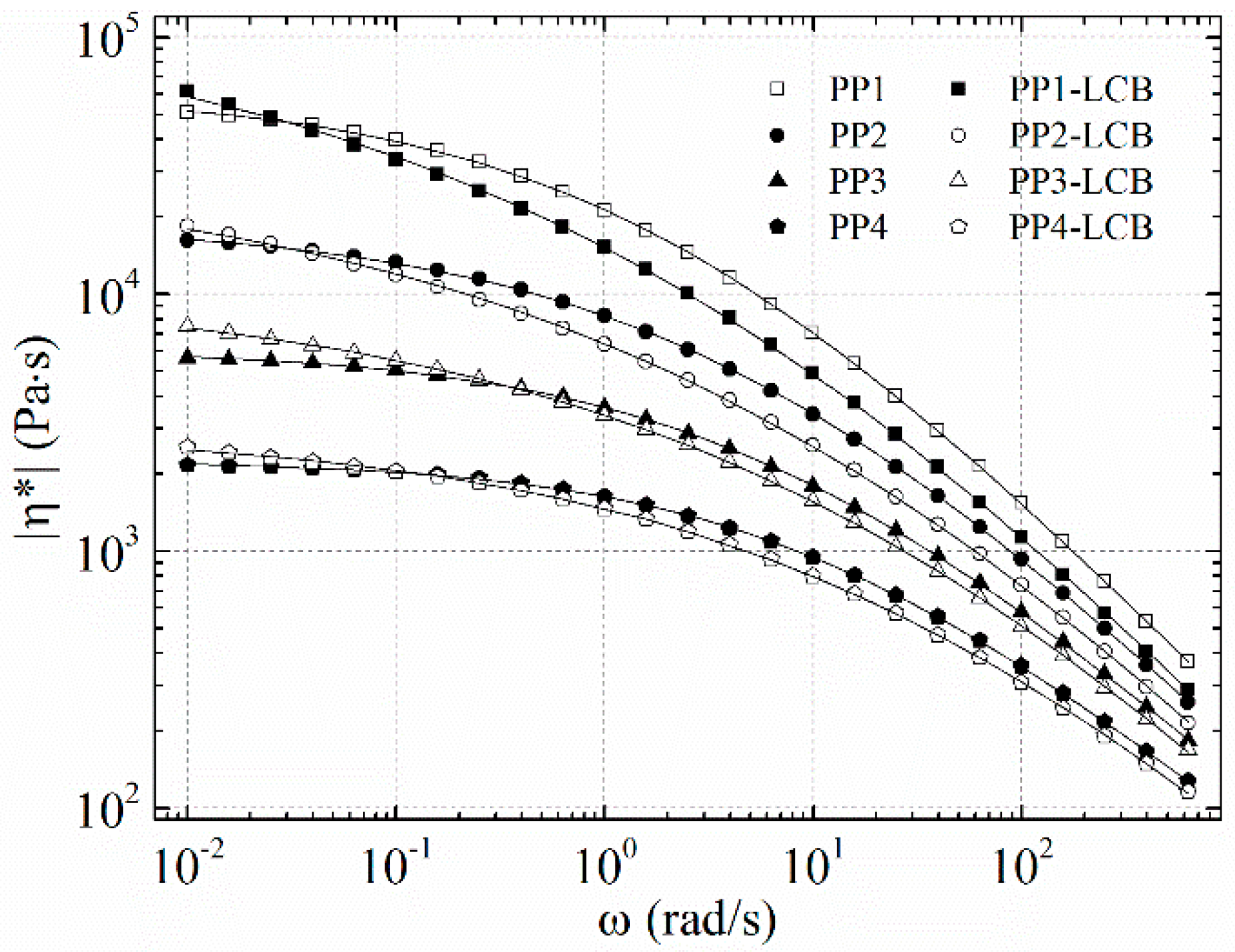

| Sample | MFI a [g/10 min] | η0 [Pa·s] | Mw [kg mol−1] | Mn [kg mol−1] | Mw/Mn |
|---|---|---|---|---|---|
| PP1 | 0.75 | 56 600 | 559 | 321 | 1.74 |
| PP2 | 2.8 | 17 700 | 394 | 198 | 1.99 |
| PP3 | 8 | 5 900 | 300 | 151 | 1.99 |
| PP4 | 21 | 2 140 | 227 | 99 | 2.29 |
| Sample | OIT | OIT |
|---|---|---|
| Industrial Grade | after Soxhlet | |
| [min] | [min] | |
| PP1 | >90 | 17 |
| PP2 | 67 | 9 |
| PP3 | 48 | 10 |
| PP4 | >90 | 11 |
| Sample | Tm [°C] | Tc [°C] | ΔHm [J·g−1] |
|---|---|---|---|
| PP 1 | 163 | 114 | 90 |
| PP 1-LCB | 164 | 128 | 98 |
| PP 2 | 161 | 113 | 92 |
| PP 2-LCB | 163 | 128 | 98 |
| PP 3 | 162 | 115 | 93 |
| PP 3-LCB | 163 | 128 | 99 |
| PP 4 | 162 | 114 | 98 |
| PP 4-LCB | 163 | 127 | 101 |
| Sample | Mw [kg mol−1] | Mn [kg mol−1] | Mw/Mn | Bn |
|---|---|---|---|---|
| PP1-LCB | 611 | 317 | 1.92 | 0.08 |
| PP2-LCB | 436 | 196 | 2.22 | 0.13 |
| PP3-LCB | 333 | 151 | 2.20 | 0.25 |
| PP4-LCB | 264 | 106 | 2.49 | 0.27 |
| Sample | η0 [Pa·s] | Bn | α |
|---|---|---|---|
| PP1-LCB | 150 200 | 0.08 | 0.45 |
| PP2-LCB | 37 100 | 0.13 | 0.46 |
| PP3-LCB | 11 500 | 0.25 | 0.49 |
| PP4-LCB | 2730 | 0.27 | 0.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Schmid, K.; Archodoulaki, V.-M. Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers 2017, 9, 442. https://doi.org/10.3390/polym9090442
Kamleitner F, Duscher B, Koch T, Knaus S, Schmid K, Archodoulaki V-M. Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers. 2017; 9(9):442. https://doi.org/10.3390/polym9090442
Chicago/Turabian StyleKamleitner, Florian, Bernadette Duscher, Thomas Koch, Simone Knaus, Klaus Schmid, and Vasiliki-Maria Archodoulaki. 2017. "Influence of the Molar Mass on Long-Chain Branching of Polypropylene" Polymers 9, no. 9: 442. https://doi.org/10.3390/polym9090442
APA StyleKamleitner, F., Duscher, B., Koch, T., Knaus, S., Schmid, K., & Archodoulaki, V.-M. (2017). Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers, 9(9), 442. https://doi.org/10.3390/polym9090442





