Synthesis, Characterization and the Solvent Effects on Interfacial Phenomena of Jatropha Curcas Oil Based Non-Isocyanate Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of JCO Based Cyclic Carbonates
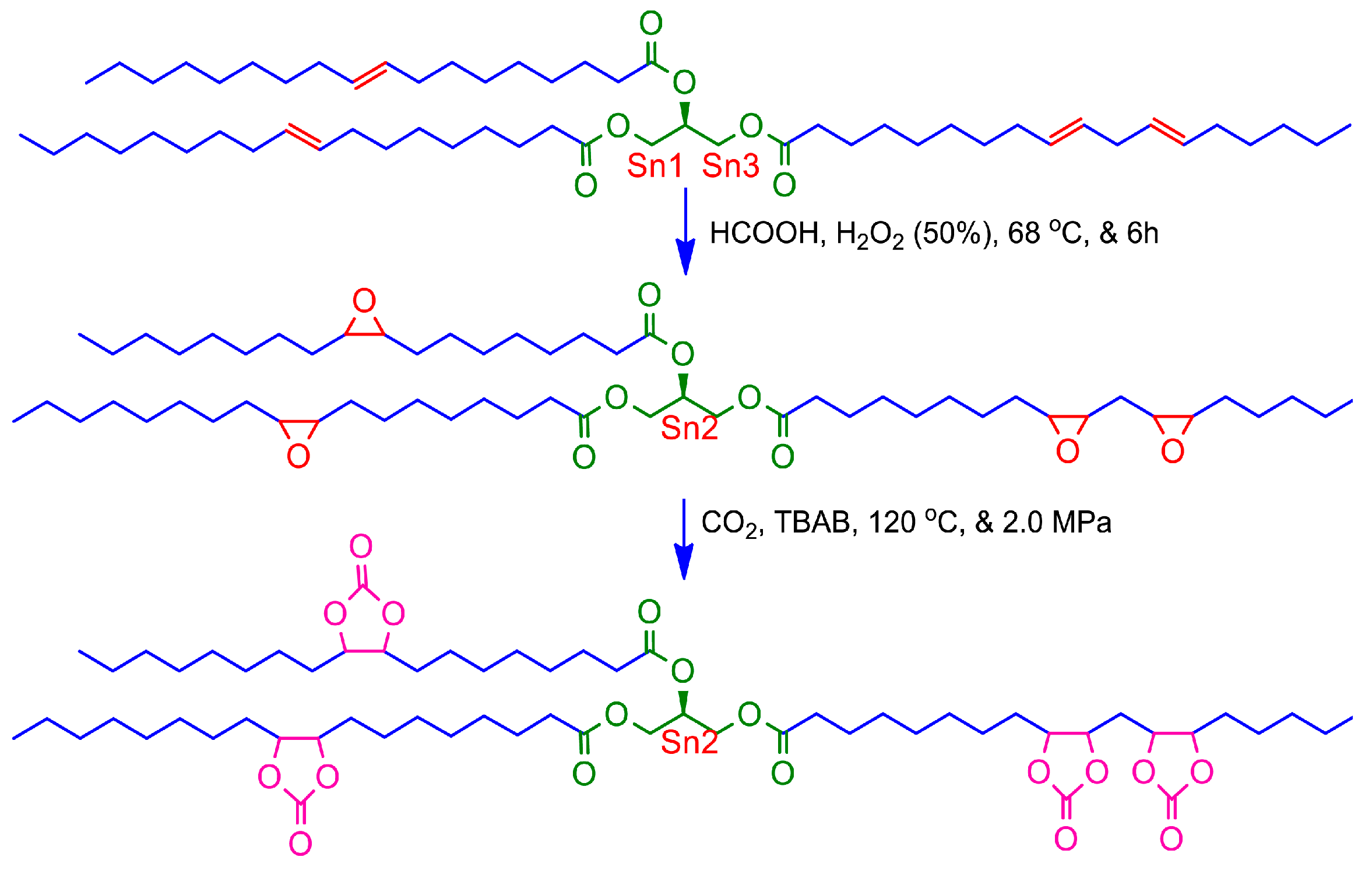
2.2.1. Preparation of EJCO
2.2.2. Preparation of CJCO
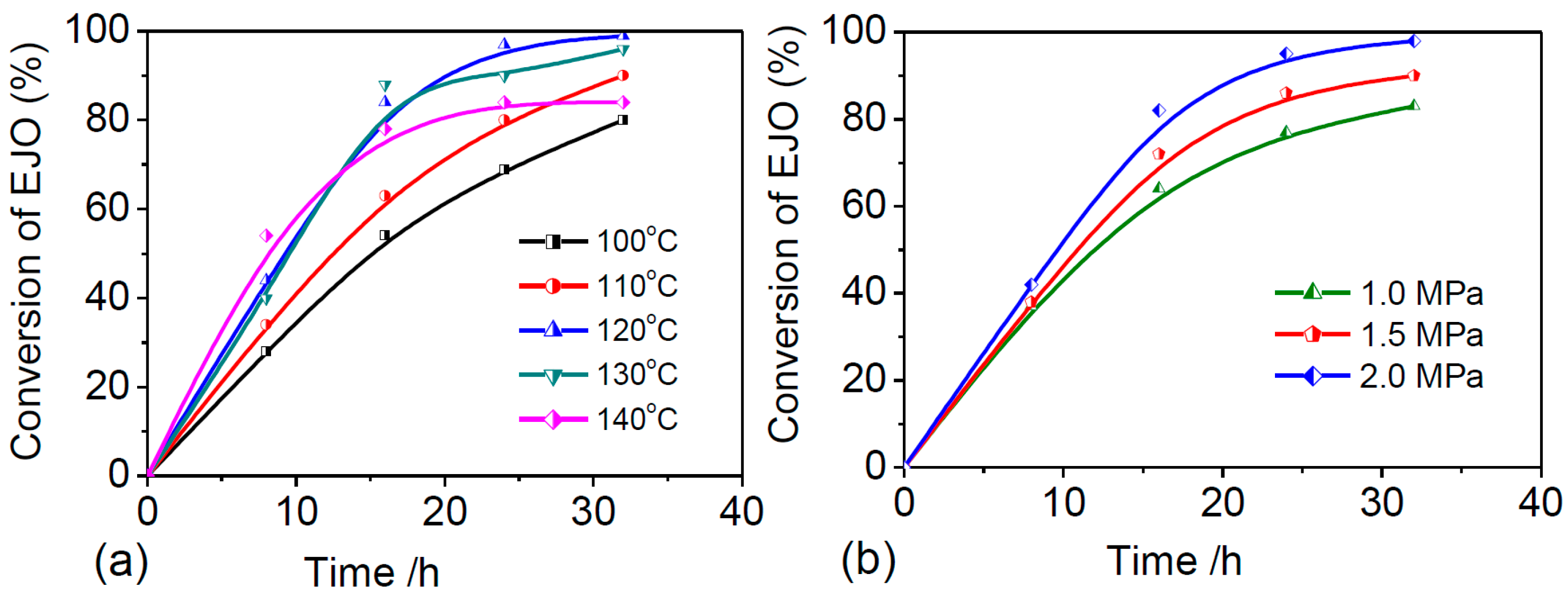
2.2.3. Optimization of the Reaction Parameters of CJCO
2.3. Synthesis of JCO-Based Catalytic Carbonated Alkyd Resin
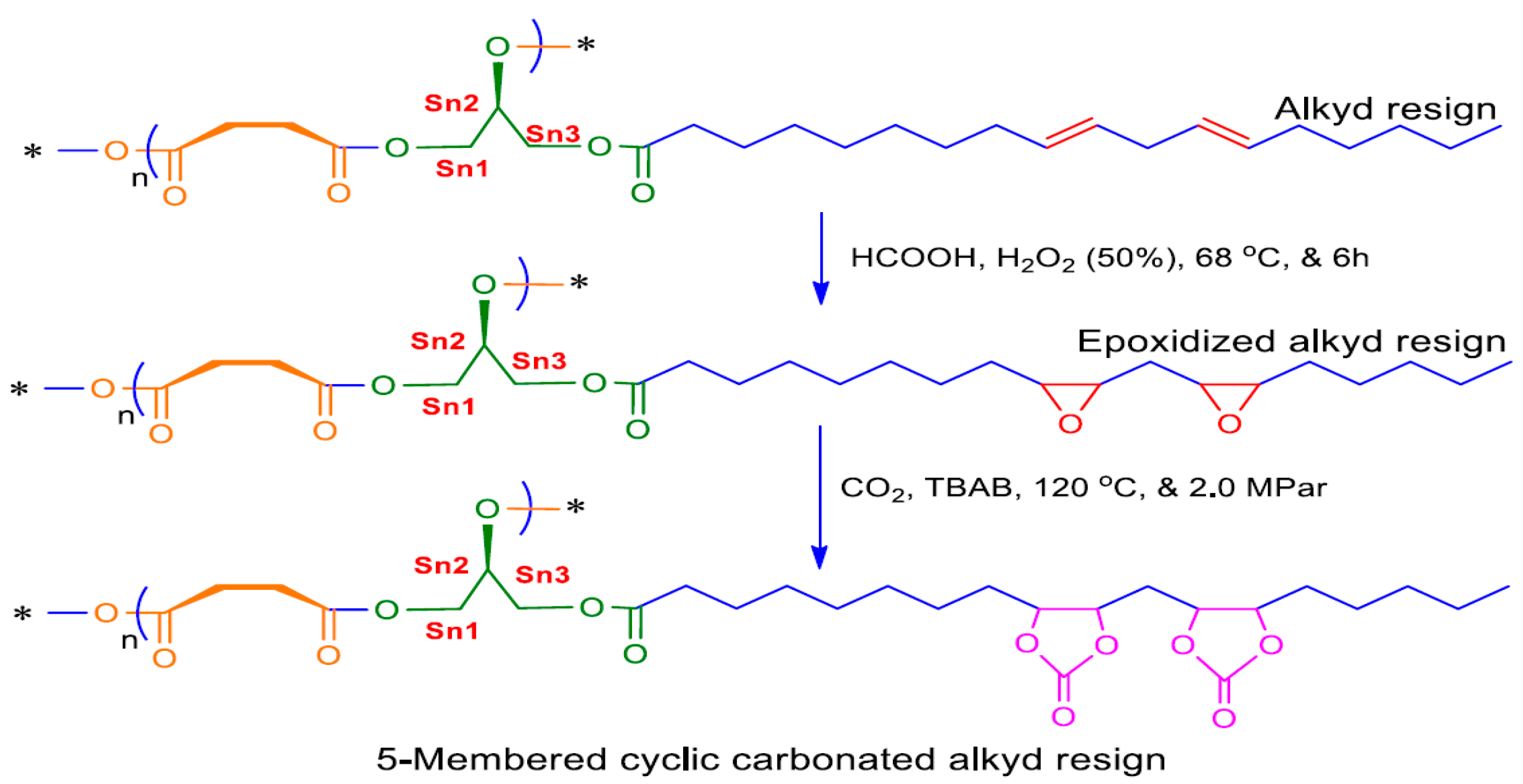
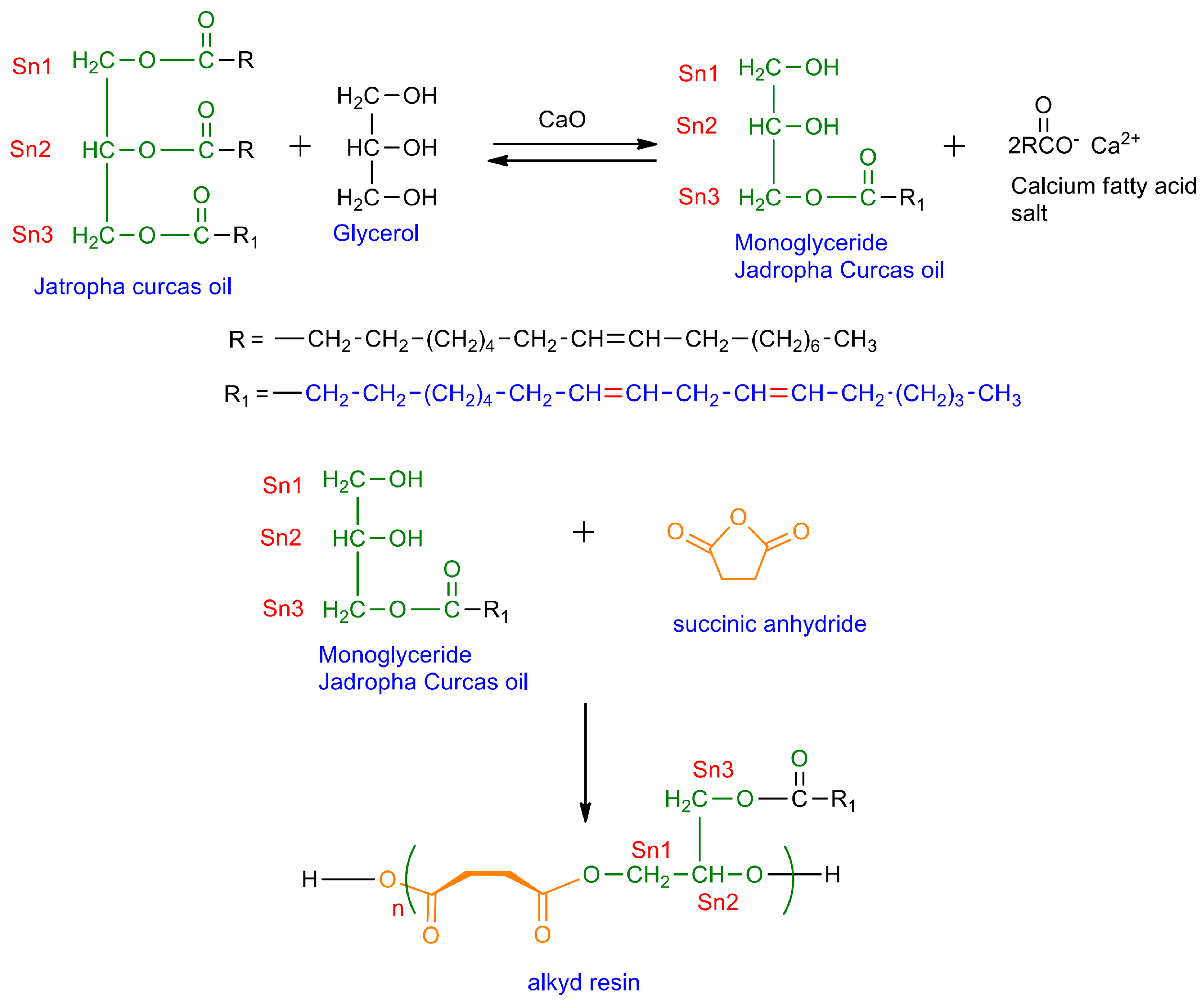
2.3.1. Preparations of JCO-Based Monoglyceride Alkyd Resin
2.3.2. Preparation of Epoxidation and Cyclic Carbonation of JCO-Based Alkyd Resin (CC-AR)
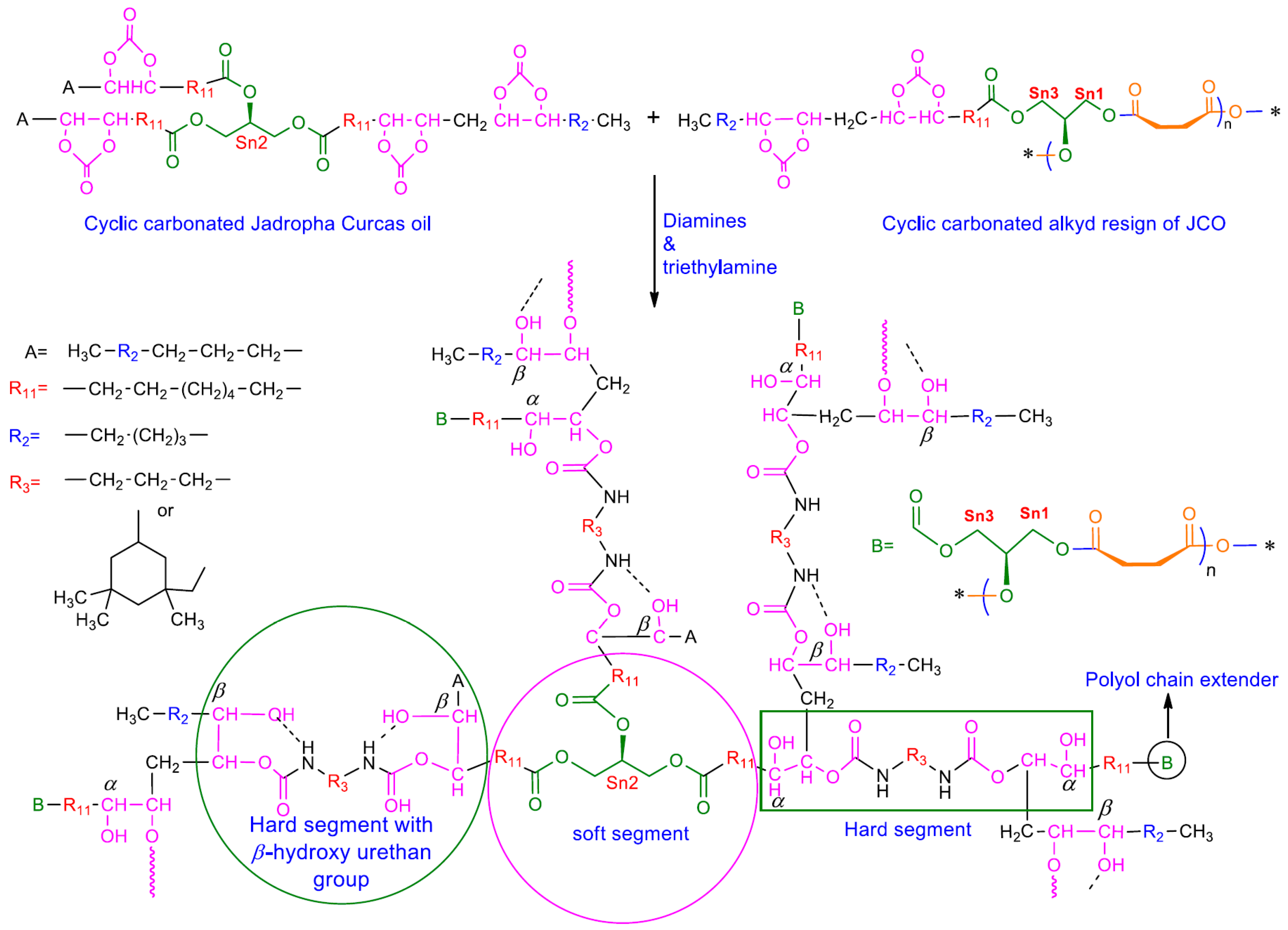
2.4. Synthesis and Characterization of CJCO and Its Blends Based on NIPU
2.5. Characterization Study
2.5.1. Molecular Weight
2.5.2. Structural Characterization
2.5.3. Thermo-Mechanical Properties
2.5.4. Solvent and Chemical Resistance Tests
2.6. Characterization of EJCO and CJCO
3. Results and Discussion
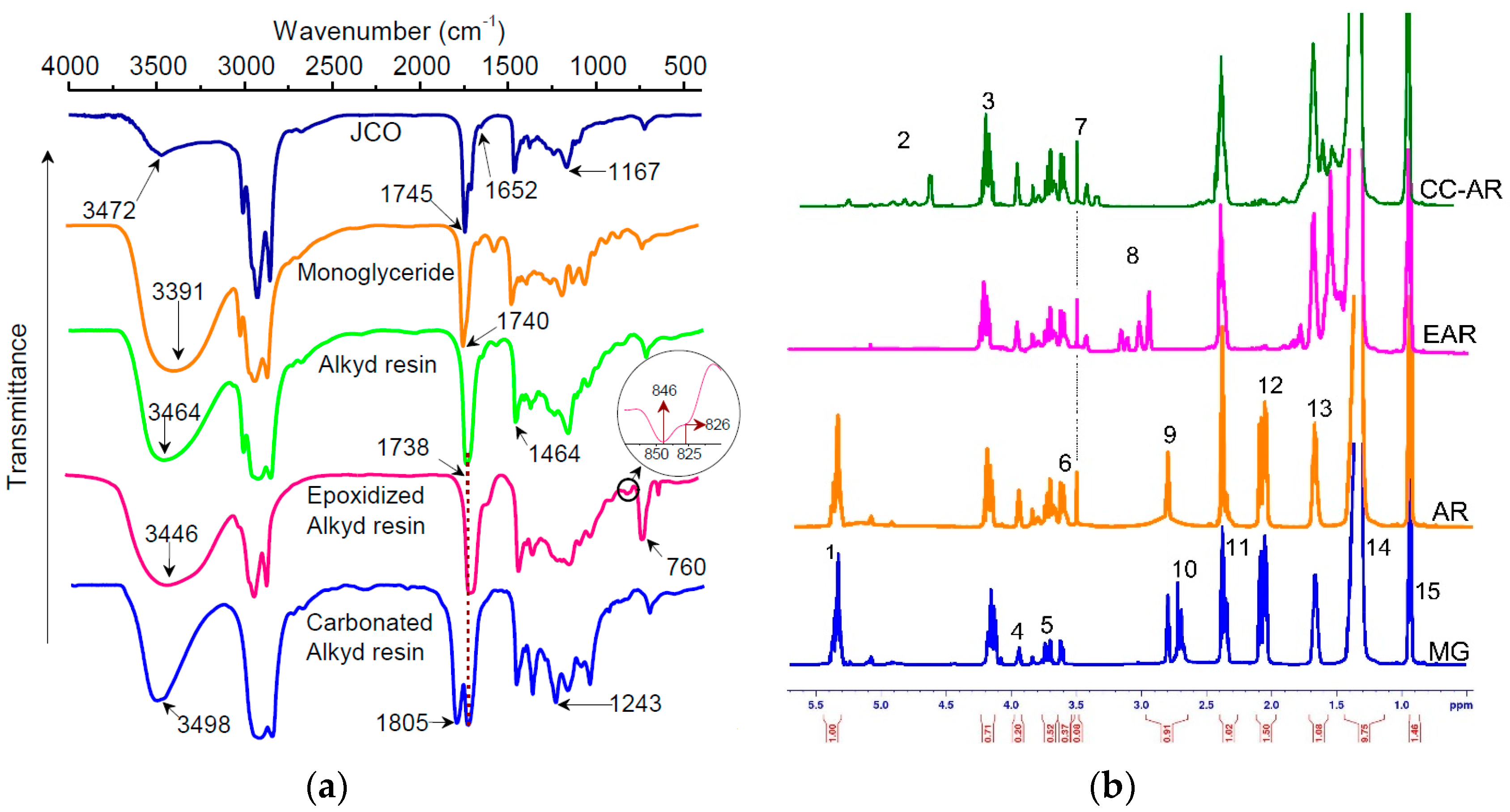
3.1. Characterization of Monoglyceride, Alkyd Resin (AR), Epoxidized (EAR), and CC-AR
3.2. Characterization of CJCO and CC-AR
3.3. Thermo-Mechanical Properties of the NIPU Film
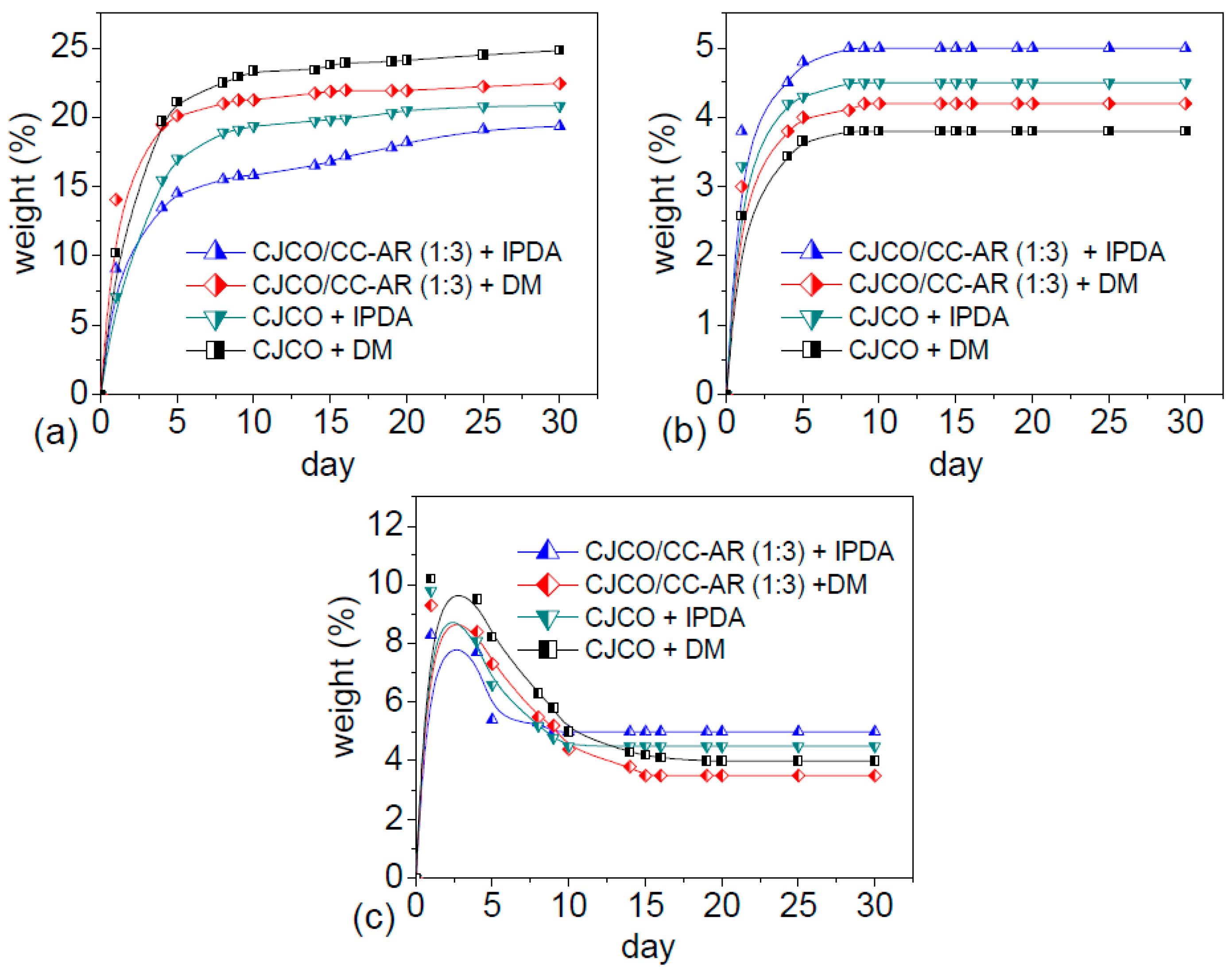
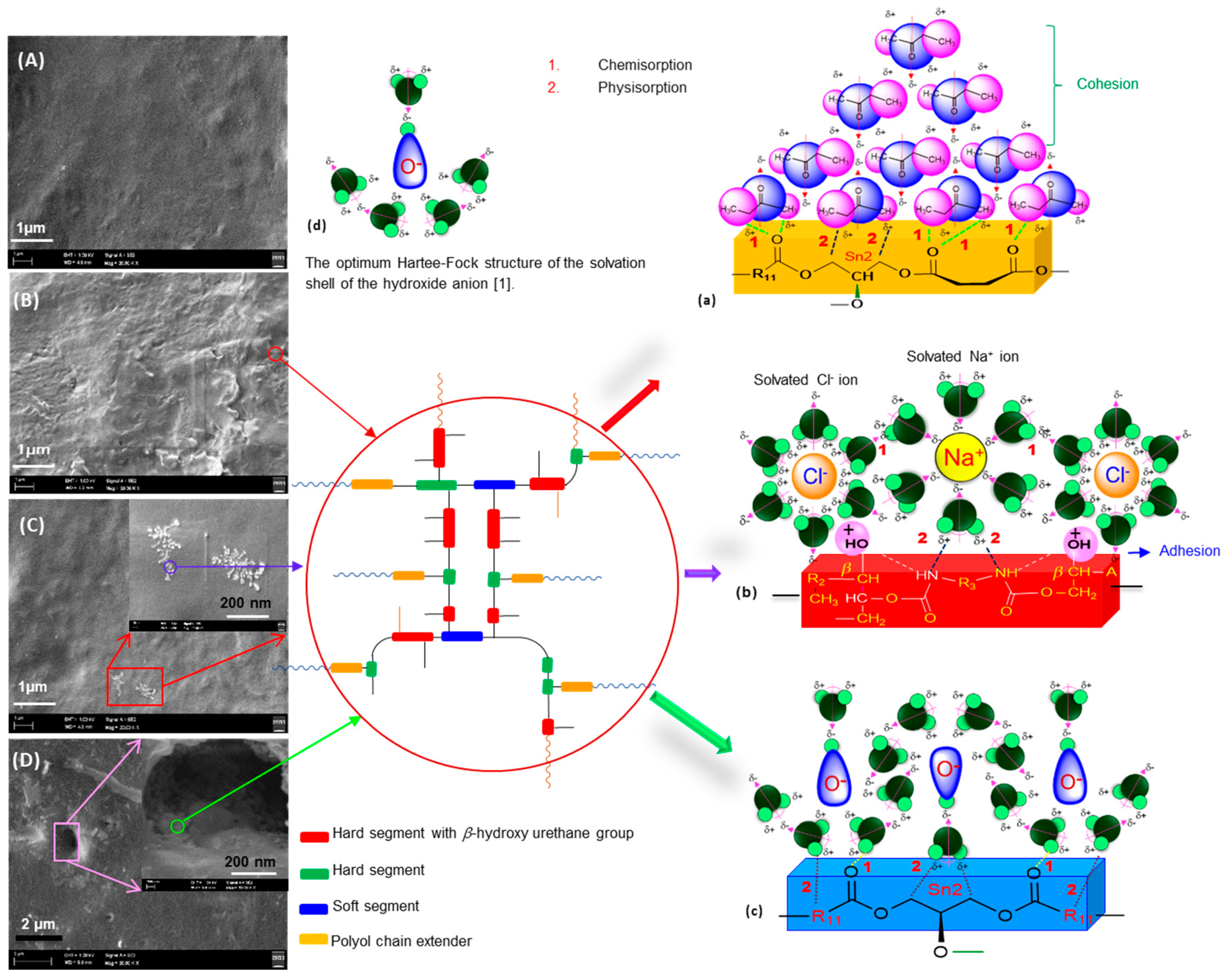
3.4. The Solvent Effect on the Surface Interfacial Phenomena
3.4.1. Solvent Resistance and Surface Interfacial Phenomena of NIPU
3.4.2. Chemical Resistance and Surface Interfacial Phenomena of NIPU
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gogoi, P.; Boruah, M.; Sharma, S.; Dolui, S.K. Blends of epoxidized alkyd resins based on jatropha oil and the epoxidized oil cured with aqueous citric acid solution: A green technology approach. ACS Sustain. Chem. Eng. 2015, 3, 261–268. [Google Scholar] [CrossRef]
- Lim, K.M.; Ching, Y.C.; Gan, S.N. Effect of palm oil bio-based plasticizer on the morphological, thermal and mechanical properties of poly(vinyl chloride). Polymers 2015, 7, 2031–2043. [Google Scholar] [CrossRef]
- de Espinosa, L.M.; Meier, M.A. Plant oils: The perfect renewable resource for polymer science? Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Y.; Hou, Z.; He, Z.; Eli, W. Synthesis of carbonated cotton seed oil and its application as lubricating base oil. J. Am. Oil Chem. Soc. 2014, 91, 143–150. [Google Scholar] [CrossRef]
- Mhd Haniffa, M.A.C.; Ching, Y.C.; Abdullah, L.C.; Poh, S.C.; Chuah, C.H. Review of bionanocomposite coating films and their applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef]
- Makkar, H.P.; Becker, K. Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. Eur. J. Lipid Sci. Technol. 2009, 111, 773–787. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An evaluation of multipurpose oil seed crop for industrial uses Jatropha curcas L: A review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Betancur-Ancona, D. The Promising Future of Jatropha curcas: Properties and Potential Applications; Nova Science Publishers Incorporated: Hauppauge, NY, USA, 2016. [Google Scholar]
- Abdulla, R.; Chan, E.S.; Ravindra, P. Biodiesel production from Jatropha curcas: A critical review. Crit. Rev. Biotechnol. 2011, 31, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Cheng, Y.-J.; Ching, Y.C.; Chang, C.M.; Hsiang, D. Green production of energetic jatropha oil from de-shelled Jatropha curcas L. seeds using carbon dioxide extraction. J. Supercrit. Fluids 2012, 66, 137–143. [Google Scholar] [CrossRef]
- Hazmi, A.S.A.; Aung, M.M.; Abdullah, L.C.; Salleh, M.Z.; Mahmood, M.H. Producing jatropha oil-based polyol via epoxidation and ring opening. Ind. Crops Prod. 2013, 50, 563–567. [Google Scholar] [CrossRef]
- Lestari, D.; Mulder, W.J.; Sanders, J.P. Jatropha seed protein functional properties for technical applications. Biochem. Eng J. 2011, 53, 297–304. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of jatropha Jatropha curcas L. oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Gogoi, P.; Boruah, M.; Bora, C.; Dolui, S.K. Jatropha curcas oil based alkyd/epoxy resin/expanded graphite (EG) reinforced bio-composite: Evaluation of the thermal, mechanical and flame retardancy properties. Prog. Org. Coat. 2014, 77, 87–93. [Google Scholar] [CrossRef]
- Gofferje, G.; Schmid, M.; Stäbler, A. Characterization of Jatropha curcas L. Protein cast films with respect to packaging relevant properties. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Boruah, M.; Gogoi, P.; Adhikari, B.; Dolui, S.K. Preparation and characterization of Jatropha curcas oil based alkyd resin suitable for surface coating. Prog. Org. Coat. 2012, 74, 596–602. [Google Scholar] [CrossRef]
- Liao, L.; Li, X.; Wang, Y.; Fu, H.; Li, Y. Effects of surface structure and morphology of nanoclays on the properties of Jatropha curcas oil-based waterborne polyurethane/clay nanocomposites. Ind. Eng. Chem. Res. 2016, 55, 11689–11699. [Google Scholar] [CrossRef]
- Hong, L.K.; Yusop, R.M.; Salih, N.; Salimon, J. Pengoptimuman tindakbalas pengepoksidaan in situ asid linoleik minyak Jatropha curcas dengan asid performik. Malays. J. Anal. Sci. 2015, 19, 144–154. [Google Scholar]
- Kamil, R.N.M.; Yusup, S.; Rashid, U. Optimization of polyol ester production by transesterification of jatropha-based methyl ester with trimethylolpropane using taguchi design of experiment. Fuel 2011, 90, 2343–2345. [Google Scholar] [CrossRef]
- Tapanes, N.C.O.; Aranda, D.A.G.; de Mesquita Carneiro, J.W.; Antunes, O.A.C. Transesterification of Jatropha curcas oil glycerides: Theoretical and experimental studies of biodiesel reaction. Fuel 2008, 87, 2286–2295. [Google Scholar] [CrossRef]
- Rosillo-Calle, F.; Pelkmans, L.; Walter, A. A global overview of vegetable oils, with reference to biodiesel. Rep. Bioenergy Task 2009, 40. [Google Scholar]
- López Téllez, G.; Vigueras-Santiago, E.; Hernández-López, S. Characterization of linseed oil epoxidized at different percentages. Superf. Y Vacío 2009, 22, 5–10. [Google Scholar]
- Da Silva, N.D.L.; Batistella Filho, C. Determination of castor oil molecular weight by vapour pressure osmometry technique. Chem. Eng. Trans. 2011, 24, 601–606. [Google Scholar]
- Doll, K.M.; Erhan, S.Z. The improved synthesis of carbonated soybean oil using supercritical carbon dioxide at a reduced reaction time. Green Chem. 2005, 7, 849–854. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Yan, S.; Wang, X.; Kang, M.; Wang, J.; Xiang, H. Catalytic synthesis of carbonated soybean oil. Catal. Lett. 2008, 123, 246–251. [Google Scholar] [CrossRef]
- Supasitmongkol, S.; Styring, P. A single centre aluminium (III) catalyst and tbab as an ionic organo-catalyst for the homogeneous catalytic synthesis of styrene carbonate. Catal. Sci. Technol. 2014, 4, 1622–1630. [Google Scholar] [CrossRef]
- Tamami, B.; Sohn, S.; Wilkes, G. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J. Appl. Polym. Sci. 2004, 92, 883–891. [Google Scholar] [CrossRef]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Soy-based polyurethanes by nonisocyanate route. J. Appl. Polym. Sci. 2008, 108, 3867–3875. [Google Scholar] [CrossRef]
- Mazo, P.C.; Rios, L.A. Improved synthesis of carbonated vegetable oils using microwaves. Chem. Eng. J. 2012, 210, 333–338. [Google Scholar] [CrossRef]
- Parzuchowski, P.G.; Jurczyk-Kowalska, M.; Ryszkowska, J.; Rokicki, G. Epoxy resin modified with soybean oil containing cyclic carbonate groups. J. Appl. Polym. Sci. 2006, 102, 2904–2914. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Chevereau, E.; Zouaoui, N.; Limousy, L.; Dutournié, P.; Déon, S.; Bourseau, P. Surface properties of ceramic ultrafiltration tio 2 membranes: Effects of surface equilibriums on salt retention. Desalination 2010, 255, 1–8. [Google Scholar] [CrossRef]
- Cacace, M.; Landau, E.; Ramsden, J. The hofmeister series: Salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 1997, 30, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Iskandar, I.Y. Influence of nano-SiO2/polyamide composites coating on thermic effect and optical properties of polyethylene film. Int. J. Mod. Phys. B 2009, 23, 1395–1400. [Google Scholar]
- Khoon, S.C.; Ching, Y.C.; Ng, C.A.; Ishenny, N.; Beg, M.T. Effect of polyurethane/nanosilica composite coating on water resistance of paper substrate. Mater. Res. Innov. 2014, 18, 368–371. [Google Scholar]
- Schumacher, R. The quartz microbalance: A novel approach to the in-situ investigation of interfacial phenomena at the solid/liquid junction (new analytical methods (40)). Angew. Chem. Int. Ed. 1990, 29, 329–343. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Zhao, T.; Ye, Z.; Huang, H. Ultrasonication-assisted rapid determination of epoxide values in polymer mixtures containing epoxy resin. Anal. Methods 2014, 6, 4257–4261. [Google Scholar] [CrossRef]
- Cornille, A.; Michaud, G.; Simon, F.; Fouquay, S.; Auvergne, R.; Boutevin, B.; Caillol, S. Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur. Polym. J. 2016, 84, 404–420. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zheng, J.L.; Burel, F.; Salmi, T.; Taouk, B.; Leveneur, S.B. Carbonation of vegetable oils: Influence of mass transfer on reaction kinetics. Ind. Eng. Chem. Res. 2015, 54, 10935–10944. [Google Scholar] [CrossRef]
- Motokura, K.; Itagaki, S.; Iwasawa, Y.; Miyaji, A.; Baba, T. Silica-supported aminopyridinium halides for catalytic transformations of epoxides to cyclic carbonates under atmospheric pressure of carbon dioxide. Green Chem. 2009, 11, 1876–1880. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, pentaerythritol- and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Buslov, F. Ultraviolet and thermostable non-isocyanate polyurethane coatings. Surf. Coat. Int. B Coat. Trans. 2005, 88, 67–71. [Google Scholar] [CrossRef]
- Babak, V.G.; Desbrieres, J.; Tikhonov, V.E. Dynamic surface tension and dilational viscoelasticity of adsorption layers of a hydrophobically modified chitosan. Colloids Surf. A 2005, 255, 119–130. [Google Scholar] [CrossRef]
- Pathan, S.; Ahmad, S. Synthesis, characterization and the effect of the s-triazine ring on physico-mechanical and electrochemical corrosion resistance performance of waterborne castor oil alkyd. J. Mater. Chem. A 2013, 1, 14227–14238. [Google Scholar] [CrossRef]
- Pramanik, S.; Kar, K.K. Functionalized poly(ether ether ketone): Improved mechanical property and acellular bioactivity. J. Appl. Polym. Sci. 2012, 123, 1100–1111. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, H.; Liu, S.; Zhang, T. The humidity-sensitive property of mcm-48 self-assembly fiber prepared via electrospinning. RSC Adv. 2014, 4, 2807–2812. [Google Scholar] [CrossRef]
- Ching, Y.C.; Chen, Y.C.; Kalam, A.; Iskandar, Y.I. Comparison use of suspended cell system and polyurethane base immobilized cell system in biological treatment of wastewater. Res. J. Chem. Environ. 2011, 15, 849–855. [Google Scholar]
- Waite, J.H. Mussel adhesion—Essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Arjona, N.; Trejo, G.; Ledesma-García, J.; Arriaga, L.; Guerra-Balcázar, M. An electrokinetic-combined electrochemical study of the glucose electro-oxidation reaction: Effect of gold surface energy. RSC Adv. 2016, 6, 15630–15638. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Okay, O. Swelling behavior of strong polyelectrolyte poly(n-t-butylacrylamide-co-acrylamide) hydrogels. Eur. Polym. J. 2003, 39, 877–886. [Google Scholar] [CrossRef]
- Ching, Y.C.; Iskander, I.Y. Effect of Polyurethane/nanosilica composites Coating on thermo-mechanical properties of polyethylene film. Mater. Technol. 2012, 27, 113–115. [Google Scholar] [CrossRef]
- Ching, Y.C.; Nurehan, S. Effect of nanosilica filled polyurethane composite coating on polypropylene substrate. J. Nanomaterials 2013, 10, 567–578. [Google Scholar] [CrossRef]
- Morawetz, H. Kinetics of intramolecular and intermolecular reactions involving two functional groups attached to polymers. Pure Appl. Chem. 1974, 38, 267–277. [Google Scholar] [CrossRef]
- Novoa, J.J.; Mota, F.; Perez del Valle, C.; Planas, M. Structure of the first solvation shell of the hydroxide anion. A model study using OH–(H2O)n (n = 4, 5, 6, 7, 11, 17) clusters. J. Phys. Chem. A 1997, 101, 7842–7853. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Nik Sulaiman, N.; Aroua, M.K.; Ching, Y.C.; Awanis, N.H. Impact of in situ physical and chemical cleaning on PVDF membrane properties and performances. Chem. Eng. Sci. 2015, 122, 426–435. [Google Scholar] [CrossRef]
- Nurul, A.S.; Awanis, H.; Nik Meriam, N.S.; Ching, Y.C. Alkaline etching treatment of pvdf membrane for water filtration. RSC Adv. 2016, 6, 22153–22160. [Google Scholar]




| Oil | C 18:3 (%) | C 18:2 (%) | C 18:1 (%) | RA | SFA | V/mPa s | Mw/g·mol−1 | AV/mg·KOH·g−1 |
|---|---|---|---|---|---|---|---|---|
| SBO | 7–10 | 51 | 23 | 0 | 14 | 54.3 | 879.4 | 2.7 |
| LSO | 56 | 17 | 24 | 0 | 10.4–11.6 | 48.4 | 887.4 [23] | 4.0 |
| JCO | 0 | 45.15 | 39.5 | 0 | 14.3 | 42.3 | 900.0 | 2.6 |
| CSO | 0.75 | 3 | 44 | 90 | 1.5 | 700 | 927.0 [24] | 2.0 |
| Oil | Nature of the Reactor System | T/(°C) | P/(MPa) | RT/(h) | C/(%) | Reference |
|---|---|---|---|---|---|---|
| JCO | Pressure reactor | 120 | 2.0 | 30 | 99 | Present study |
| ESBO | Flow of CO2 | 110 | 0.101 | 70 | 94 | Tamami et al. [27] |
| ESBO | MI with flow of CO2 | 120 | 0.101 | 40 | 95 | Mazo and Rios [29] |
| ECSO | Autoclave | 140 | 3.000 | 24 | 100 | Zhang et al. [4] |
| ESBO | Autoclave/sc CO2 | 100 | 10.300 | 20 | 94 | Doll and Erhan [22] |
| ESBO | Pressure reactor | 140 | 5.650 | 20 | 100 | Javni et al. [28] |
| ELO | Pressure reactor | 140 | 3.000 | 20 | 100 | Bähr and Mülhaupt [43] |
| Carbonate of JCO | a M (g·mol−1) | b Viscosity 25 °C/mPa·s | Conversion % | c Carbonate Content/wt % |
|---|---|---|---|---|
| CJCO | 1684 | 2900 ± 430 | 99 | 24.9 |
| CC-AR | 798 | 3400 ± 520 | 97 | 20.2 |
| Amine | Carbonate | w/w | CC/wt % | Amine/wt % | MR of A:C | Tg/(°C) | E-modules (MPa) | TS (MPa) | Break (%) |
|---|---|---|---|---|---|---|---|---|---|
| DM | CJCO | 1 | 24.9 | 17.8 | 6:1 | 23 | 4 ± 2 | 5 ± 1 | 230 ± 40 |
| IPDA | CJCO | 1 | 24.9 | 34.3 | 9:1 | 54 | 150 ± 30 | 17 ± 2 | 67 ± 28 |
| DM | CJCO/CC-AR | 1:1 | 22.6 | 14.7 | 5:2 | 22 | 12 ± 3 | 11 ± 2 | 210 ± 20 |
| CJCO/CC-AR | 1:2 | 21.8 | 20.5 | 3:1 | 25 | 70 ± 10 | 8 ± 1 | 130 ± 10 | |
| CJCO/CC-AR | 1:3 | 21.4 | 27.3 | 3:1 | 21 | 220 ± 40 | 10 ± 2 | 80 ± 30 | |
| CJCO/CC-AR | 1:4 | 21.1 | 22.3 | 7:3 | 27 | 108 ± 10 | 5 ± 2 | 54 ± 30 | |
| IPDA | CJCO/CC-AR | 1:1 | 22.6 | 35.6 | 4:1 | 48 | 27 ± 5 | 4 ± 0 | 170 ± 10 |
| CJCO/CC-AR | 1:2 | 21.8 | 30.4 | 3:1 | 45 | 390 ± 20 | 6 ± 0 | 70 ± 10 | |
| CJCO/CC-AR | 1:3 | 21.4 | 30.8 | 4:1 | 44 | 680 ± 140 | 7 ± 2 | 30 ± 20 | |
| CJCO/CC-AR | 1:4 | 21.1 | 24.8 | 7:3 | 42 | 259 ± 30 | 4 ± 2 | 160 ± 20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haniffa, M.A.C.M.; Ching, Y.C.; Chuah, C.H.; Kuan, Y.C.; Liu, D.-S.; Liou, N.-S. Synthesis, Characterization and the Solvent Effects on Interfacial Phenomena of Jatropha Curcas Oil Based Non-Isocyanate Polyurethane. Polymers 2017, 9, 162. https://doi.org/10.3390/polym9050162
Haniffa MACM, Ching YC, Chuah CH, Kuan YC, Liu D-S, Liou N-S. Synthesis, Characterization and the Solvent Effects on Interfacial Phenomena of Jatropha Curcas Oil Based Non-Isocyanate Polyurethane. Polymers. 2017; 9(5):162. https://doi.org/10.3390/polym9050162
Chicago/Turabian StyleHaniffa, Mhd. Abd. Cader M., Yern Chee Ching, Cheng Hock Chuah, Yong Ching Kuan, De-Shin Liu, and Nai-Shang Liou. 2017. "Synthesis, Characterization and the Solvent Effects on Interfacial Phenomena of Jatropha Curcas Oil Based Non-Isocyanate Polyurethane" Polymers 9, no. 5: 162. https://doi.org/10.3390/polym9050162
APA StyleHaniffa, M. A. C. M., Ching, Y. C., Chuah, C. H., Kuan, Y. C., Liu, D.-S., & Liou, N.-S. (2017). Synthesis, Characterization and the Solvent Effects on Interfacial Phenomena of Jatropha Curcas Oil Based Non-Isocyanate Polyurethane. Polymers, 9(5), 162. https://doi.org/10.3390/polym9050162





