Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Hydrogels
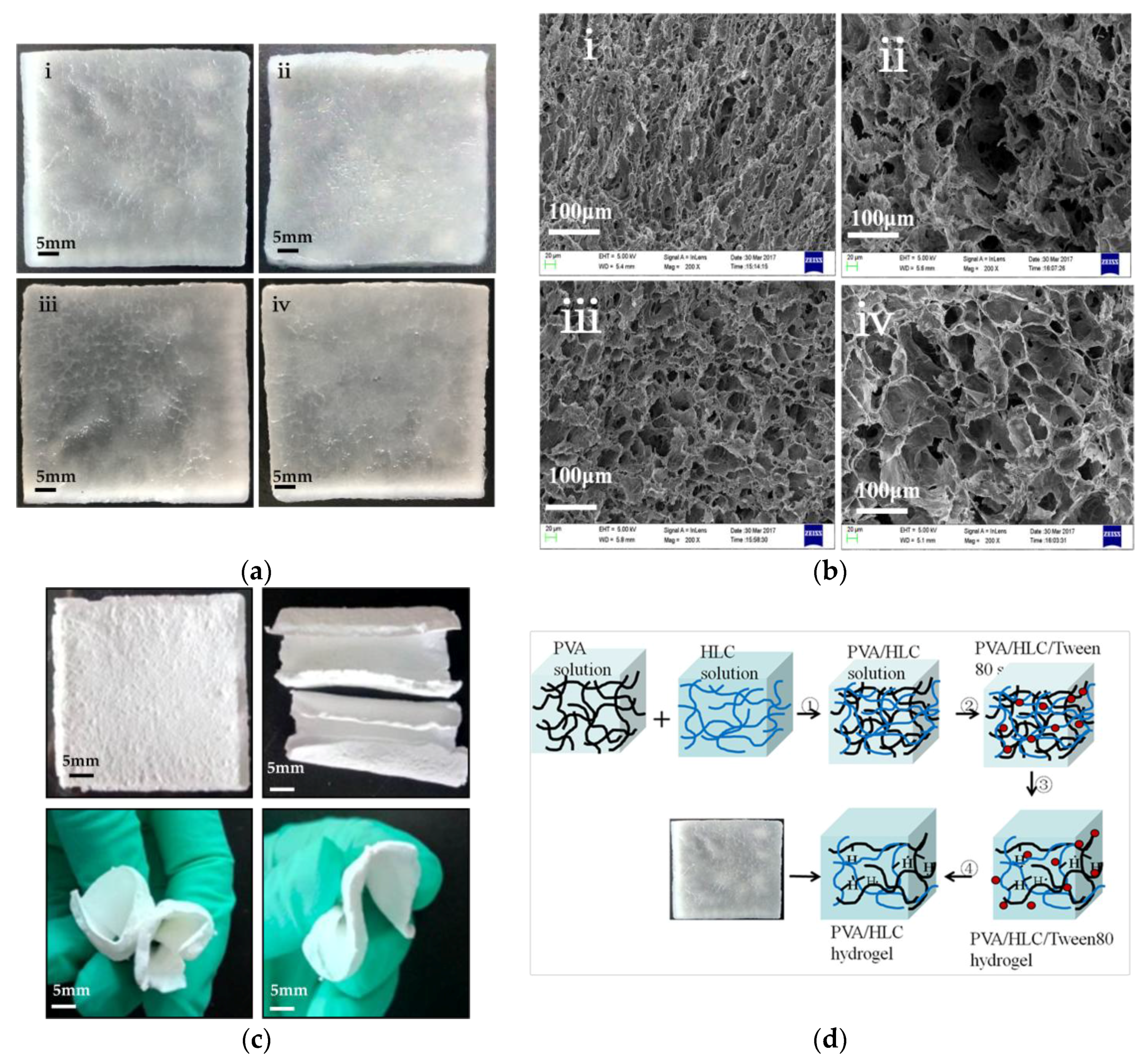
2.3. Microstructure Analysis of Lyophilized Hydrogels
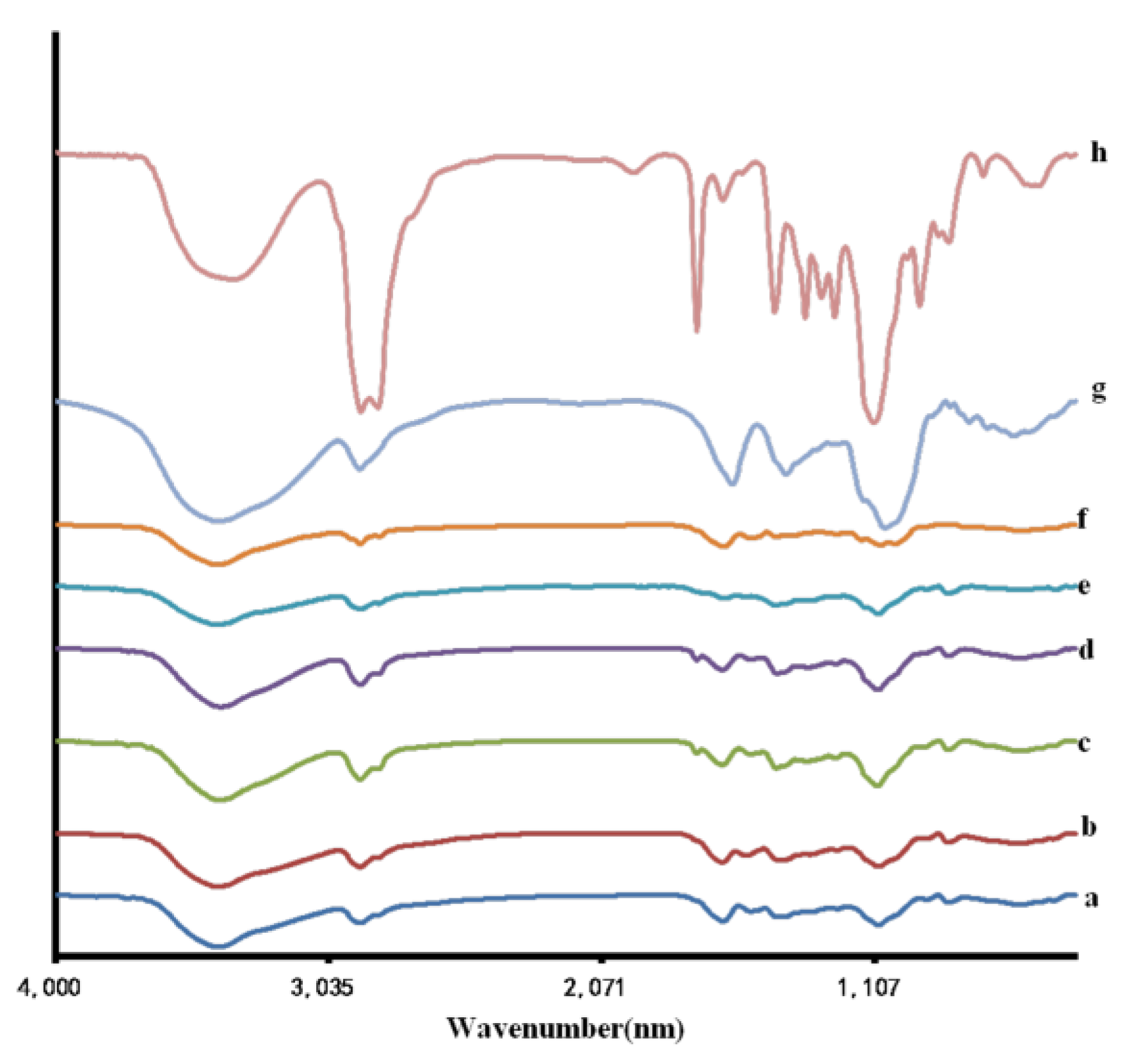
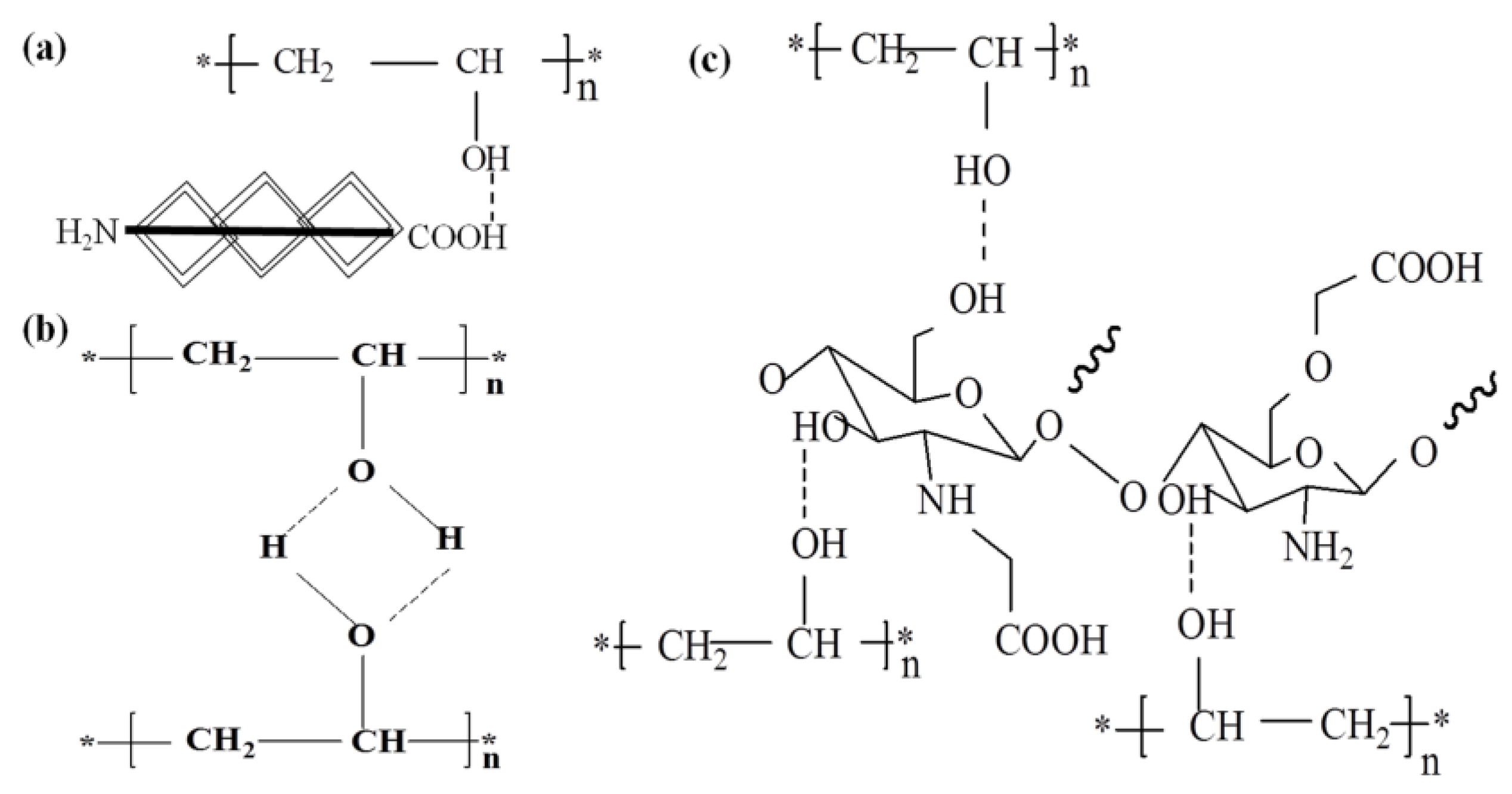
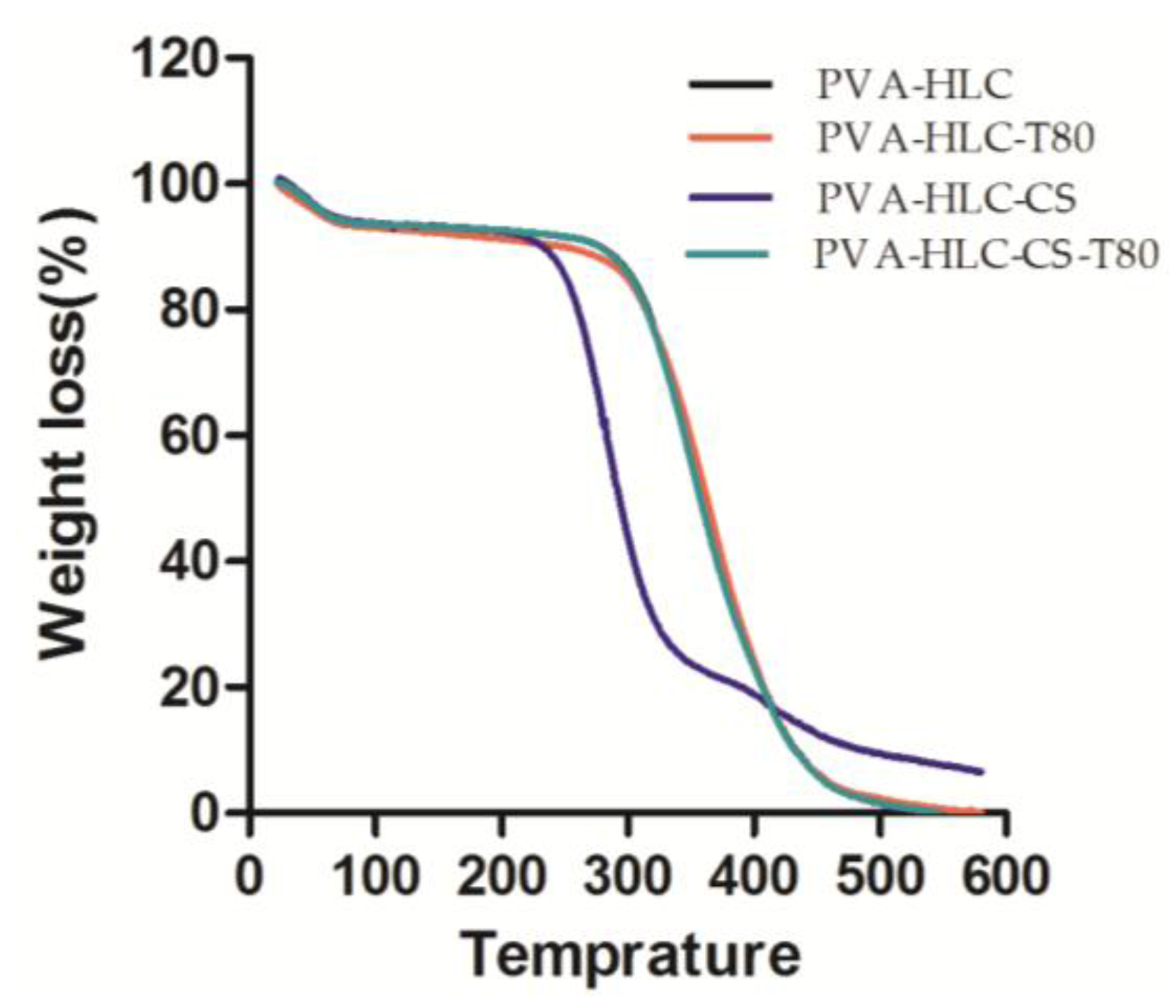
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy and Thermal Gravimetric Analysis (TGA)
2.5. Density and Porosity of the Lyophilized Hydrogels
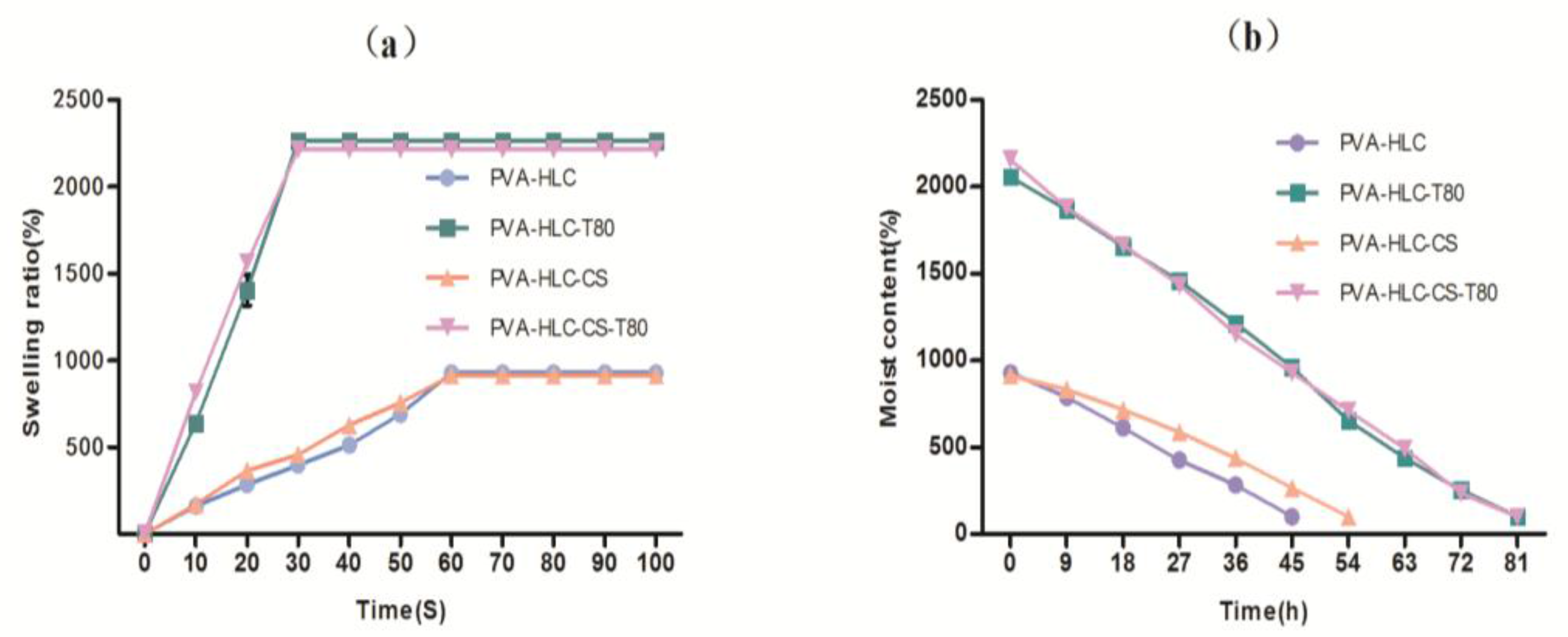
2.6. Swelling Kinetics
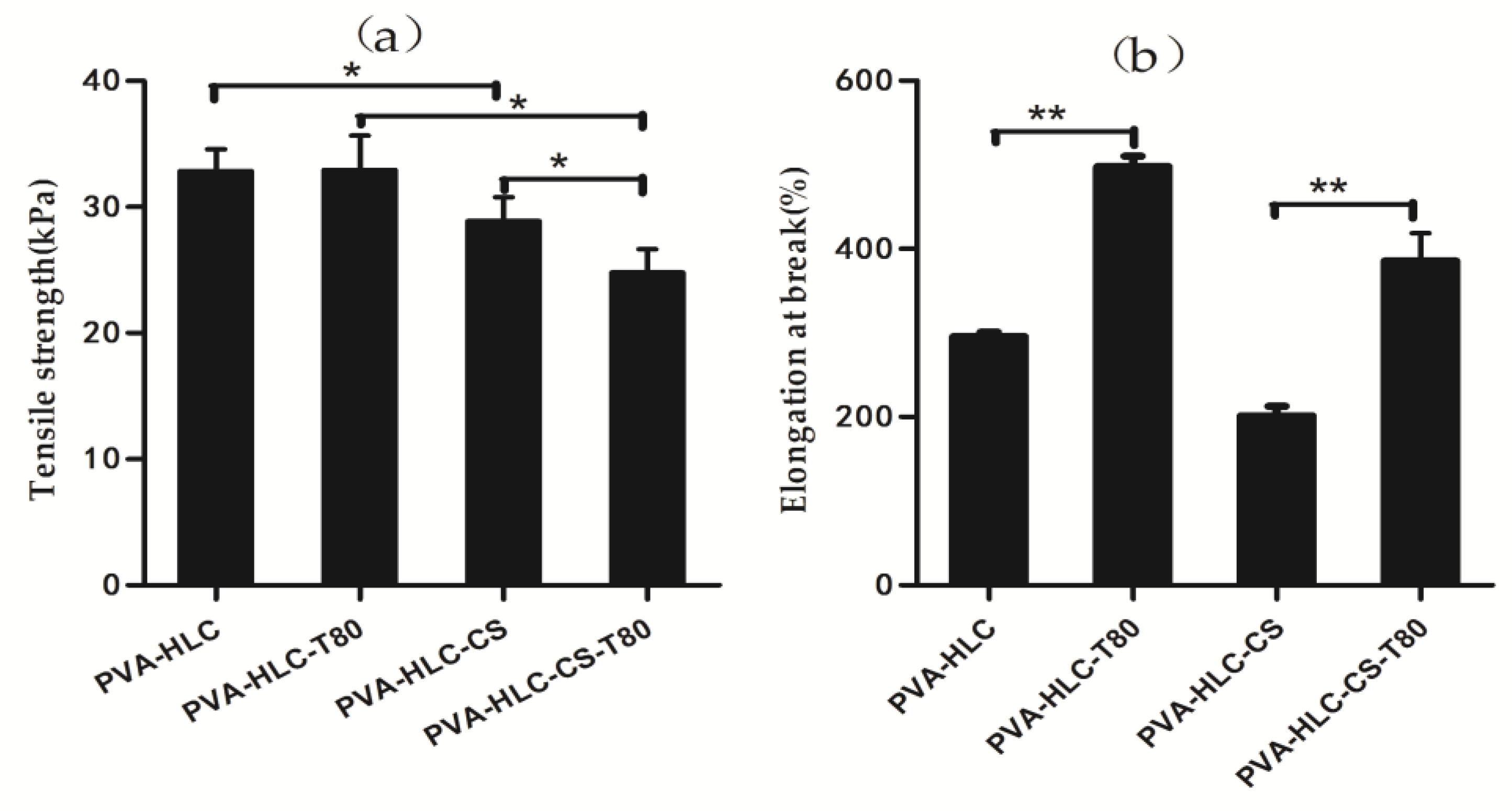
2.7. Tensile Property of the Hydrogels
2.8. Moisture Vapor Transmission Rate
2.9. In Vitro Degradation Rate of the Hydrogels
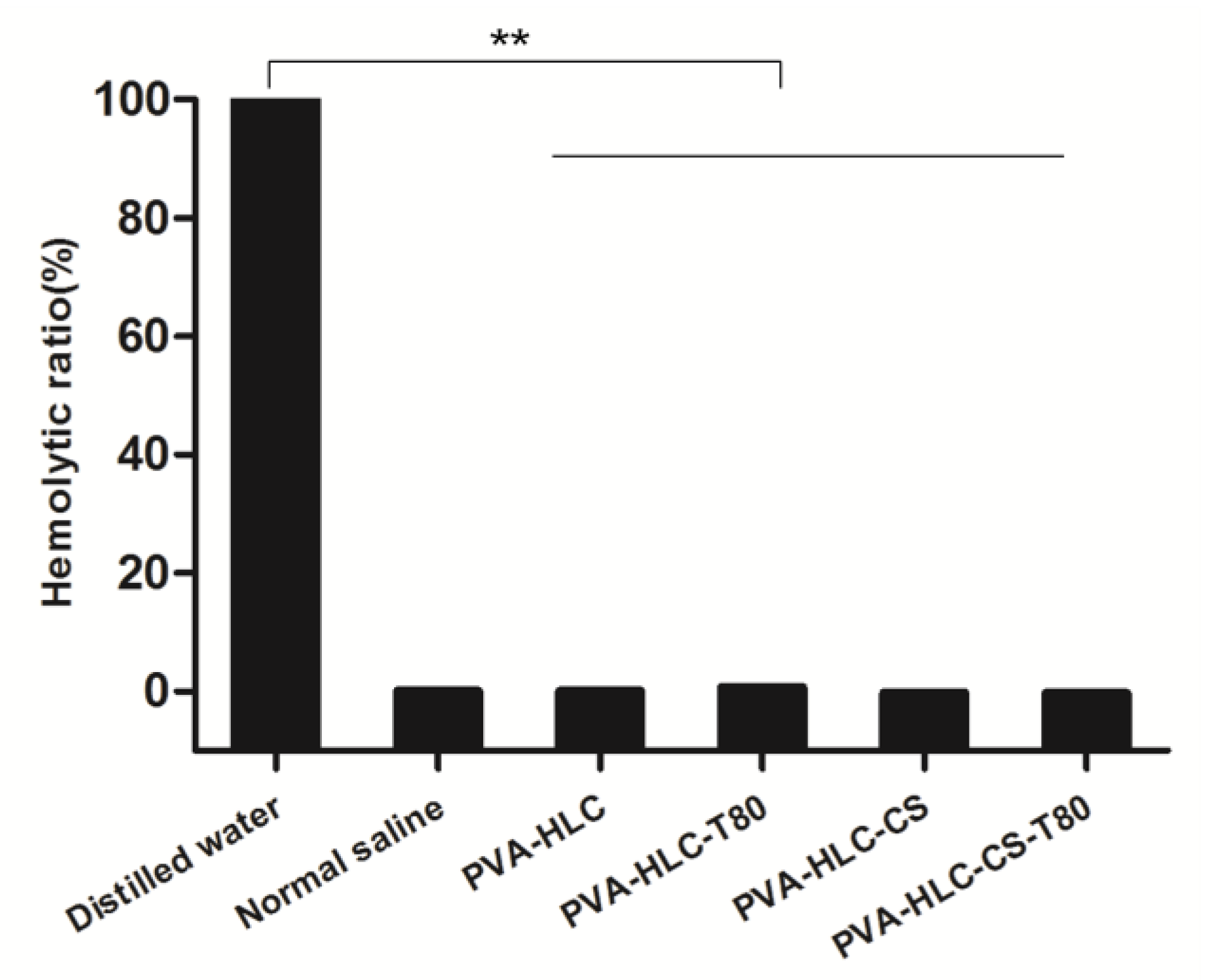
2.10. In Vitro Hemolytic Activity Test of the Hydrogels
2.11. Bacterial Barrier Properties of the Hydrogels
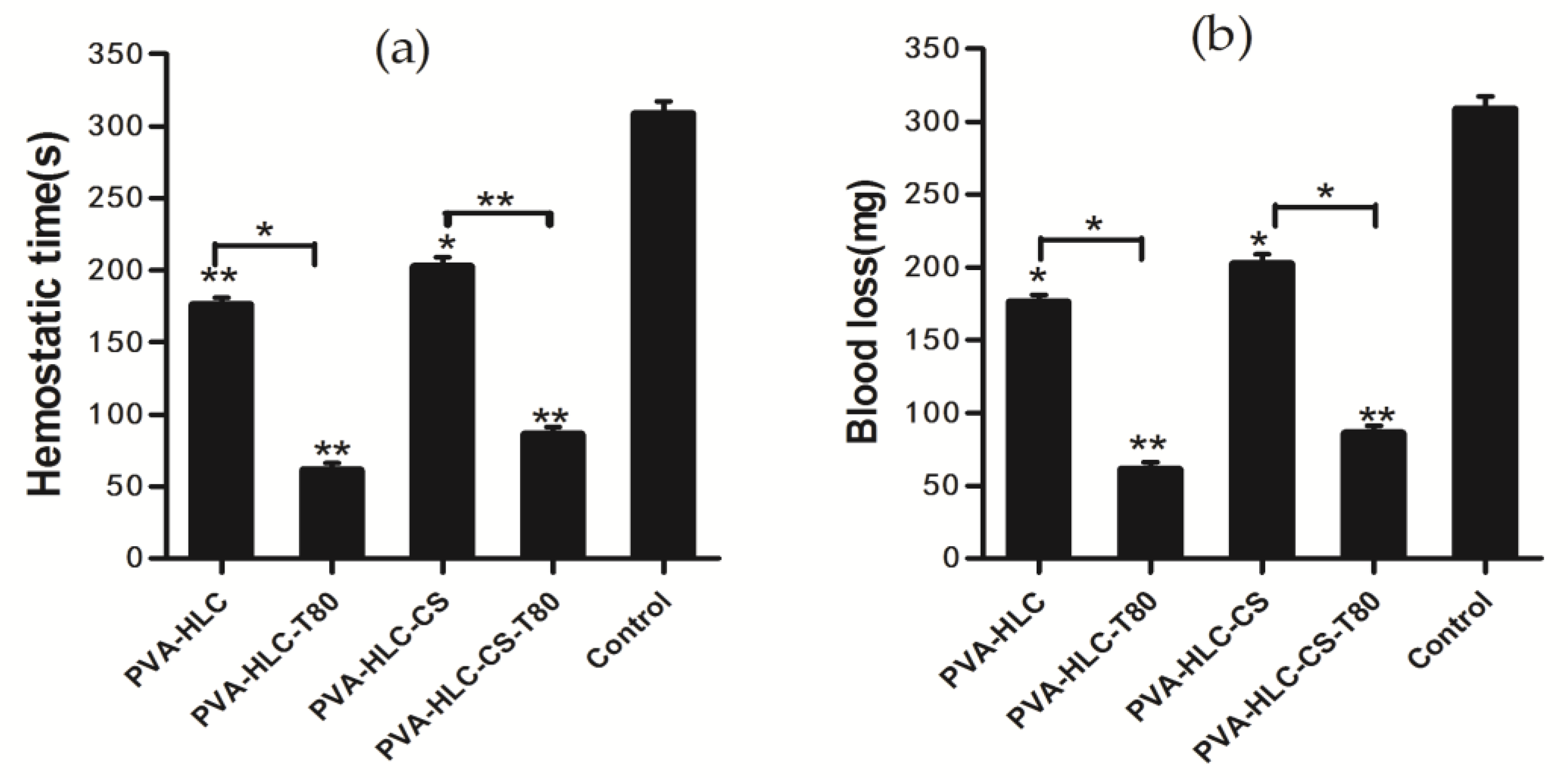
2.12. Hemostatic Property of the Hydrogel
2.13. Cytotoxicity Testing of Hydrogels
2.14. In Vitro Biocompatibility of the Hydrogel
2.14.1. MTT Assay
2.14.2. Microscopic Analysis
2.15. In Vivo Histocompatibility Evaluation
2.15.1. Implantation of Hydrogels
2.15.2. H&E Staining and TEM Analysis
2.16. In Vivo Studies in Rabbit Animal Model
2.16.1. Surgical Procedure
2.16.2. Macroscopic and Histological Evaluation
2.16.3. Transmission Electron Microscope Analysis
2.17. Statistical Analysis
3. Results
3.1. Macrostructure and Microstructure of the Hydrogels
3.2. FT-IR Spectroscopic and TGA Analysis
3.3. Density and Porosity of the Hydrogels
3.4. Swelling Kinetics
3.5. Tensile Property of Hydrogels
3.6. Moisture Vapour Transmission Rate (MVTR)
3.7. In Vitro Degradation Rate of the Hydrogel Dressing
3.8. Hemolytic Activity Test of the Hydrogels
3.9. Bacterial Barrier Properties
3.10. Hemostasis Activity
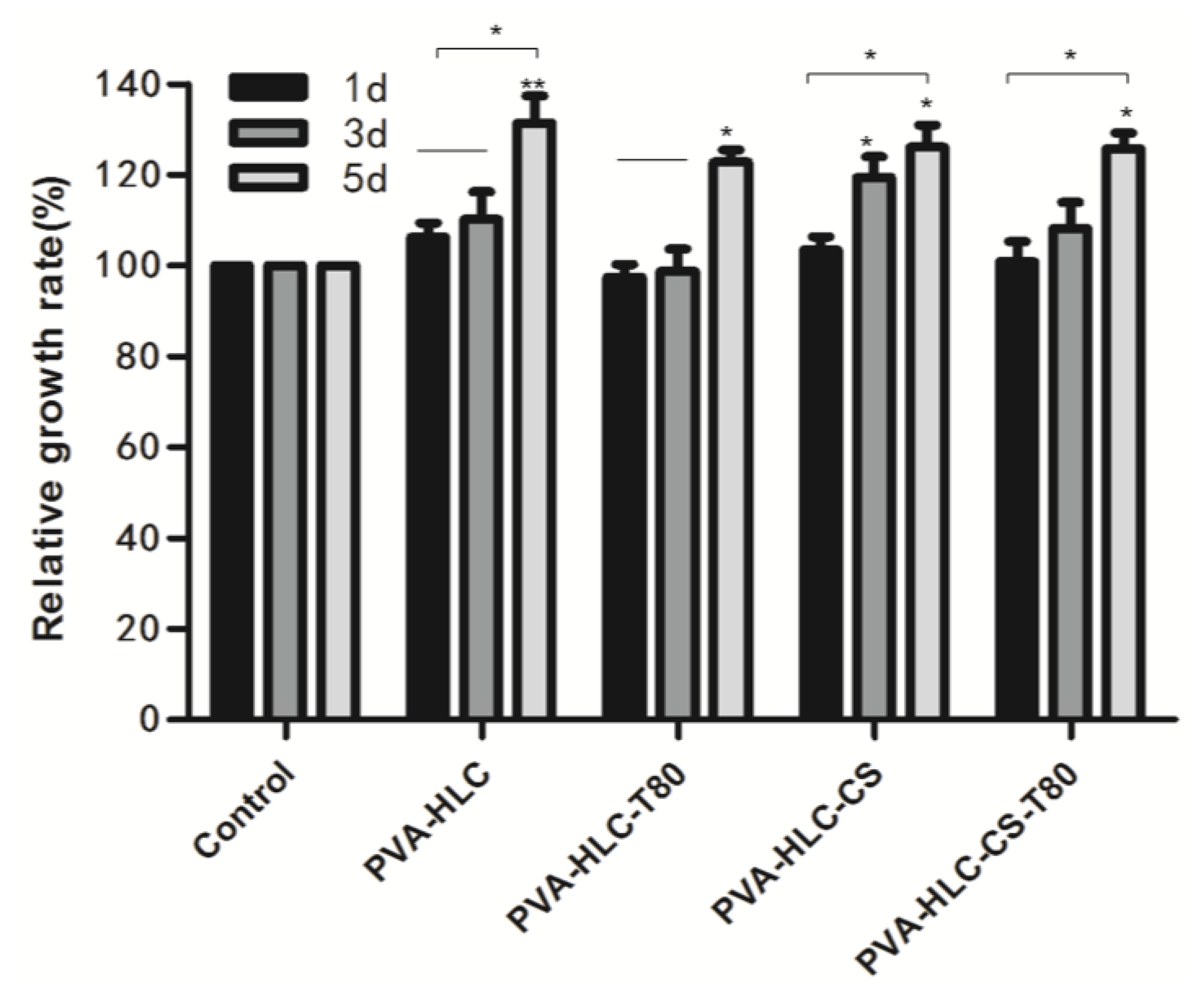
3.11. Cytotoxicity of Hydrogels
3.12. In Vitro Biocompatibility of the Hydrogel
3.12.1. Cell Proliferation
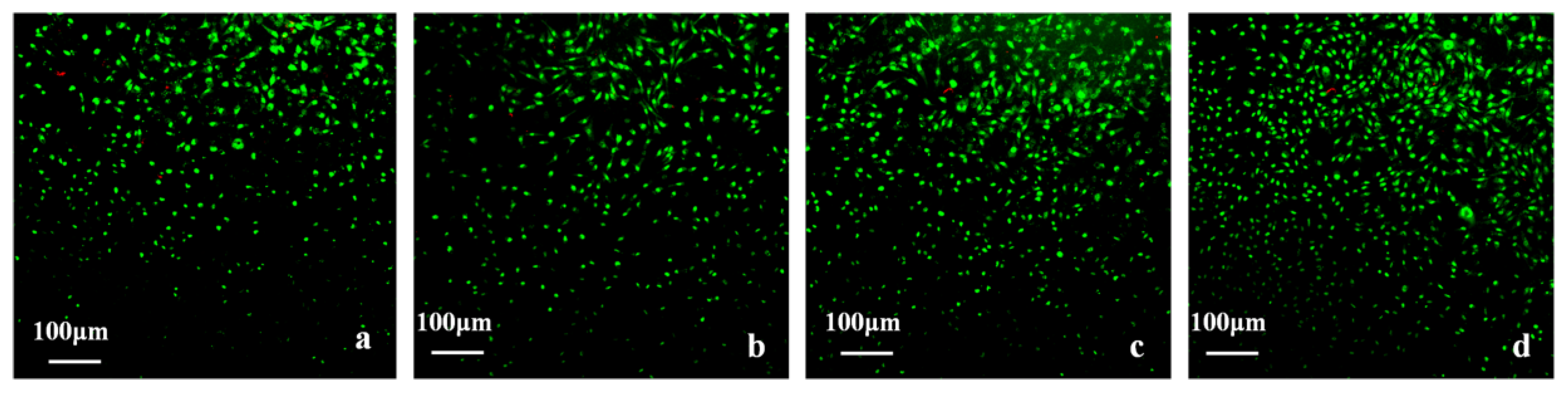
3.12.2. Cell Viability
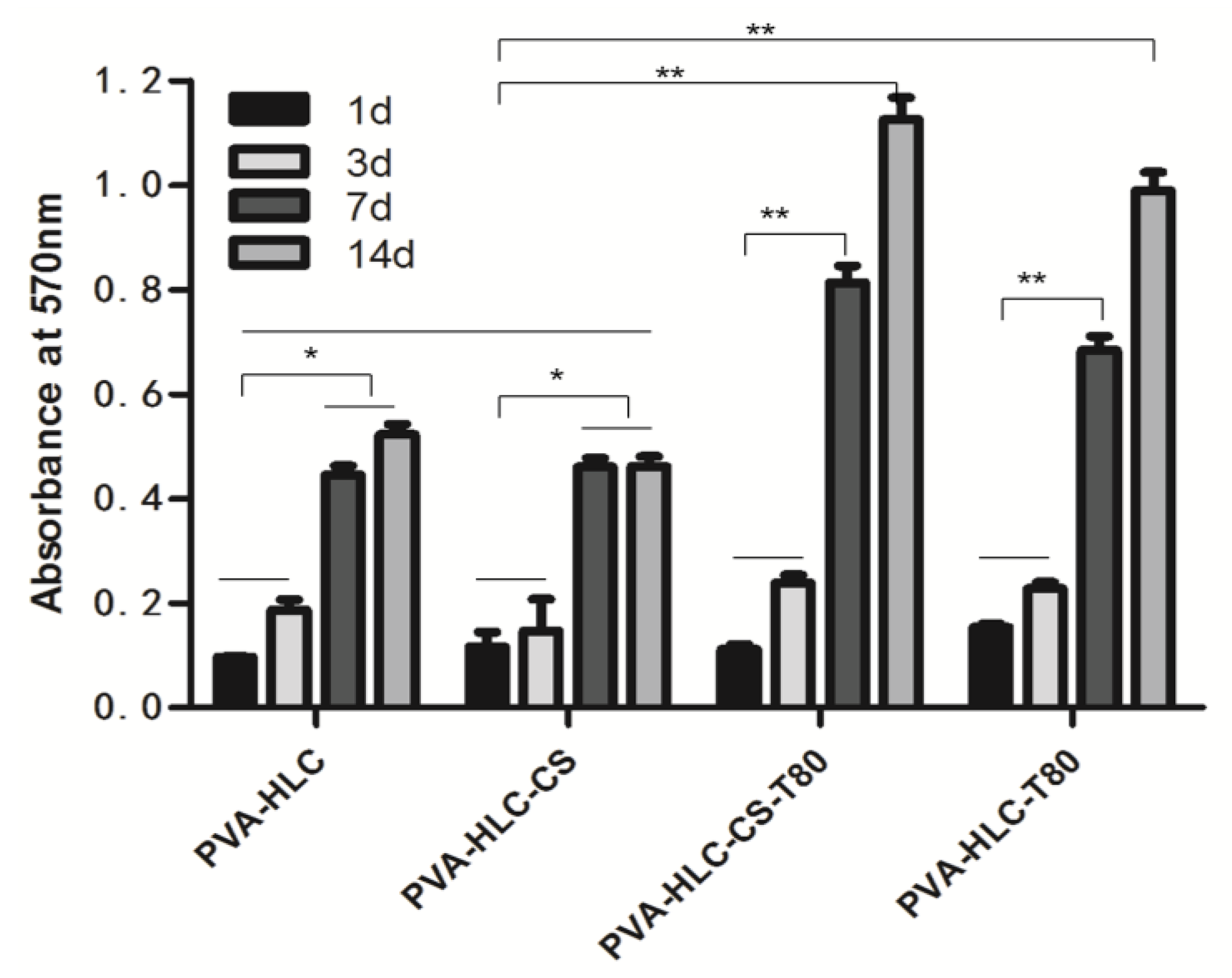
3.13. In Vivo Histocompatibility Evaluation
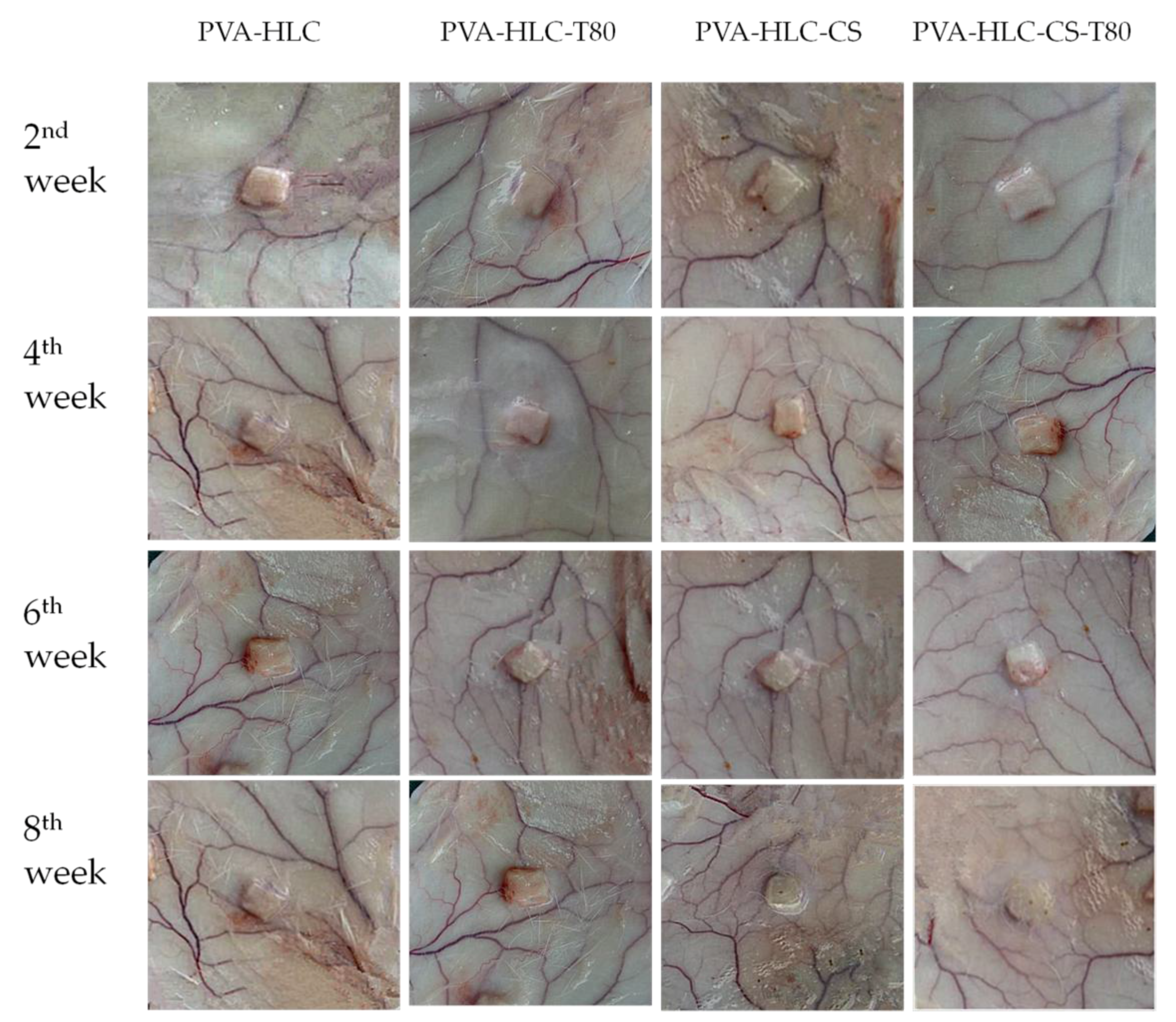
3.13.1. Macroscopic Observation of Implanted Hydrogels and Surrounding Tissue
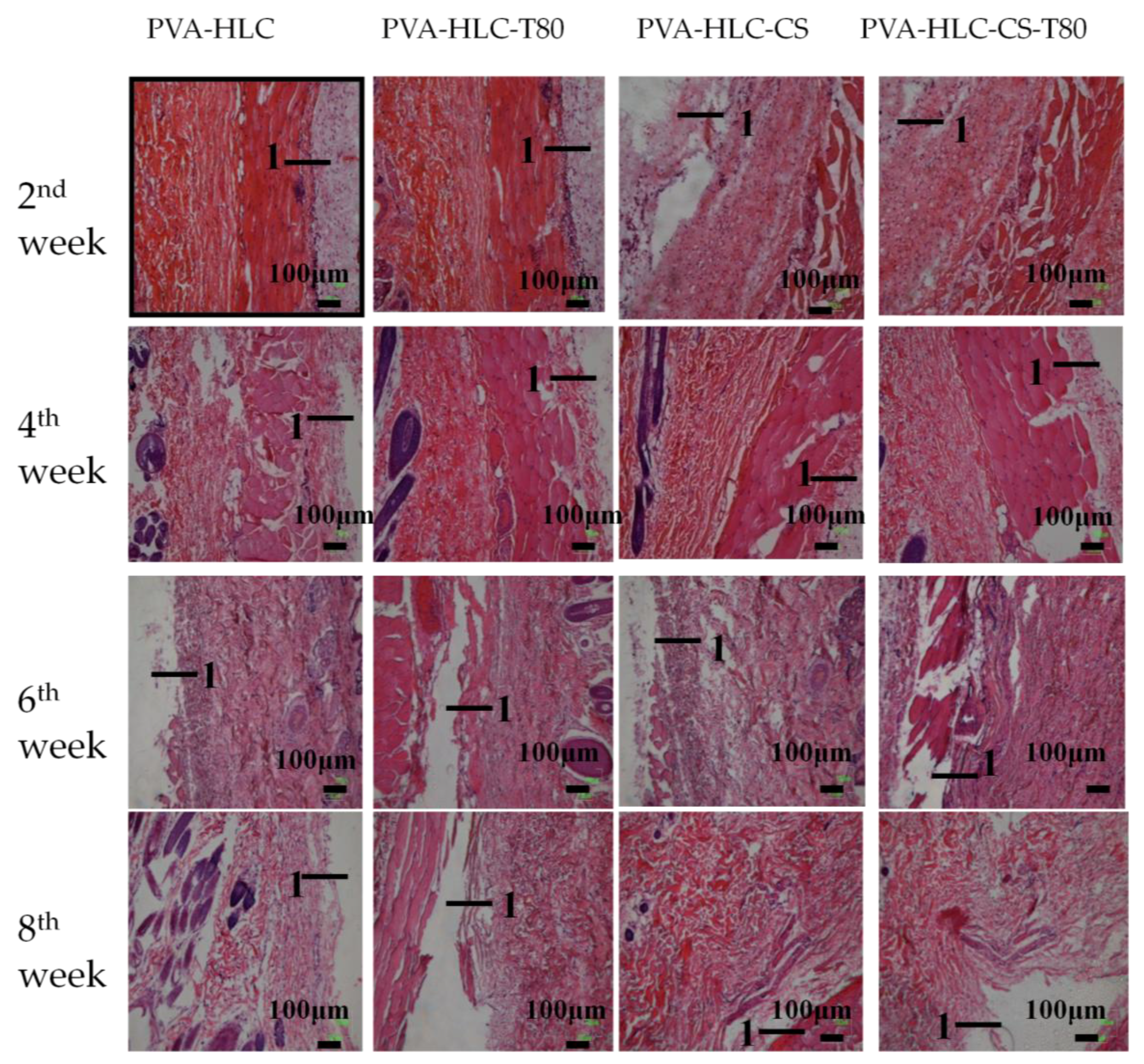
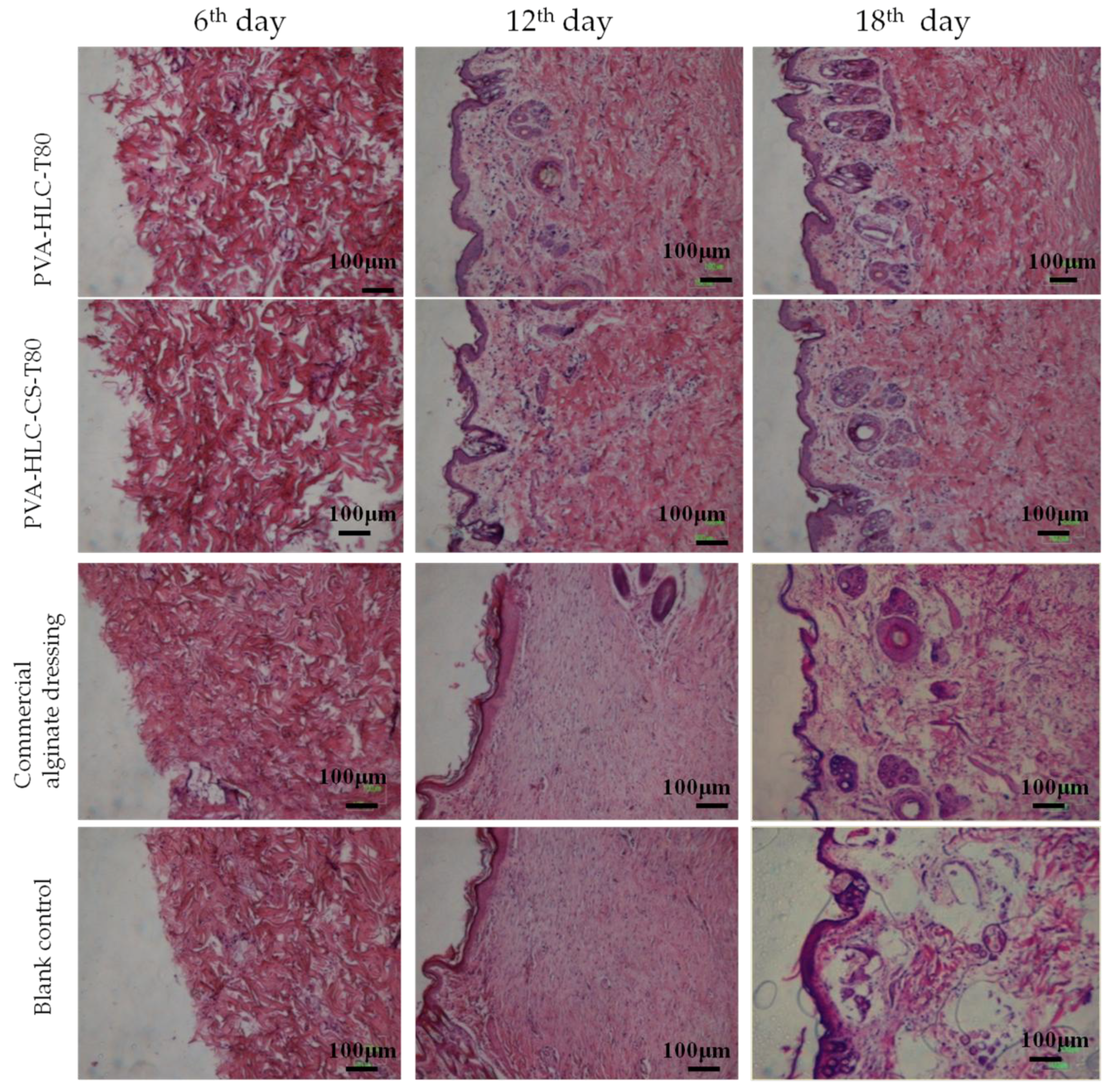
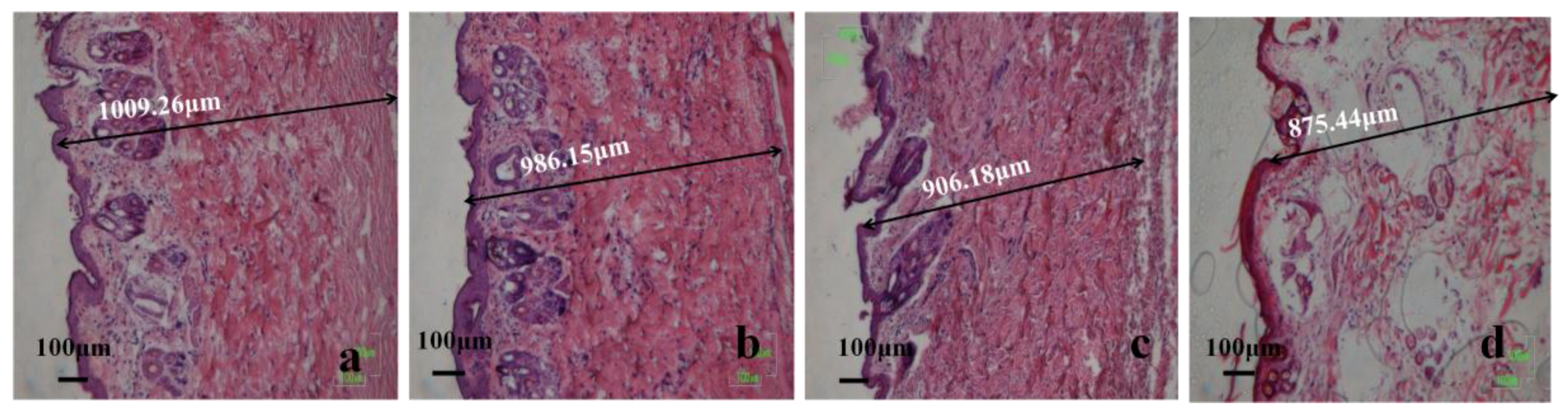
3.13.2. H&E Staining Analysis
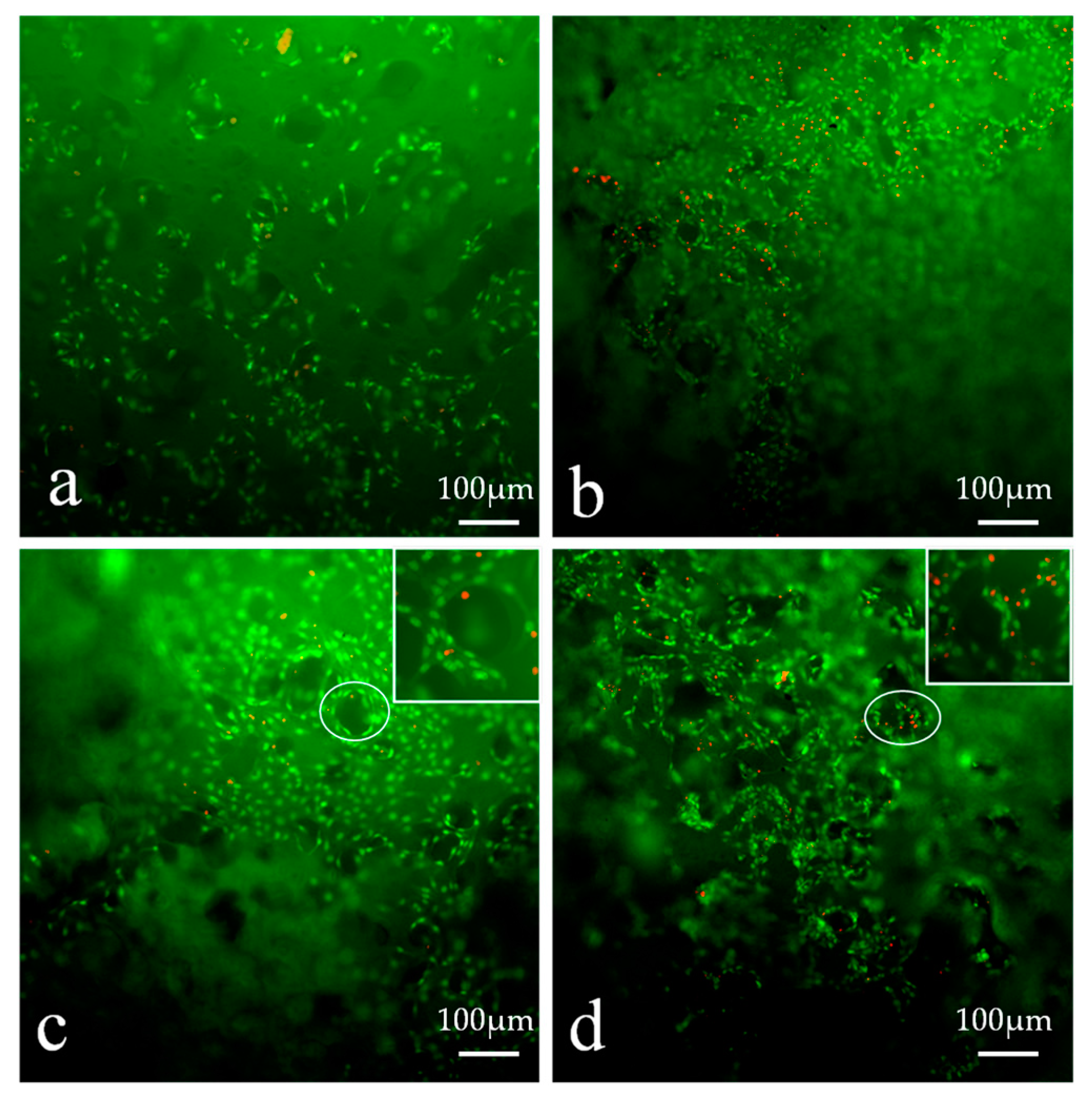
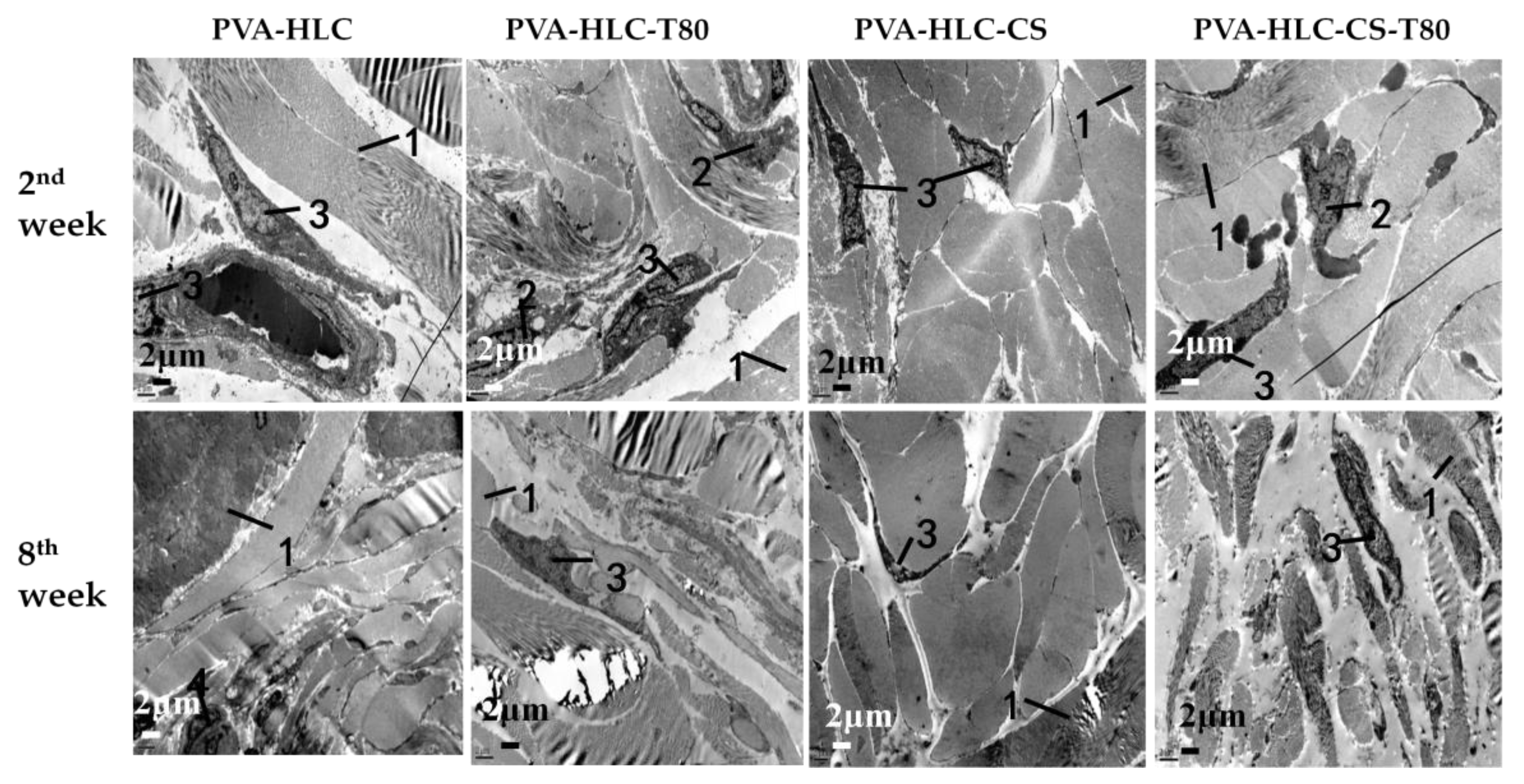
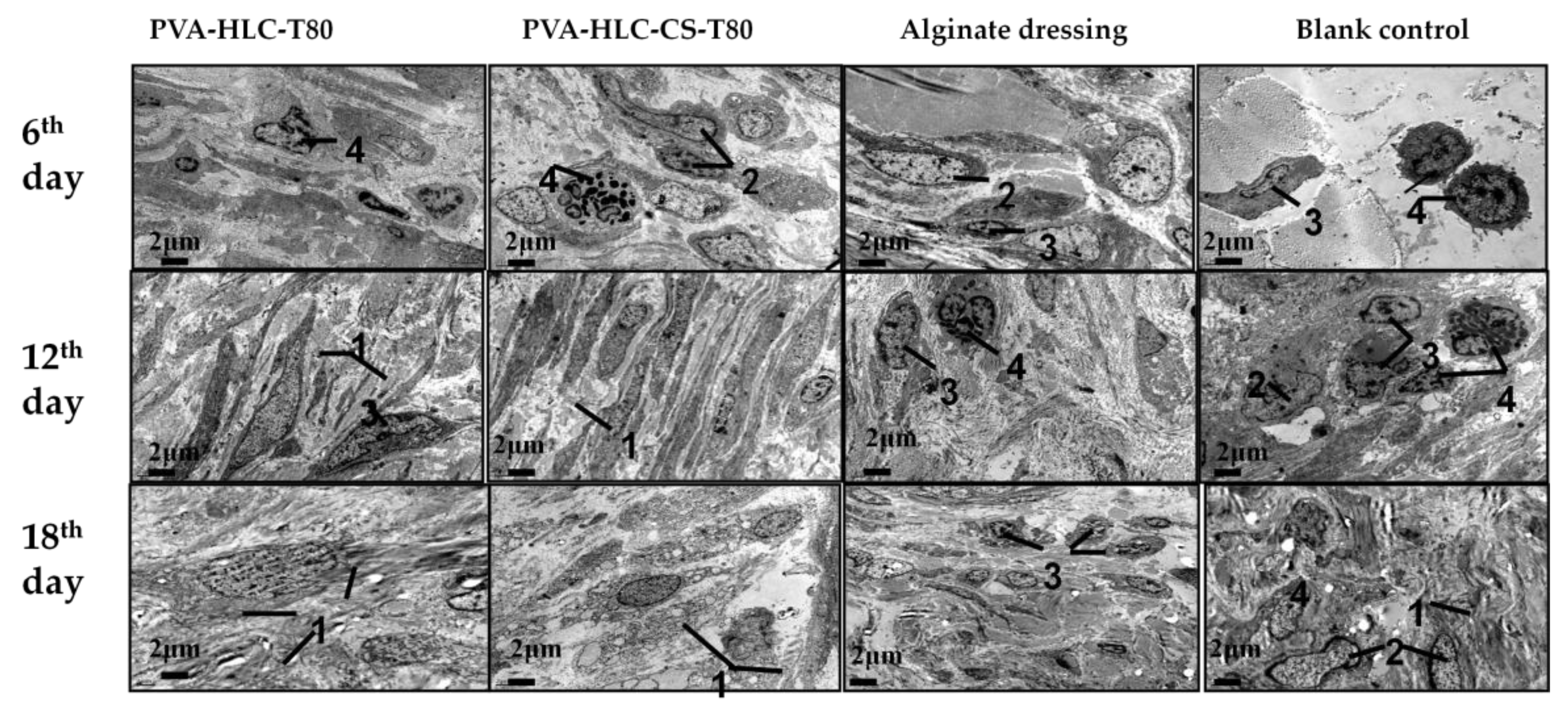
3.13.3. TEM Analysis
3.14. In Vivo Studies in Rabbit Animal Model
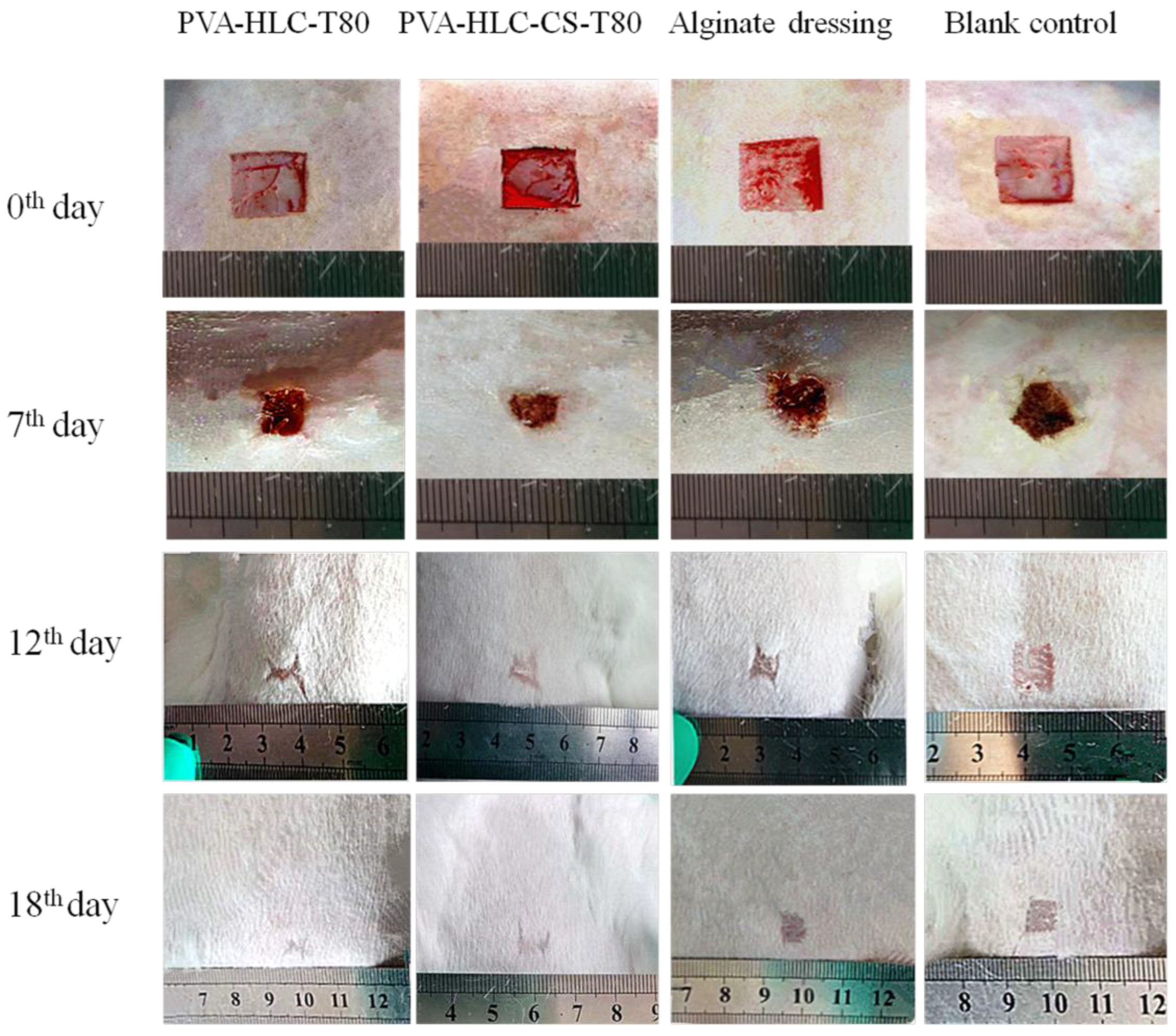
3.14.1. Observation of Wound Contraction and Healing
3.14.2. Histomorphological Evaluation of Wound Regeneration
3.14.3. TEM Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Papini, R. Management of burn injuries of various depths. BMJ 2004, 329, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: Pva-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C.; Xie, X.-M. Studies on the properties and formation mechanism of flexible nanocomposite hydrogels from cellulose nanocrystals and poly(acrylic acid). J. Mater. Chem. 2012, 22, 22467–22480. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Duan, J.F.; Xu, F.; Sun, R.C. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly(ethylene glycol) nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Sharma, P.C. Chitosan and alginate wound dressings—A short review. Trends Biomater. Artif. Organs 2004, 18, 18–23. [Google Scholar]
- Nho, Y.C.; Park, J.S.; Lim, Y.M. Preparation of hydrogel by radiation for the healing of diabetic ulcer. Radiat. Phys. Chem. 2014, 94, 176–180. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Fan, D.D.; Ma, X.X.; Mi, Y.; Luo, Y.E.; Zhu, C.H.; Zhu, X.L.; Xue, W.J. A genetic algorithm for the optimization of the thermoinduction protocol for high-level production of recombinant human-like collagen from Escherichia coli. Biotechnol. Appl. Biochem. 2011, 58, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.E.; Fan, D.D.; Shang, L.A.; Shi, H.J.; Ma, X.X.; Mi, Y.; Zhao, G.F. Analysis of metabolic flux in Escherichia coli expressing human-like collagen in fed-batch culture. Biotechnol. Lett. 2008, 30, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fan, D.; Duan, Z.; Xue, W.; Shang, L.; Chen, F.; Luo, Y. Initial investigation of novel human-like collagen/chitosan scaffold for vascular tissue engineering. J. Biomed. Mater. Res. Part A 2009, 89, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Winterowd, J.G.; Allen, G.G.; Bothwell, M.A.; Rubel, E.W. Nerve growth factor: Increased angiogenesis without improved nerve regeneration. Otolaryngol. Head Neck Surg. 1991, 105, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Cui, F.; Lv, Q.; Ma, J.; Feng, Q.; Xu, L.; Fan, D. Preparation of fibroin/recombinant human-like collagen scaffold to promote fibroblasts compatibility. J. Biomed. Mater. Res. Part A 2008, 84A, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, D.; Ma, X.; Zhu, C.; Luo, Y.; Liu, B.; Chen, L. A novel injectable pH/temperature sensitive cs-HLC/β-GP hydrogel: The gelation mechanism and its properties. Soft Mater. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Ng, K.W.; Torzilli, P.A.; Warren, R.F.; Maher, S.A. Characterization of a macroporous polyvinyl alcohol scaffold for the repair of focal articular cartilage defects. J. Biomed. Mater. Res. Part A 2014, 8, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Arun, U.; Remya, S.; Nair, P.D. A biodegradable and biocompatible pva-citric acid polyester with potential applications as matrix for vascular tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20 (Suppl. 1), S259–S269. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids Surf. B 2012, 100, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Agostino, A.D.; La Gatta, A.; Busico, T.; De Rosa, M.; Schiraldi, C. Semi-interpenetrated hydrogels composed of PVA and hyaluronan or chondroitin sulphate: Chemico-physical and biological characterization. J. Biotechnol. Biomater. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Kishi, R.; Miura, T.; Kihara, H.; Asano, T.; Shibata, M.; Yosomiya, R. Fast ph-thermo-responsive copolymer hydrogels with micro-porous structures. J. Appl. Polym. Sci. 2003, 89, 75–84. [Google Scholar] [CrossRef]
- Lu, G.D.; Yan, Q.Z.; Ge, C.C. Preparation of porous polyacrylamide hydrogels by frontal polymerization. Polym. Int. 2007, 56, 1016–1020. [Google Scholar] [CrossRef]
- Kazimierska-Drobny, K.; El Fray, M.; Kaczmarek, M. Determination of mechanical and hydraulic properties of pva hydrogels. Mater. Sci. Eng. C 2015, 48, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Queen, D.; Evans, J.H.; Gaylor, J.D.; Courtney, J.M.; Reid, W.H. Preclinical assessment of burn wound dressings. Burns Incl. Therm. Inj. 1986, 12, 161–166. [Google Scholar] [CrossRef]
- Materials, P.; Materials, E.I.; Specimens, P. Standard Test Method for Tensile Properties of Plastics 1; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; pp. 1–16. [Google Scholar]
- Concepts, R. E96-93 Standard Test Methods for Water-Vapor Transmission of Materials. Annual Book of ASTM Standards. 2014. Available online: http://users.encs.concordia.ca/~raojw/crd/reference/reference000277.htmL (accessed on 26 October 2017).
- Yan, D.; Hu, S.; Zhou, Z.; Zeenat, S.; Cheng, F.; Li, Y.; Feng, C.; Cheng, X.; Chen, X. Different chemical groups modification on the surface of chitosan nonwoven dressing and the hemostatic properties. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, L.; Fan, D.; Xue, W.; Zhu, C.; Li, X.; Liu, Y.; Liu, W.; Ma, P.; Wang, Y. Physicochemical properties and biological behavior of injectable crosslinked hydrogels composed of pullulan and recombinant human-like collagen. J. Mater. Sci. 2017, 52, 1–15. [Google Scholar] [CrossRef]
- Mbarki, M.; Sharrock, P.; Fiallo, M.; ElFeki, H. Hydroxyapatite bioceramic with large porosity. Mater. Sci. Eng. C 2017, 76, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Low, W.L.; Mohd Amin, M.C.I.; Radecka, I.; Raj, P.; Kenward, K. Current trends in the development of wound dressings, biomaterials and devices. Pharm. Pat. Anal. 2013, 2, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Fansler, R.F.; Taheri, P.; Cullinane, C.; Sabates, B.; Flint, L.M. Polypropylene mesh closure of the complicated abdominal wound. Am. J. Surg. 1995, 170, 15–18. [Google Scholar] [CrossRef]
- Ruixin, L.; Cheng, X.; Yingjie, L.; Hao, L.; Caihong, S.; Weihua, S.; Weining, A.; Yinghai, Y.; Xiaoli, Q.; Yunqiang, X. Degradation behavior and compatibility of micro, nanoha/chitosan scaffolds with interconnected spherical macropores. Int. J. Biol. Macromol. 2017, 103, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano zno composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Carraway, J.W.; Kent, D.; Young, K.; Cole, A.; Friedman, R.; Ward, K.R. Comparison of a new mineral based hemostatic agent to a commercially available granular zeolite agent for hemostasis in a swine model of lethal extremity arterial hemorrhage. Resuscitation 2008, 78, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linkin. Biomolecules 2004, 5, 284–294. [Google Scholar]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Dong, R.; Ge, J.; Guo, B.; Ma, P. Biocompatible, biodegradable, and electroactive polyurethane-urea elastomers with tunable hydrophilicity for skeletal muscle tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 28273–28280. [Google Scholar]
- Caldeira, J.; Sousa, A.; Sousa, D.M.; Barros, D. 2—Extracellular matrix constitution and function for tissue regeneration and repair a 2—Barbosa, mário a. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Martins, M.C.L., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 29–72. [Google Scholar]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 3, 834–893. [Google Scholar] [CrossRef] [PubMed]



| Hydrogels | Content of Different Components (wt %) | |||
|---|---|---|---|---|
| PVA | HLC | CS | T80 | |
| PVA-HLC | 6.67 | 1.67 | ||
| PVA-HLC-T80 | 6.67 | 1.67 | 2.4 | |
| PVA-HLC-CS | 5.55 | 1.11 | 0.55 | |
| PVA-HLC-CS-T80 | 5.55 | 1.11 | 0.55 | 2.4 |
| The Hydrogels | Standards (mm) | Density (g·cm−3) | Porosity |
|---|---|---|---|
| PVA-HLC | 38 × 38 × 3 | 0.1260 ± 0.002 | 39.88% ± 0.079 |
| PVA-HLC-T80 | 38 × 38 × 3 | 0.0478 ± 0.010 ** | 85.01% ± 0.571 ** |
| PVA-HLC-CS | 38 × 38 × 3 | 0.1098 ± 0.008 | 38.20% ± 0.077 |
| PVA-HLC-CS-T80 | 38 × 38 × 3 | 0.0534 ± 0.005 ** | 82.56% ± 0.093 ** |
| Sample | Water Vapor Transmission Rate (g·m−2·d−1) |
|---|---|
| PVA-HLC | 923.2 ± 7.1668 ** |
| PVA-HLC-T80 | 2398.4 ± 3.1355 ** |
| PVA-HLC-CS | 921.1 ± 3.3391 ** |
| PVA-HLC-CS-T80 | 2887.4 ± 4.3629 ** |
| Control | 4102.5 ± 2.5176 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers 2017, 9, 727. https://doi.org/10.3390/polym9120727
Pan H, Fan D, Cao W, Zhu C, Duan Z, Fu R, Li X, Ma X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers. 2017; 9(12):727. https://doi.org/10.3390/polym9120727
Chicago/Turabian StylePan, Hong, Daidi Fan, Wei Cao, Chenhui Zhu, Zhiguang Duan, Rongzhan Fu, Xian Li, and Xiaoxuan Ma. 2017. "Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects" Polymers 9, no. 12: 727. https://doi.org/10.3390/polym9120727
APA StylePan, H., Fan, D., Cao, W., Zhu, C., Duan, Z., Fu, R., Li, X., & Ma, X. (2017). Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers, 9(12), 727. https://doi.org/10.3390/polym9120727





