Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TiO2 Paste Preparation
2.3. Preparation of Nanocrystalline TiO2 Films (Photoanodes) and Platinum Counter Electrodes
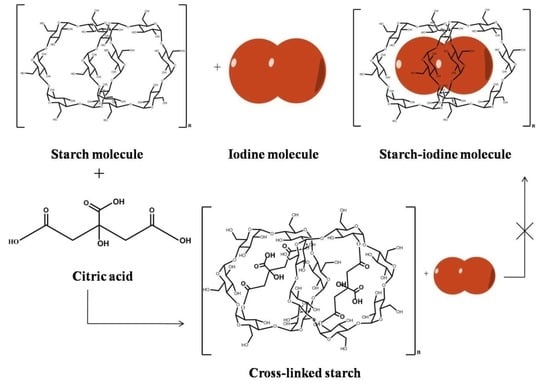
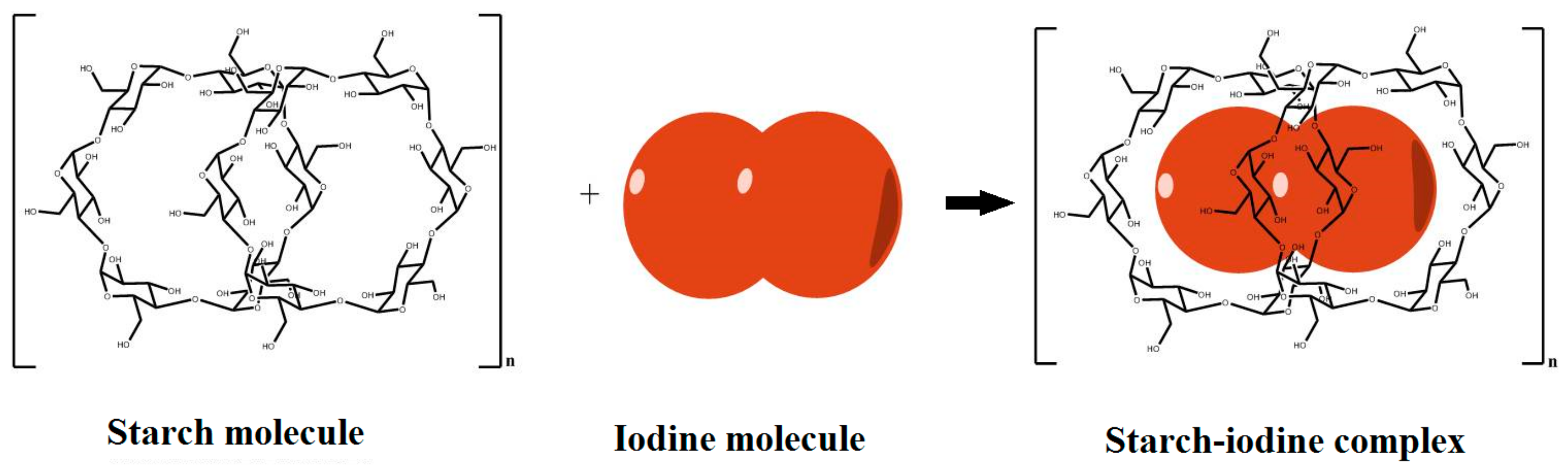
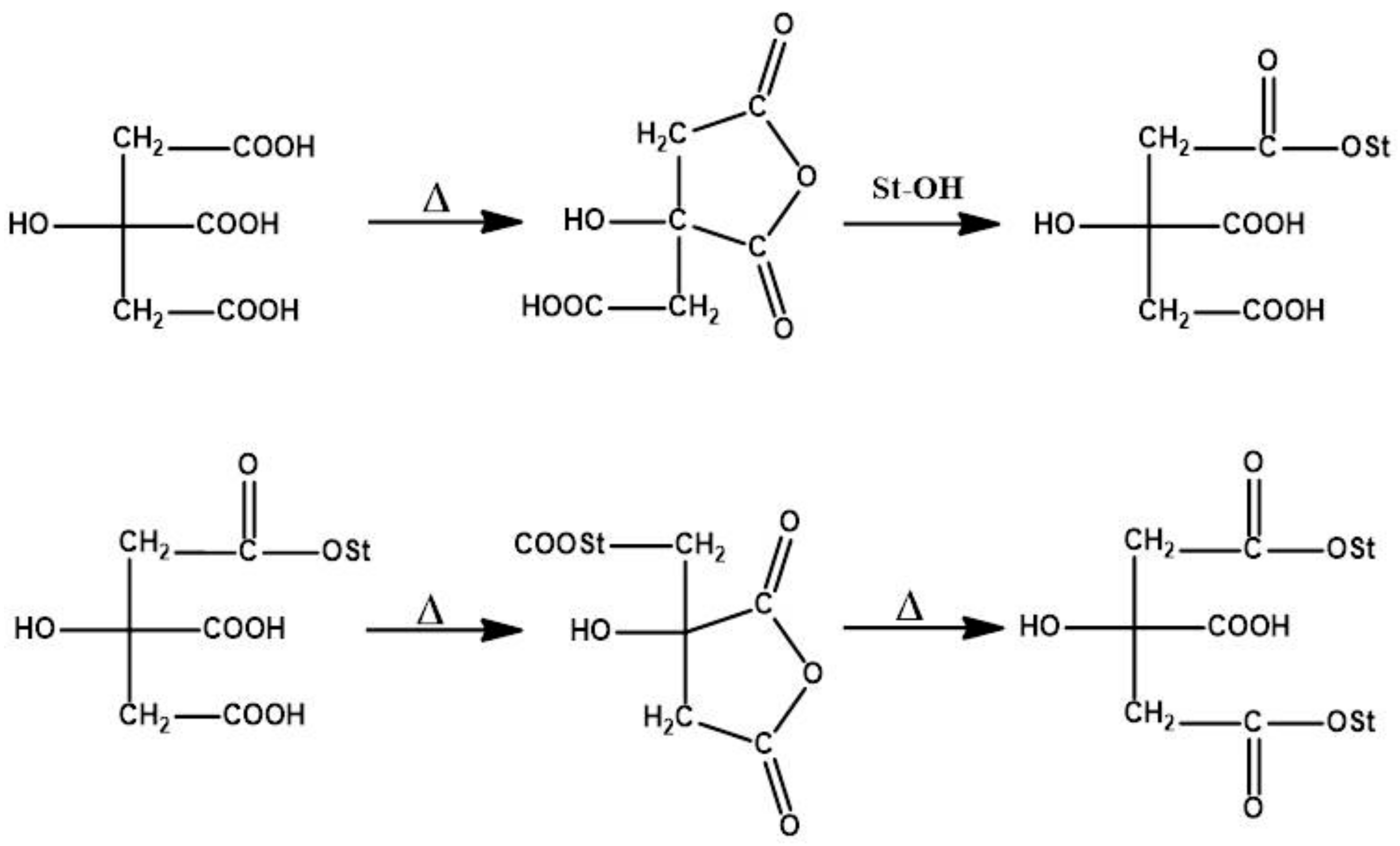
2.4. Preparation of Cross-Linked Starch
2.5. Preparation of Starch-Based Polymer Gel Electrolytes
2.6. Cell Fabrication
2.7. Characterization Techniques
3. Results
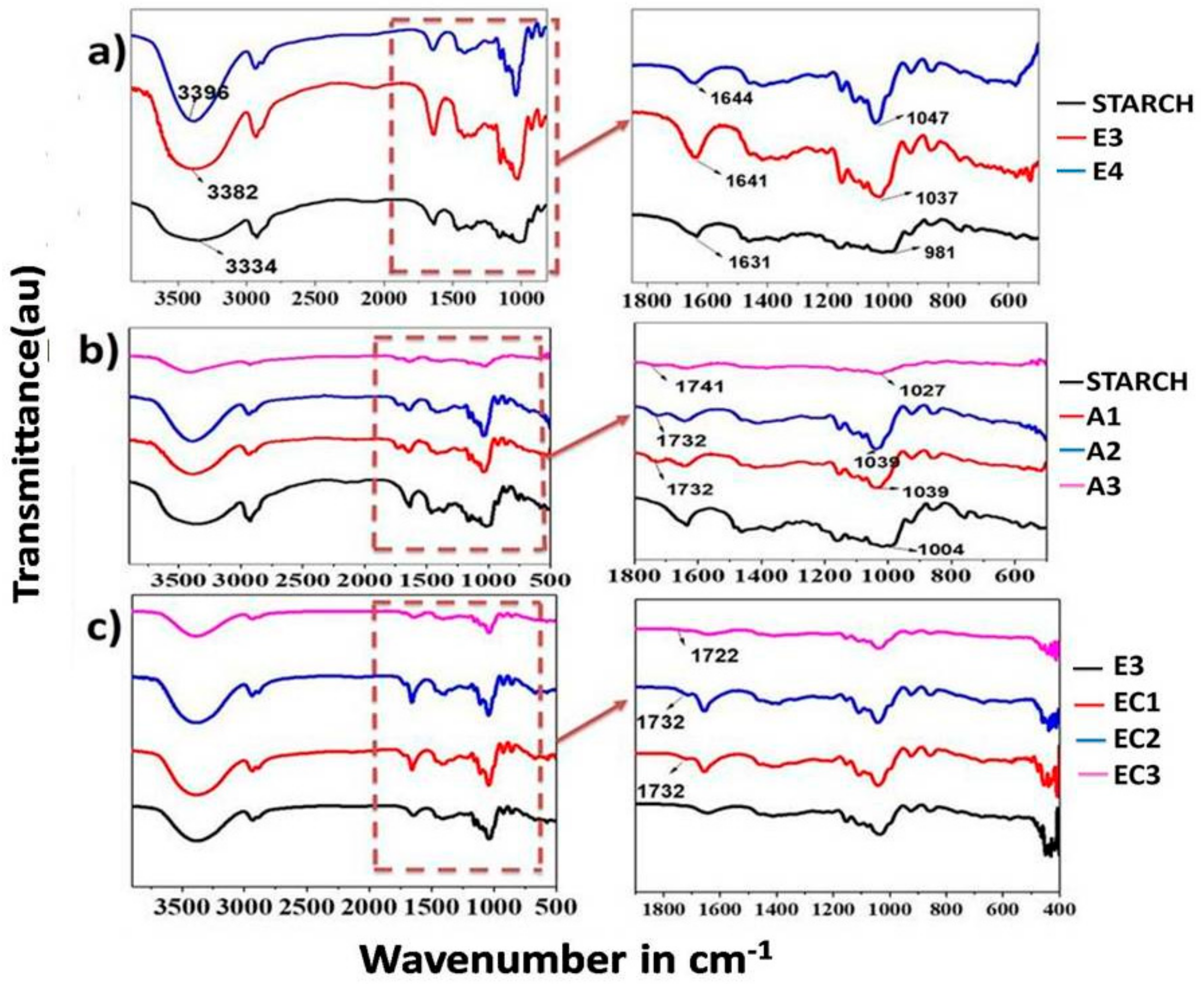
3.1. Fourier-Transform Infrared Spectroscopy
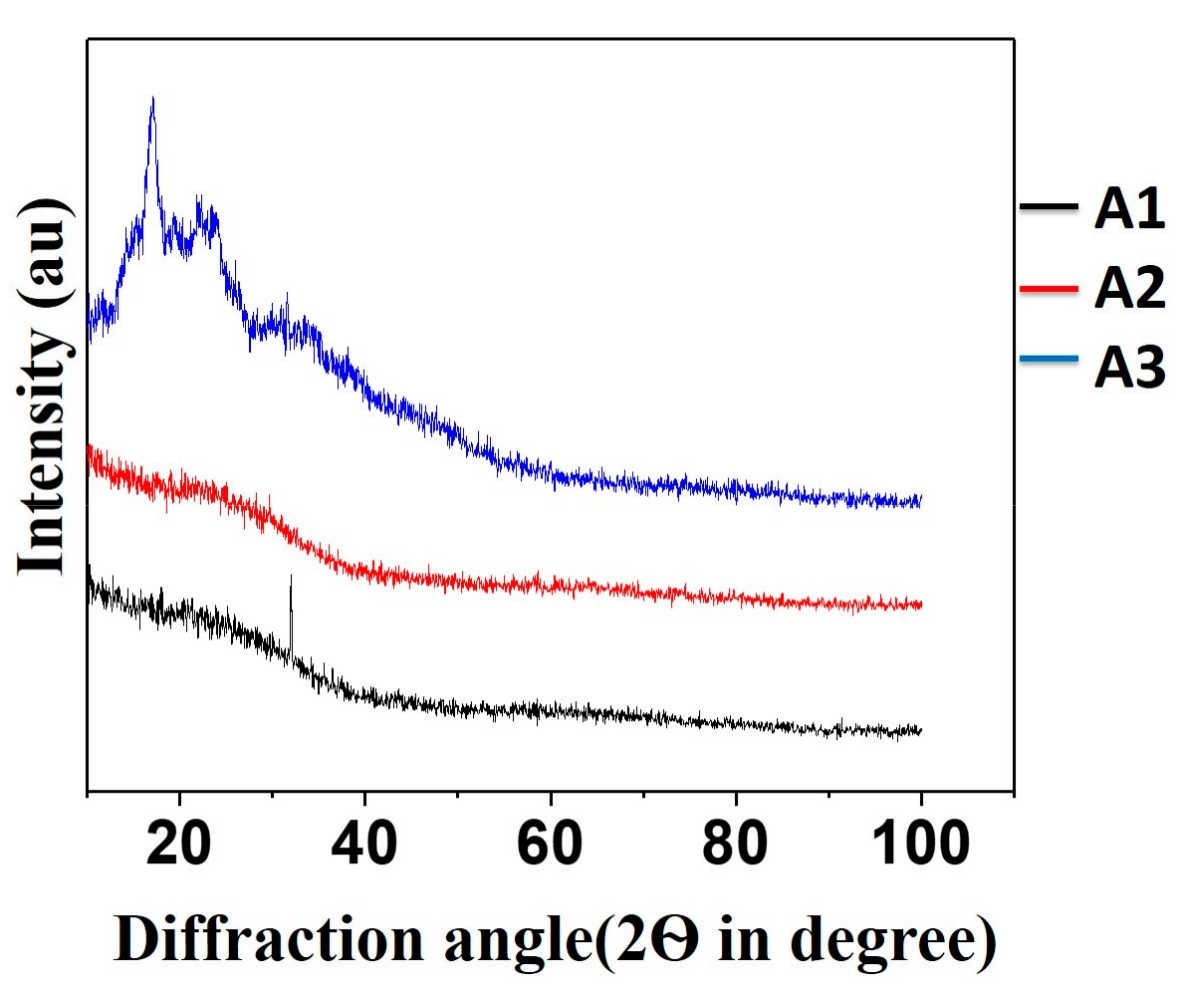
3.2. X-ray Diffraction
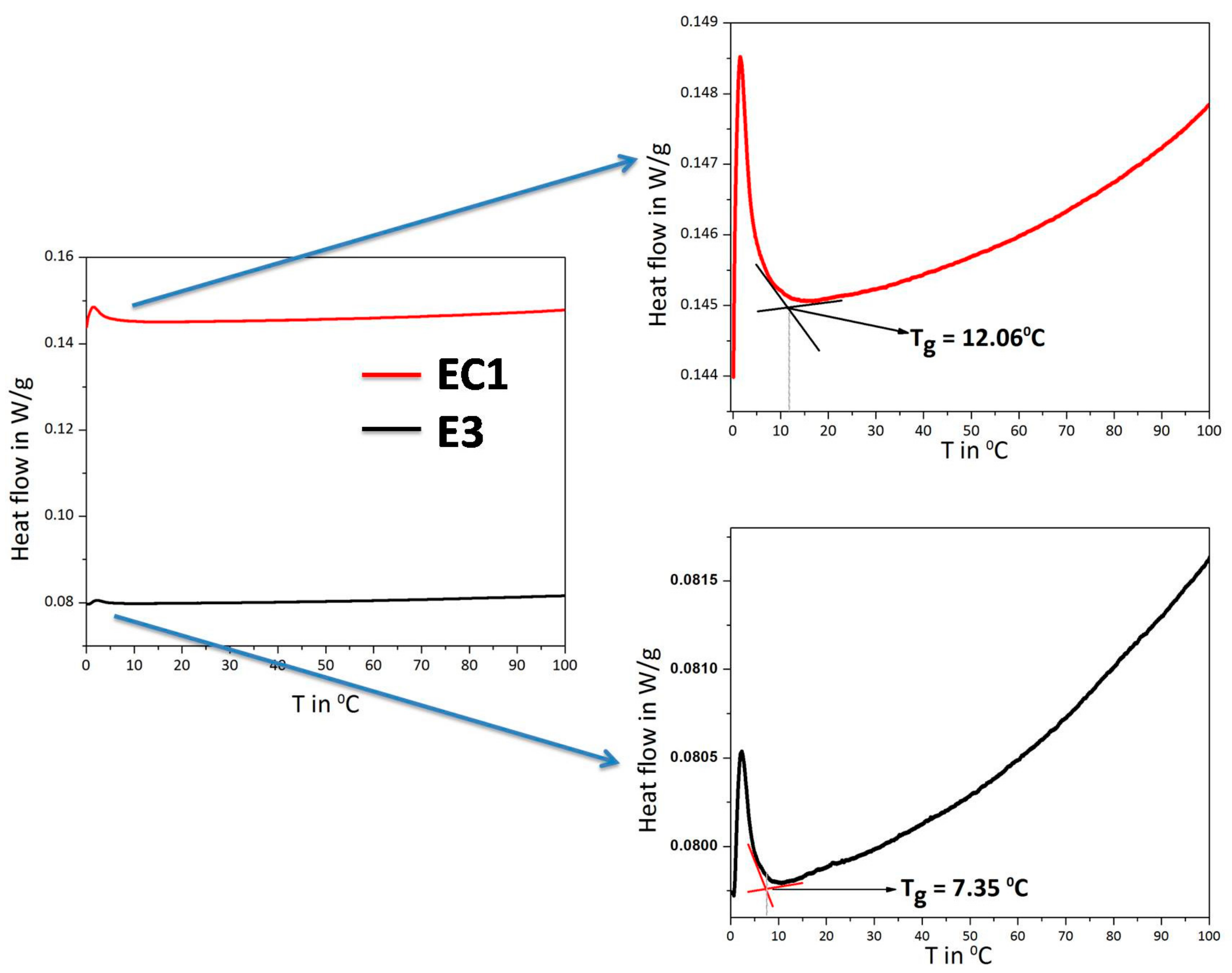
3.3. Differential Scanning Calorimetry (DSC)
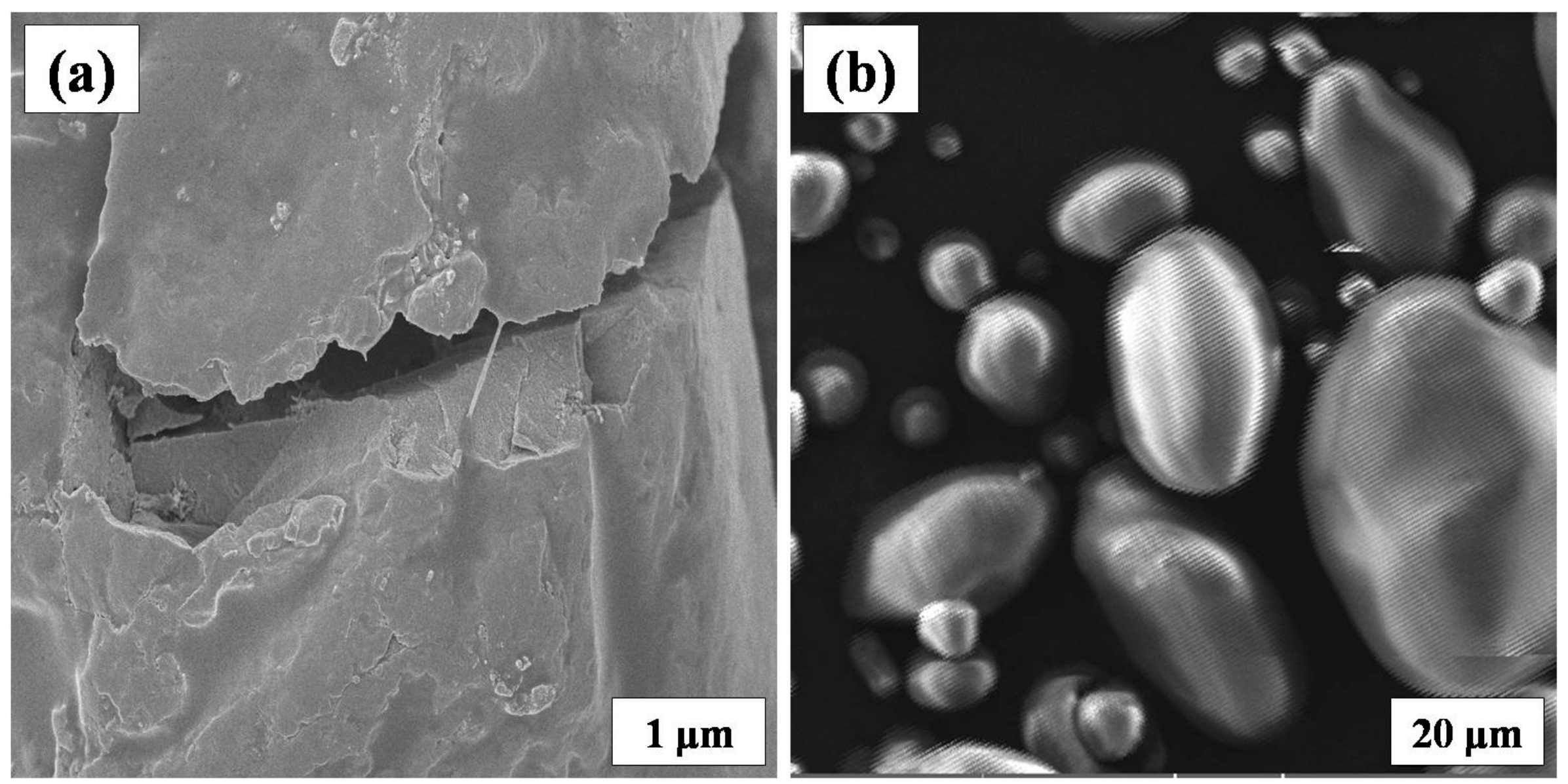
3.4. Scanning Electron Microscopy
3.5. Conductivity Measurements of Prepared Gel Polymer Electrolytes
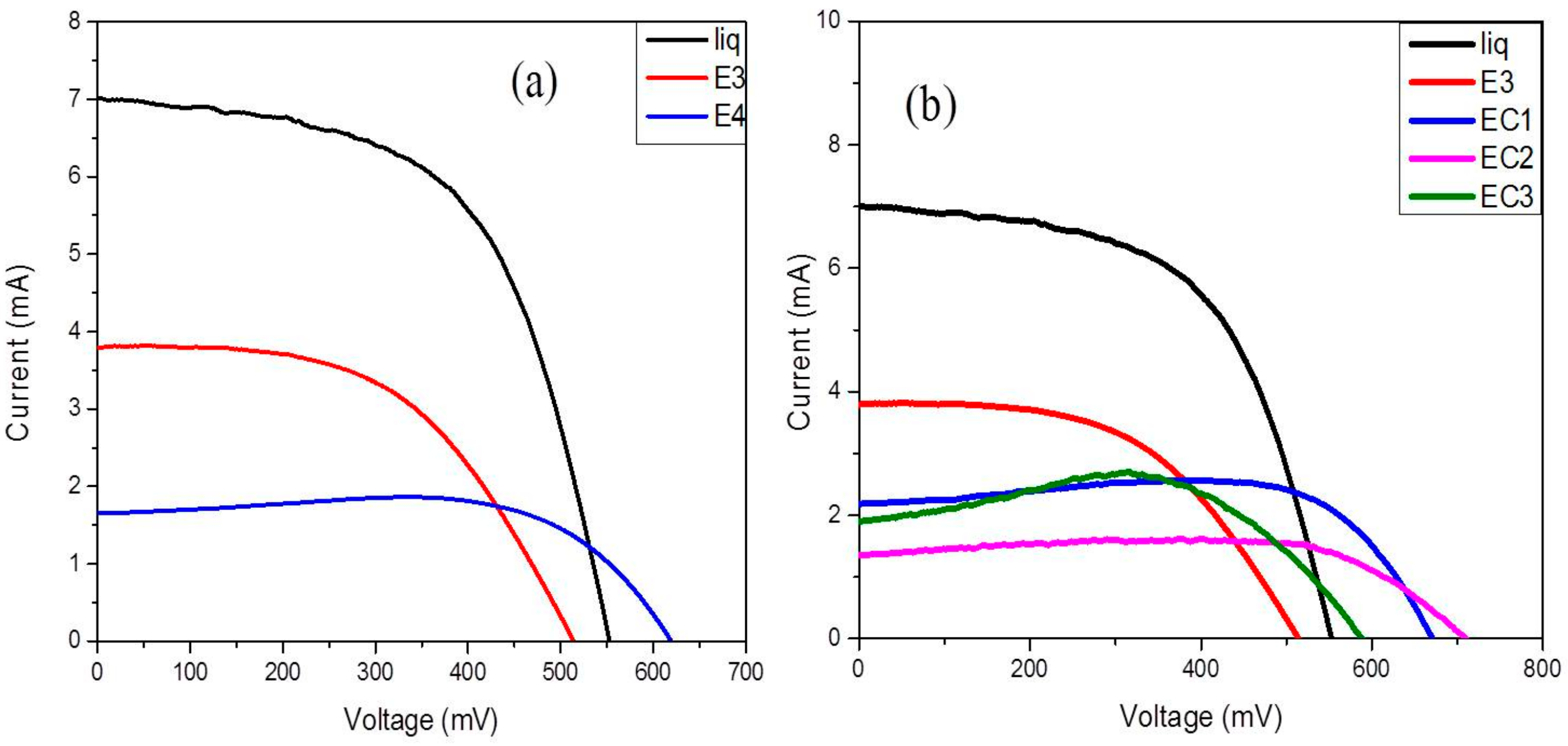
3.6. Photovoltaic Measurements
3.7. Electrochemical Impedance Spectroscopy
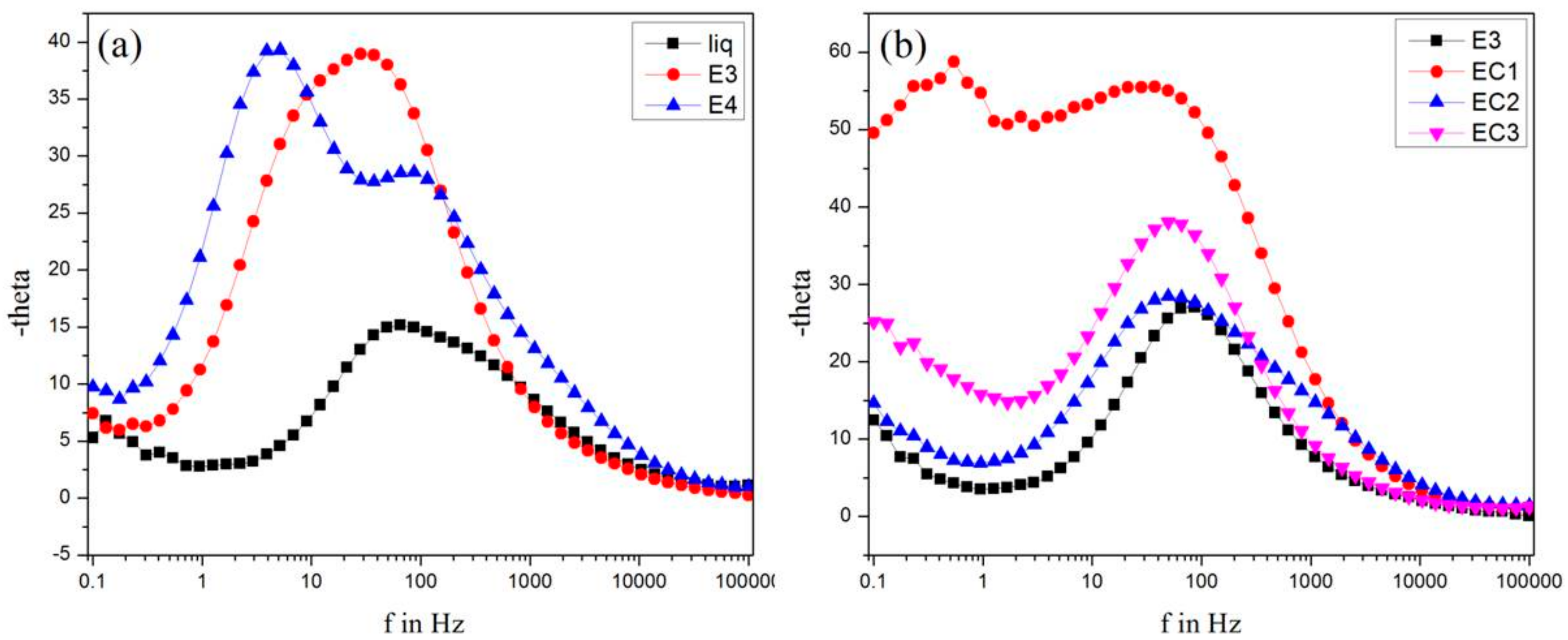
3.8. Electron Lifetime Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoekstra, A.Y.; Wiedmann, T.O. Humanity’s unsustainable environmental footprint. Science 2014, 344, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Hepbasli, A. A key review on exergetic analysis and assessment of renewable energy resources for a sustainable future. Renew. Sustain. Energy Rev. 2008, 12, 593–661. [Google Scholar] [CrossRef]
- GrȨtzel, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Mehmood, U.; Rahman, S.; Harrabi, K.; Hussein, I.A.; Reddy, B.V.S. Recent Advances in Dye Sensitized Solar Cells. Adv. Mater. Sci. Eng. 2014, 2014, 974782. [Google Scholar] [CrossRef]
- Song, S.A. Improved Light Conversion Efficiency of Dye-Sensitized Solar Cell by Dispersing Submicron-Sized Granules into the Nano-Sized TiO2 Layer. Arch. Metall. Mater. 2015, 60, 1467–1471. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Fa, W.; Zhang, Y.; Huang, B. Synthesis of a grafted cellulose gel electrolyte in an ionic liquid ([Bmim]I) for dye-sensitized solar cells. Carbohydr. Polym. 2016, 86, 1216–1220. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharya, B.; Nagaraled, R.K.; Kima, K.W.; Rheea, H.W. Synthesis, characterization and application of biopolymer-ionic liquid composite membranes. Synth. Met. 2010, 160, 139–142. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837. [Google Scholar] [CrossRef]
- Pavithra, N.; Asiri, A.M.; Anandan, S. Fabrication of dye sensitized solar cell using gel polymer electrolytes consisting poly(ethylene oxide)-acetamide composite. J. Power Sources 2015, 286, 346–353. [Google Scholar] [CrossRef]
- Wu, J.; Hao, S.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Li, P.; Yin, S.; Sato, T. An all solid-state dye-sensitized solar cell-based Poly(N-alkyl-4-vinyl-pyridine iodide) electrolyte with efficiency of 5.64%. J. Am. Chem. Soc. 2008, 130, 11568–11569. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeha, B.; Almasia, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Mendoza, A.J. Tartaric acid cross-linking of starch: Effects of reaction conditions on the maximum tensile strength of cast plastic films. J. Stud. Sci. Technol. 2015, 8, 41–47. [Google Scholar] [CrossRef]
- Isah, S.; Oshodi, A.A.; Atasie, V.N. Physicochemical properties of cross linked acha (digitariaexilis) starch with citric acid. Chem. Int. 2017, 3, 150–157. [Google Scholar]
- Maa, X.; Chang, P.R.; Yu, J.; Stumborg, M. Properties of biodegradable citric acid-modified granular starch/thermoplastic pea starch composites. Carbohydr. Polym. 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Pawlicka, A.; Sabadini, A.C.; Raphael, E.; Dragunski, D.C. Ionic Conductivity Thermogravimetry Measurements of Starch-Based Polymeric Electrolytes. Mol. Cryst. Liq. Cryst. 2008, 485, 804–816. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Fanga, J.M.; Fowlera, P.A.; Tomkinsona, T.; Hillb, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and Characterization of Citric Acid-Modified Starch Nanoparticles/Plasticized-Starch Composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhya, N.R.; Gupta, A.; Sengupta, S.; Sahoo, S.; Mohanty, A.; Misra, M. Preparation and Characterization of Cross-Linked Starch/Poly(vinyl alcohol) Green Films with Low Moisture Absorption. Ind. Eng. Chem. Res. 2010, 49, 2176–2185. [Google Scholar] [CrossRef]
- Li, J.; Berke, T.G.; Glover, D.V. Variation for thermal properties of starch in tropical maize germ plasm. Cereal Chem. 1994, 71, 87–90. [Google Scholar]
- Stutz, H.; Illers, K.H.; Mertes, J. A generalized theory for the glass transition temperature of crosslinked and uncrosslinked polymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1483–1498. [Google Scholar] [CrossRef]
- Eich, M.; Reck, B.; Yoon, D.Y.; Willson, C.G.; Bjorklund, G.C. Novel second-order nonlinear optical polymers via chemical crosslinking induced vitrification under electric field. J. Appl. Phys. 1989, 66, 3241. [Google Scholar] [CrossRef]
- Aram, E.; Ehsani, M.; Khonakdar, H.A. Improvement of ionic conductivity and performance of quasi-solid-state dye sensitized solar cell using PEO/PMMA gel electrolyte. Thermochim. Acta 2015, 65, 61–67. [Google Scholar] [CrossRef]
- Wang, H.; Bell, J.; Desilvestro, J.; Bertoz, M.; Evans, G. Effect of Inorganic Iodides on Performance of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 15125–15131. [Google Scholar] [CrossRef]
- Pavithra, N.; Velayutham, D.; Sorrentino, A.; Anandan, S. Poly(ethylene oxide) polymer matrix coupled with urea as gel electrolyte for dye sensitized solar cell applications. Synth. Met. 2017, 226, 62–70. [Google Scholar] [CrossRef]
- Koops, S.E.; O’Regan, B.C.; Barnes, P.R.F.; Durrant, J.R. Parameters Influencing the Efficiency of Electron Injection in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, M.G. The Characterization of Nanocrystalline Dye-Sensitized Solar Cells with Flexible Metal Substrates by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2006, 154, B68–B71. [Google Scholar] [CrossRef]
- Angaridis, P.A.; Ferentinos, E.; Charalambidis, G.; Ladomenou, K.; Nikolaou, V.; Biswas, S.; Sharma, G.D.; Coutsolelo, A.G. Pyridyl vs. bipyridyl anchoring groups of porphyrin sensitizers for dye sensitized solar cells. RSC Adv. 2016, 6, 22187–22203. [Google Scholar] [CrossRef]


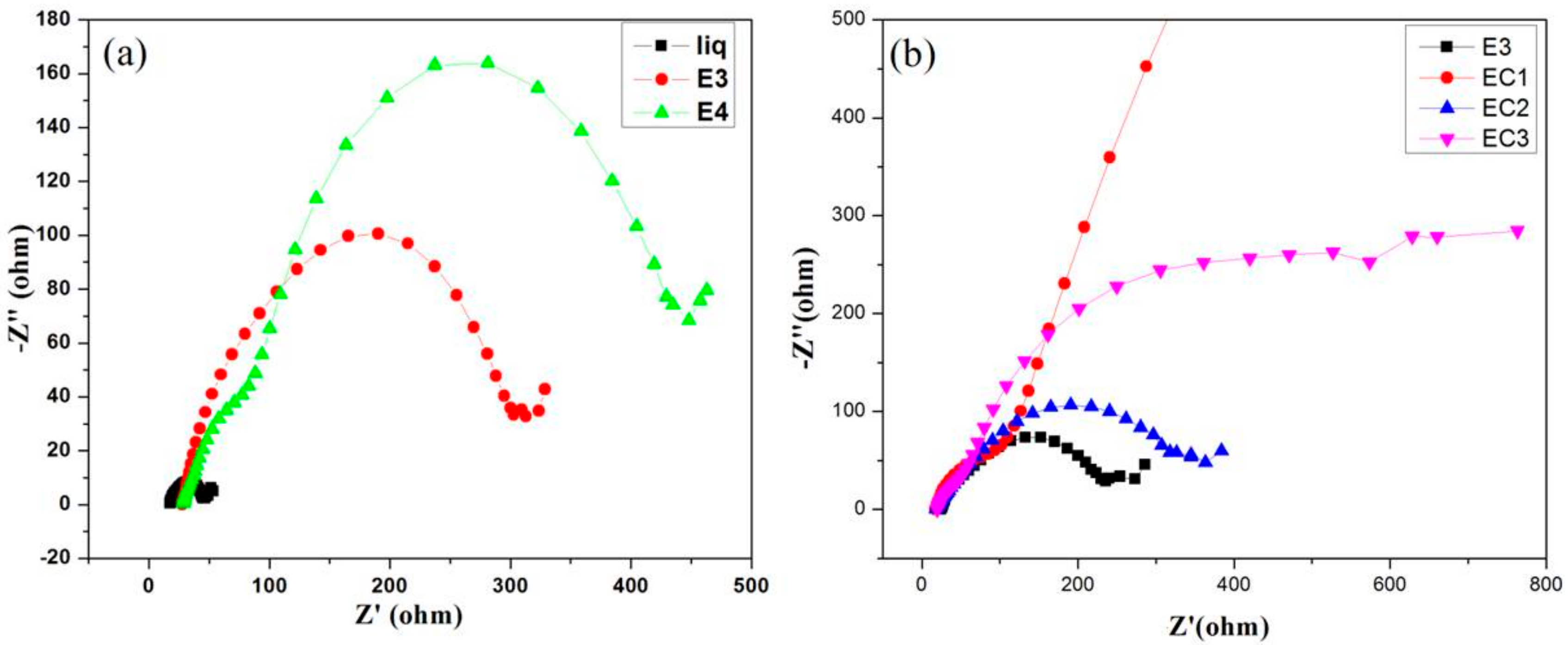
| Electrolyte | Rb (ohm) | Conductivity (S cm−1) |
|---|---|---|
| Liquid | 29.74 | 1.72 |
| E3 | 30.01 | 1.61 |
| E4 | 31.53 | 1.58 |
| EC1 | 84.69 | 0.59 |
| EC2 | 129.15 | 0.38 |
| EC3 | 164.1 | 0.30 |
| Electrolyte | VOC | JSC | FF (%) | η (%) | RCT (ohm) | τ (s) |
|---|---|---|---|---|---|---|
| Liquid | 552.41 | 7.06 | 57 | 2.6 | 33.82 | 2.2 × 10−3 |
| E3 | 514.77 | 3.98 | 50 | 1.2 | 184.12 | 3.2 × 10−3 |
| E4 | 624.72 | 1.55 | 78 | 0.89 | 277.97 | 3.5 × 10−3 |
| EC1 | 672.02 | 2.17 | 82 | 1.4 | 308.62 | 3.72 × 10−3 |
| EC2 | 708.46 | 1.33 | 84 | 0.93 | 193.80 | 3.52 × 10−3 |
| EC3 | 586.53 | 1.89 | 85 | 1.1 | 302.95 | 3.48 × 10−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, P.; Sasidharan, A.; David, V.; Sambandam, A. Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications. Polymers 2017, 9, 667. https://doi.org/10.3390/polym9120667
Nagaraj P, Sasidharan A, David V, Sambandam A. Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications. Polymers. 2017; 9(12):667. https://doi.org/10.3390/polym9120667
Chicago/Turabian StyleNagaraj, Pavithra, Asija Sasidharan, Velayutham David, and Anandan Sambandam. 2017. "Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications" Polymers 9, no. 12: 667. https://doi.org/10.3390/polym9120667
APA StyleNagaraj, P., Sasidharan, A., David, V., & Sambandam, A. (2017). Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications. Polymers, 9(12), 667. https://doi.org/10.3390/polym9120667