Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymers Descriptions
2.2. Polymer Solution Preparation
2.3. Viscosity Measurement
3. Results and Discussion
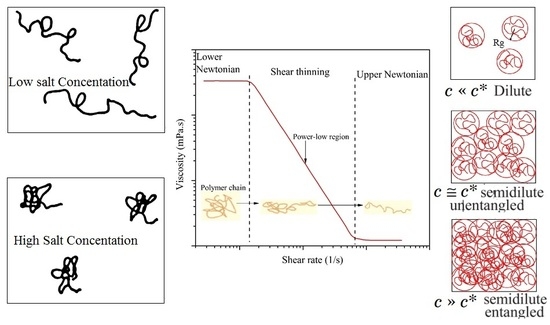
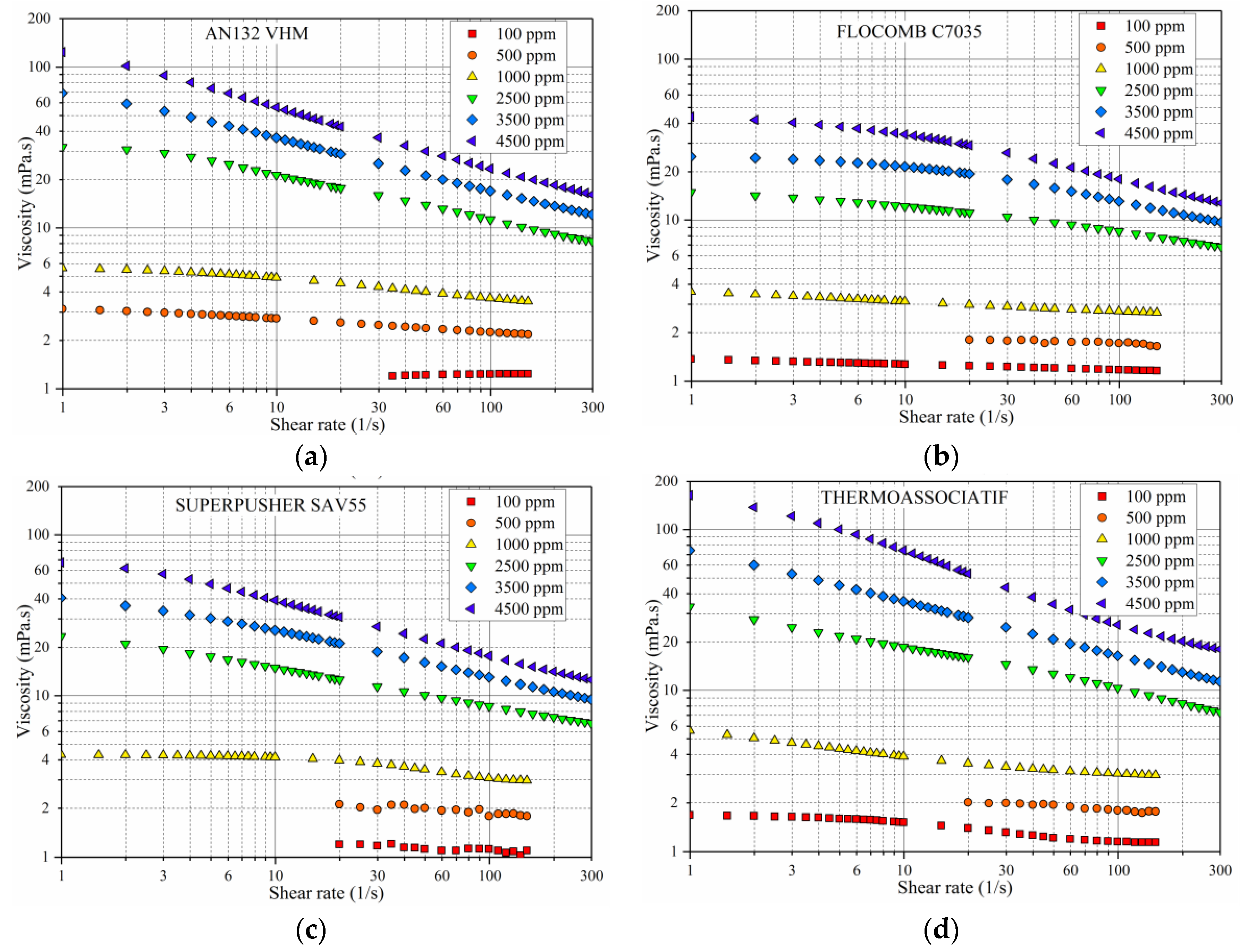
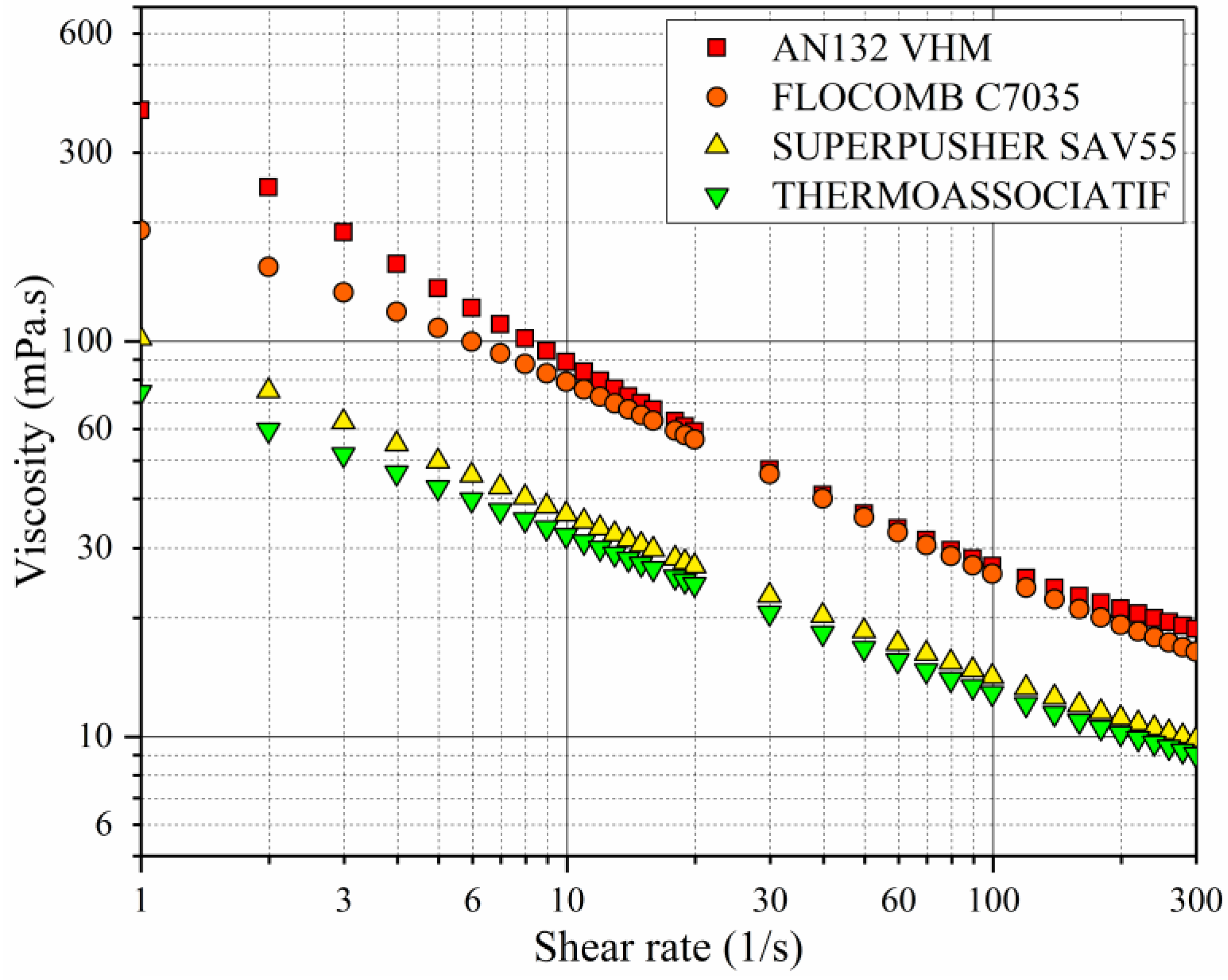
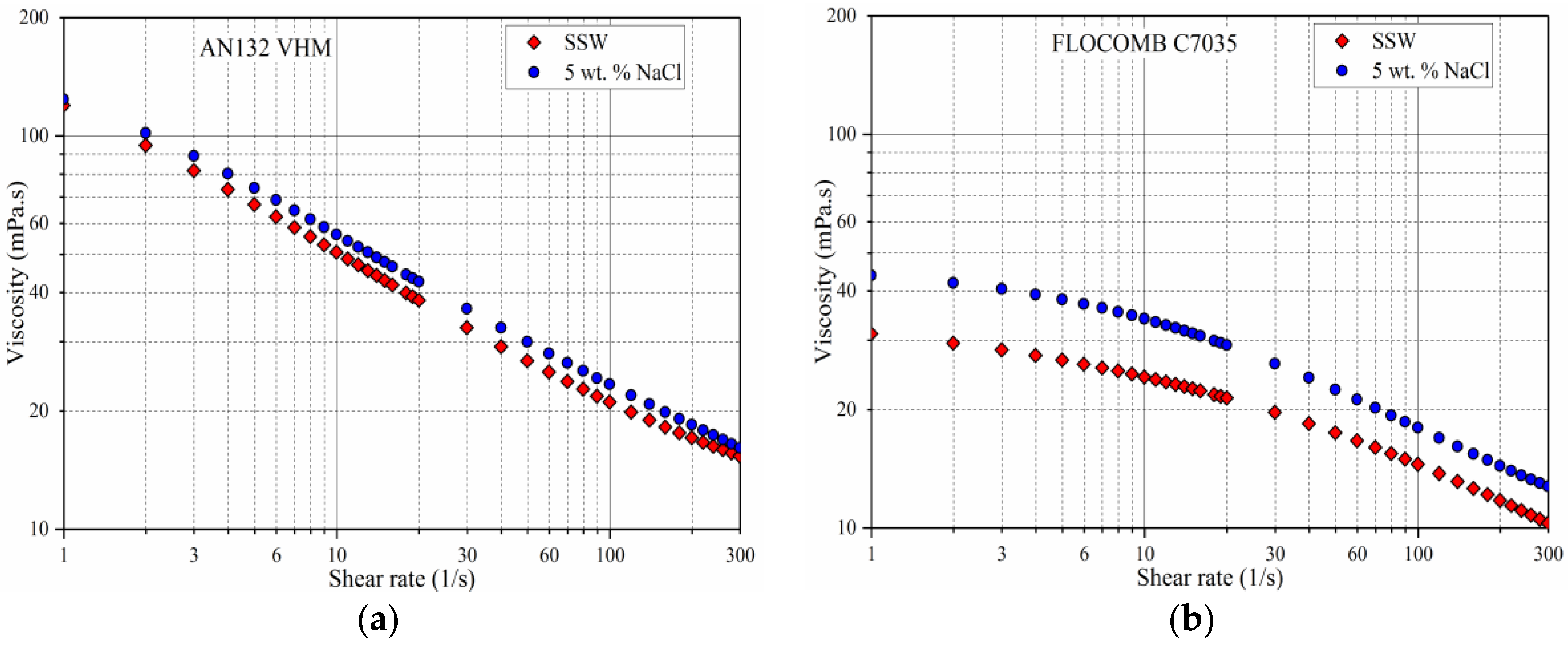
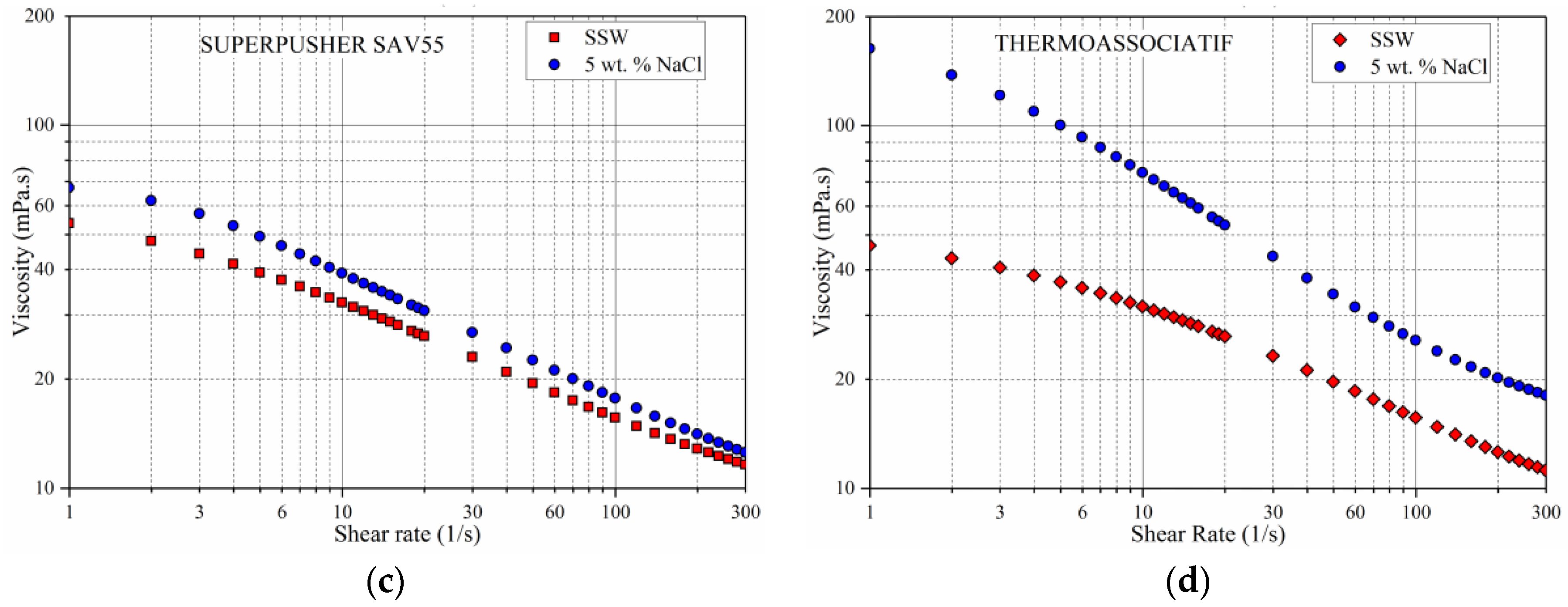
3.1. Shear Rate Dependency of Polymer Viscosity
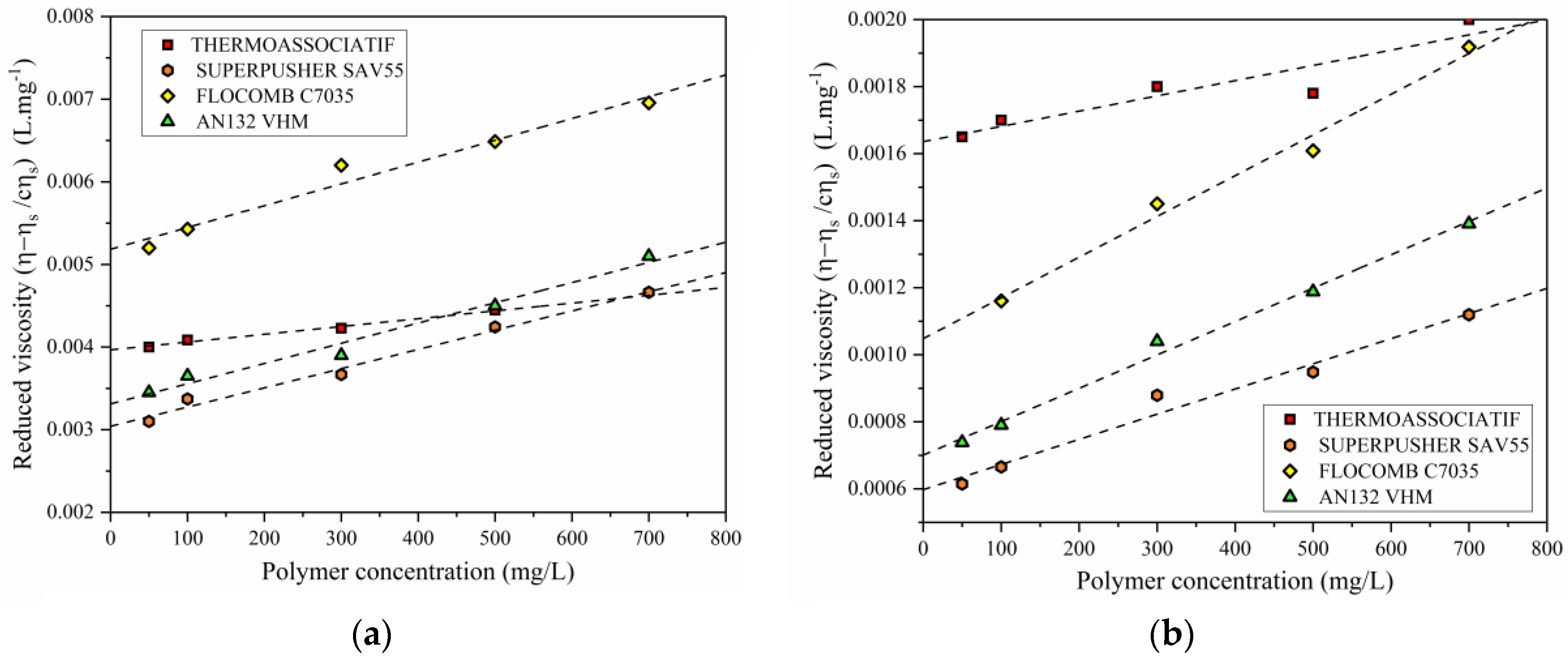
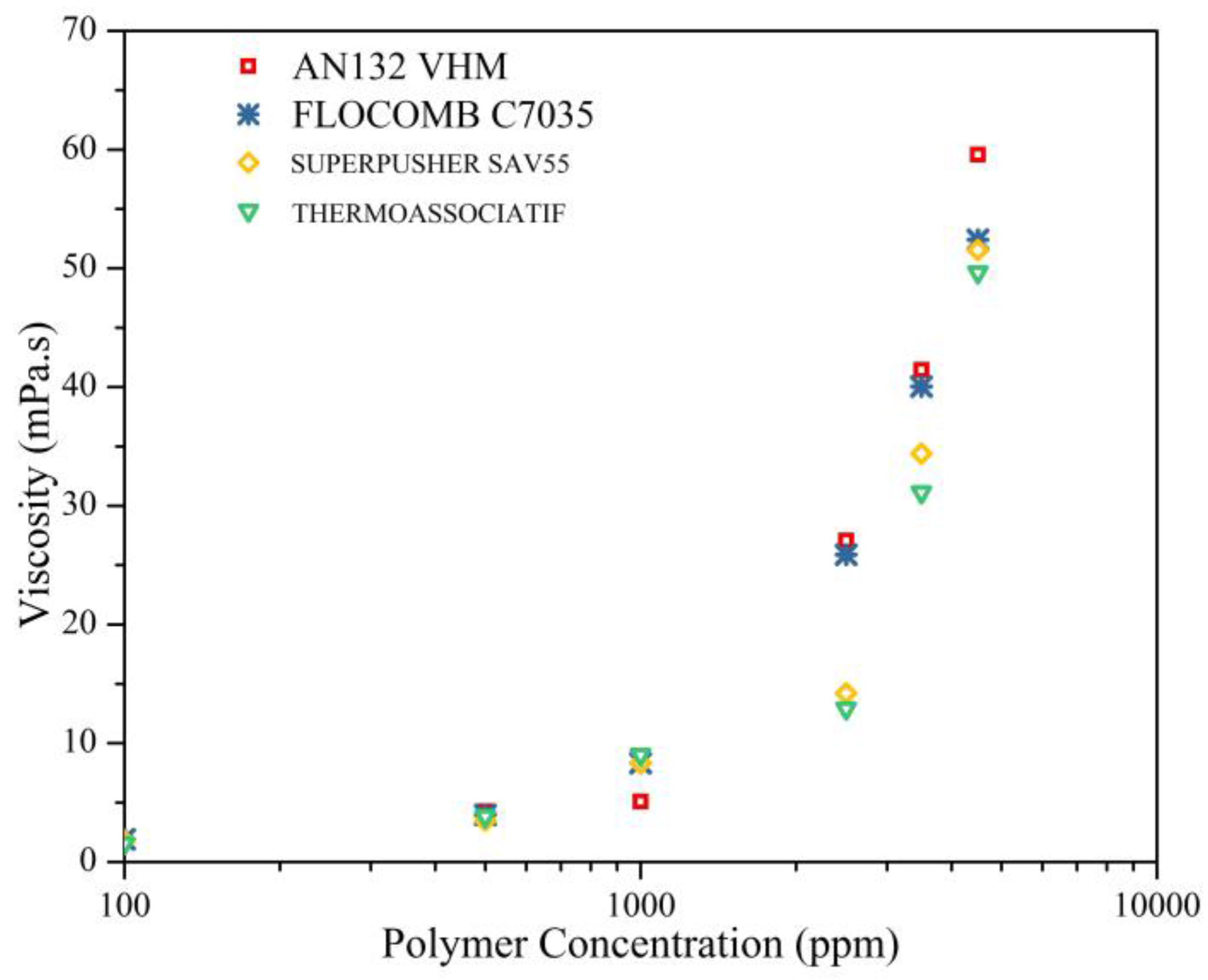
3.2. The Effect of Polymer Concentration on the Polymer Viscosity
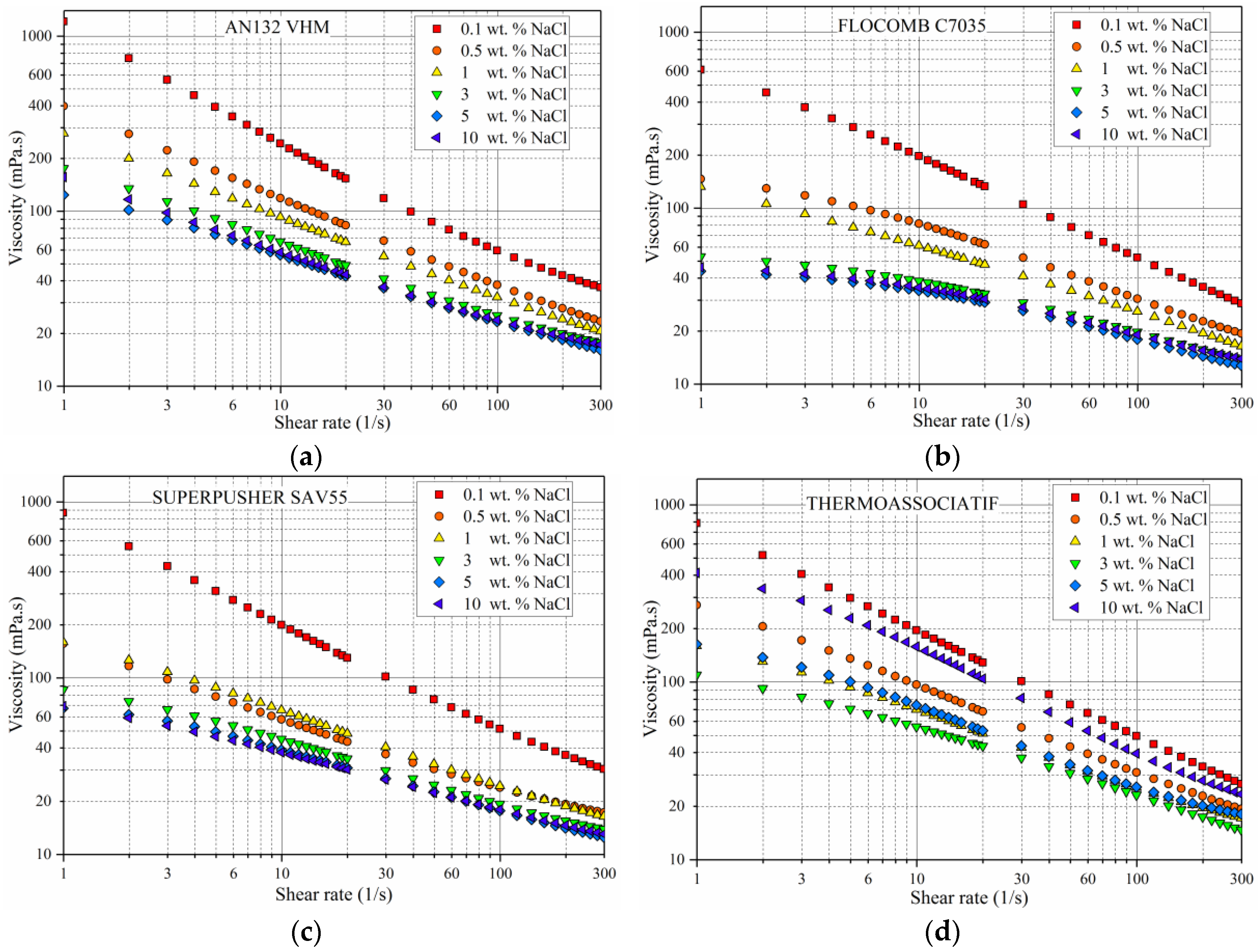
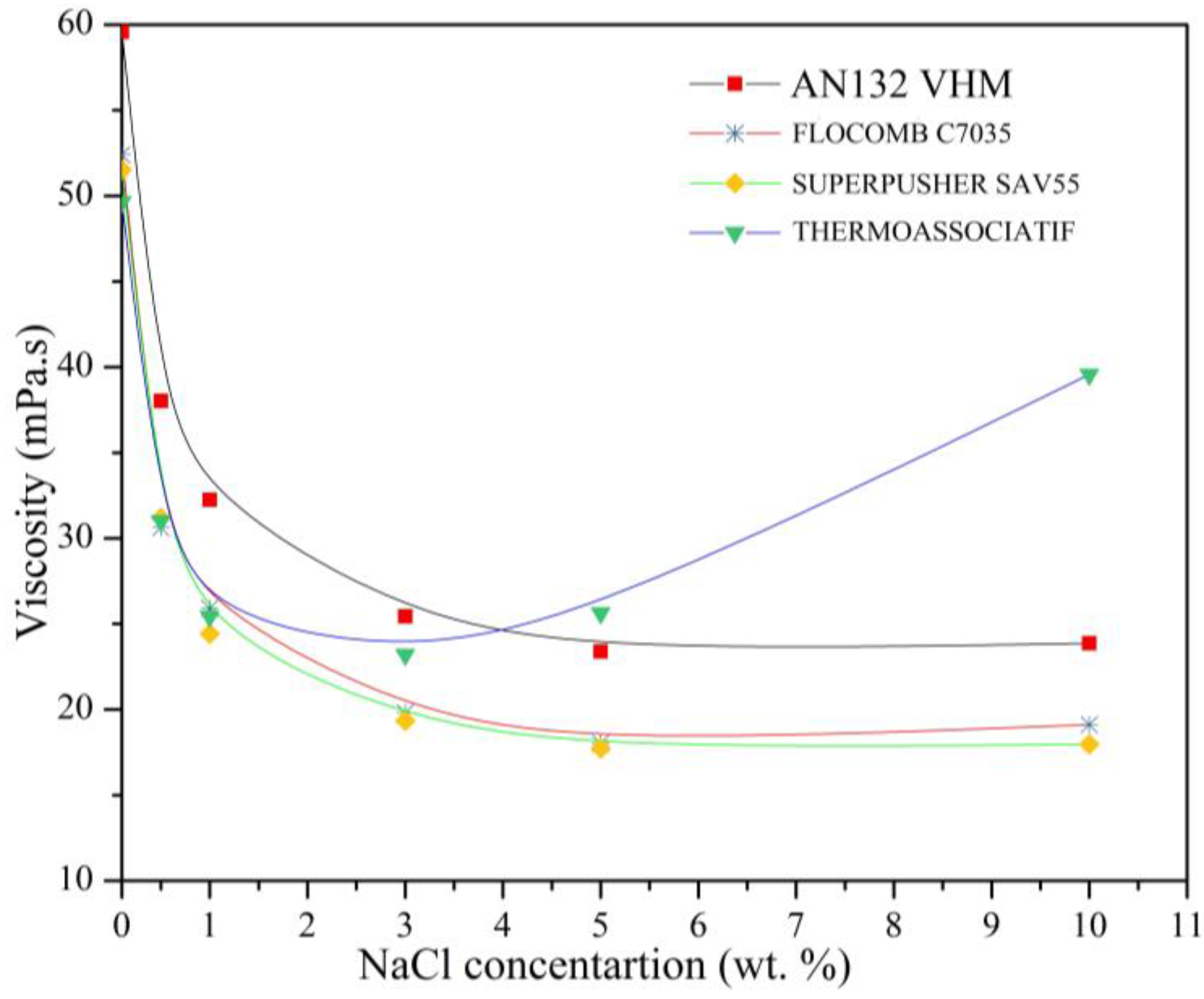
3.3. Effect of NaCl Concentration on the Polymer Viscosity
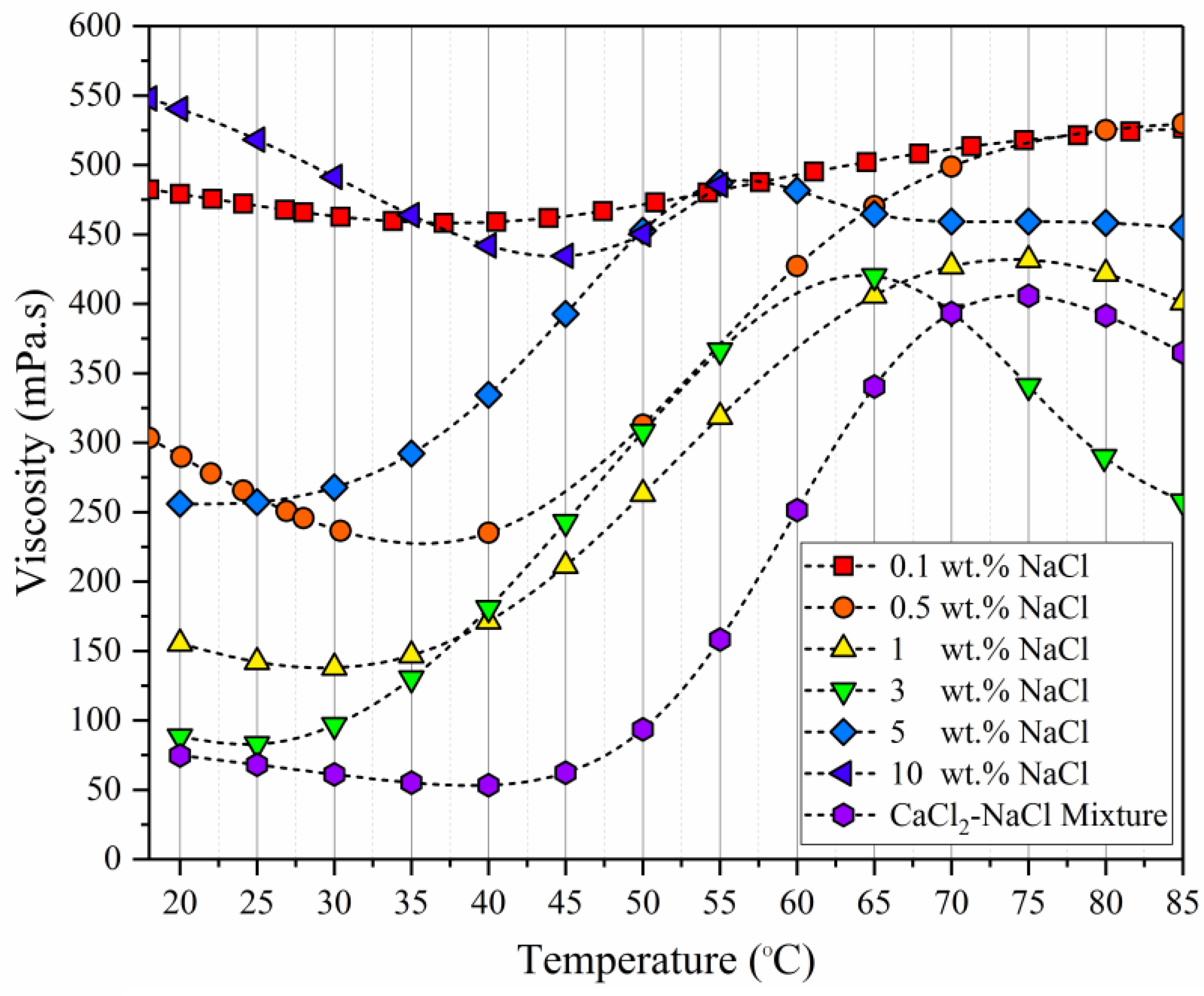
3.4. Effect off Divalent Ions Concentrations on the Polymer Viscosity
4. Conclusions
- All polymers experienced viscosity loss due to the addition of both mono- and divalent ions, although the THERMOASSOCIATIF polymer showed a uniquely complex salt-responsive behavior in salt concentrations above 3 wt %, whereas it showed thermoassociative characteristics at all of the salinities.
- Out of the four polymers tested, THERMOASSOCIATIF was the most resistant to monovalent ions hence a likely candidate for polymer flooding of high salinity reservoirs.
- Replacing a monovalent ion with a divalent ion while keeping the ionic strength constant did not influence the rheological behavior of THERMOASSOCIATIF polymer, except for significantly lowering its viscosity, thus caution must be exercised when considering it for EOR applications to reservoirs containing brines with divalent ions. Other three polymers showed a higher chemical stability in the presence of divalent ions as their viscosity reduction was small.
- All of the polymers showed shear thinning behavior (pseudoplasticity) at high polymer concentrations. The lower the polymer concentration, the lower was the pseudoplasticity that was ultimately approaching the Newtonian behavior at very low concentrations.
- All of the polymers showed an increase in C* (critical overlap concentration), with an increase in salinity from 0.1 to 5 wt % of NaCl. By the same token, the C* lowered with an increase in molecular weight and/or anionicity.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.; Dong, M.; Zhao, S. Which one is more important in chemical flooding for enhanced court heavy oil recovery, lowering interfacial tension or reducing water mobility? Energy Fuels 2010, 24, 1829–1836. [Google Scholar] [CrossRef]
- Hashmet, M.R.; Onur, M.; Tan, I.M. Empirical correlations for viscosity of polyacrylamide solutions with the effects of temperature and shear rate. II. J. Dispers. Sci. Technol. 2013, 35, 1685–1690. [Google Scholar] [CrossRef]
- Hashmet, M.R.; Onur, M.; Tan, I.M. Empirical correlations for viscosity of polyacrylamide solutions with the effects of salinity and hardness. J. Dispers. Sci. Technol. 2013, 35, 510–517. [Google Scholar] [CrossRef]
- Akbari, S.; Mahmood, S.; Tan, I.; Ling, O.; Ghaedi, H. Effect of aging, antioxidant, and mono- and divalent ions at high temperature on the rheology of new polyacrylamide-based co-polymers. Polymers 2017, 9, 480. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery, 1st ed.; Blackie & Son: Glasgow, UK, 1991. [Google Scholar]
- Sheng, J. Modern Chemical Enhance Oil Recovery: Theory and Practice; Gulf Professional: London, UK; Oxford, UK, 2011; pp. 101–102. [Google Scholar]
- Moradi-Araghi, A.; Doe, P.H. Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures. Soc. Pet. Eng. J. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Seright, R.S.; Campbell, A.; Mozley, P.; Han, P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. Soc. Pet. Eng. J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Choi, B.; Jeong, M.S.; Lee, K.S. Temperature-dependent viscosity model of hpam polymer through high-temperature reservoirs. Polym. Degrad. Stab. 2014, 110, 225–231. [Google Scholar] [CrossRef]
- Hu, Z.; Haruna, M.; Gao, H.; Nourafkan, E.; Wen, D. Rheological properties of partially hydrolyzed polyacrylamide seeded by nanoparticles. Ind. Eng. Chem. Res. 2017, 56, 3456–3463. [Google Scholar] [CrossRef]
- Ma, Q.; Shuler, P.J.; Aften, C.W.; Tang, Y. Theoretical studies of hydrolysis and stability of polyacrylamide polymers. Polym. Degrad. Stab. 2015, 121, 69–77. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, J.; Yan, L.; Zhao, M. Adsorption behavior of cocamidopropyl betaine under conditions of high temperature and high salinity. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Algharaib, M.; Alajmi, A.; Gharbi, R. Improving polymer flood performance in high salinity reservoirs. J. Pet. Sci. Eng. 2014, 115, 17–23. [Google Scholar] [CrossRef]
- Al-Bahar, M.A.; Merrill, R.; Peake, W.; Jumaa, M.; Oskui, R. Evaluation of IOR potential within kuwait. In Proceedings of the Abu Dhabi International Conference and Exhibition, Abu Dhabi, UAE, 10–13 October 2004. [Google Scholar]
- Algharaib, M.K.; Alajmi, A.F.F.; Gharbi, R. Enhancing recovery in high salinity oil reservoirs through optimized polymer flood. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 15–17 November 2011. [Google Scholar]
- Ibrahim, Z.B.; Manap, A.A.A.; Hamid, P.A.; Hon, V.Y.; Lim, P.H.; Wyatt, K. Laboratory aspect of chemical EOR processes evaluation for Malaysian oilfields. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 11–13 September 2006. [Google Scholar]
- Shell International Petroleum Company Limited. The Petroleum Handbook; Shell International Petroleum Co.: London, UK, 1966. [Google Scholar]
- Wilton, R.R.; Torabi, F. Rheological assessment of the elasticity of polymers for enhanced heavy oil recovery. In Proceedings of the SPE Heavy Oil Conference-Canada, Calgary, AB, Canada, 11–13 June 2013. [Google Scholar]
- Floerger, S. Enhancing Polymer Flooding Performance 30 Years of Experience in EOR. Available online: http://www.snf.us/wp-content/uploads/2014/08/EOR-Oil-30-Years-of-EOR1.pdf (accessed on 28 September 2012).
- Al-Hashmi, A.R.; Divers, T.; Al-Maamari, R.S.; Favero, C.; Thomas, A. Improving polymer flooding efficiency in Oman oil fields. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 21–23 March 2016. [Google Scholar]
- Gaillard, N.; Giovannetti, B.; Leblanc, T.; Thomas, A.; Braun, O.; Favero, C. Selection of customized polymers to enhance oil recovery from high temperature reservoirs. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 18–20 November 2015. [Google Scholar]
- Rashidi, M.; Blokhus, A.M.; Skauge, A. Viscosity study of salt tolerant polymers. J. Appl. Polym. Sci. 2010, 117, 1551–1557. [Google Scholar] [CrossRef]
- Leblanc, T.; Braun, O.; Thomas, A.; Divers, T.; Gaillard, N.; Favero, C. Rheological properties of stimuli-responsive polymers in solution to improve the salinity and temperature performances of polymer-based chemical enhanced oil recovery technologies. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar]
- Li, X.E.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Dai, C.; Xu, Z.; Wu, Y.; Zou, C.; Wu, X.; Wang, T.; Guo, X.; Zhao, M. Design and study of a novel thermal-resistant and shear-stable amphoteric polyacrylamide in high-salinity solution. Polymers 2017, 9, 296. [Google Scholar] [CrossRef]
- Jamshidi, H.; Rabiee, A. Synthesis and characterization of acrylamide-based anionic copolymer and investigation of solution properties. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Travas-Sejdic, J.; Easteal, A. Study of free-radical copolymerization of acrylamide with 2-acrylamido-2-methyl-1-propane sulphonic acid. J. Appl. Polym. Sci. 2000, 75, 619–628. [Google Scholar] [CrossRef]
- Levitt, D.; Pope, G.A. Selection and screening of polymers for enhanced-oil recovery. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 19–23 April 2008. [Google Scholar]
- Yerramilli, S.S.; Zitha, P.L.J.; Yerramilli, R.C. Novel insight into polymer injectivity for polymer flooding. In Proceedings of the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands, 5–7 June 2013. [Google Scholar]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Al-Shammari, B.; Al-Fariss, T.; Al-Sewailm, F.; Elleithy, R. The effect of polymer concentration and temperature on the rheological behavior of metallocene linear low density polyethylene (mlldpe) solutions. J. King Saud Univ. Eng. Sci. 2011, 23, 9–14. [Google Scholar] [CrossRef]
- Ait-Kadi, A.; Carreau, P.J.; Chauveteau, G. Rheological properties of partially hydrolyzed polyacrylamide solutions. J. Rheol. 1987, 31, 537–561. [Google Scholar] [CrossRef]
- Aho, J.; Syrjälä, S. On the measurement and modeling of viscosity of polymers at low temperatures. Polym. Test. 2008, 27, 35–40. [Google Scholar] [CrossRef]
- Vermolen, E.C.M.; Van Haasterecht, M.J.T.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M.A. Pushing the envelope for polymer flooding towards high-temperature and high-salinity reservoirs with polyacrylamide based ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Szabo, M.T. An evaluation of water-soluble polymers for secondary oil recovery—Parts 1 and 2. J. Pet. Technol. 1979, 31, 553–570. [Google Scholar] [CrossRef]
- Akbari, S.; Mahmood, S.M.; Tan, I.M.; Bharadwaj, A.M.; Hematpour, H. Experimental investigation of the effect of different process variables on the viscosity of sulfonated polyacrylamide copolymers. J. Pet. Explor. Prod. Technol. 2017, 7, 87–101. [Google Scholar] [CrossRef]
- Ghannam, M.T. Rheological properties of aqueous polyacrylamide/nacl solutions. J. Appl. Polym. Sci. 1999, 72, 1905–1912. [Google Scholar] [CrossRef]
- Samanta, A.; Bera, A.; Ojha, K.; Mandal, A. Effects of alkali, salts, and surfactant on rheological behavior of partially hydrolyzed polyacrylamide solutions. J. Chem. Eng. Data 2010, 55, 4315–4322. [Google Scholar] [CrossRef]
- Spildo, K.; Sæ, E.I.Ø. Effect of charge distribution on the viscosity and viscoelastic properties of partially hydrolyzed polyacrylamide. Energy Fuels 2015, 29, 5609–5617. [Google Scholar] [CrossRef]
- Cao, P.-F.; Mangadlao, J.D.; Advincula, R.C. Stimuli-responsive polymers and their potential applications in oil-gas industry. Polym. Rev. 2015, 55, 706–733. [Google Scholar] [CrossRef]
- Quan, H.; Li, Z.; Huang, Z. Self-assembly properties of a temperature- and salt-tolerant amphoteric hydrophobically associating polyacrylamide. RSC Adv. 2016, 6, 49281–49288. [Google Scholar] [CrossRef]
- Lu, J.H.; Wu, L.; Letey, J. Effects of soil and water properties on anionic polyacrylamide sorption. Soil Sci. Soc. Am. J. 2002, 66, 578–584. [Google Scholar] [CrossRef]
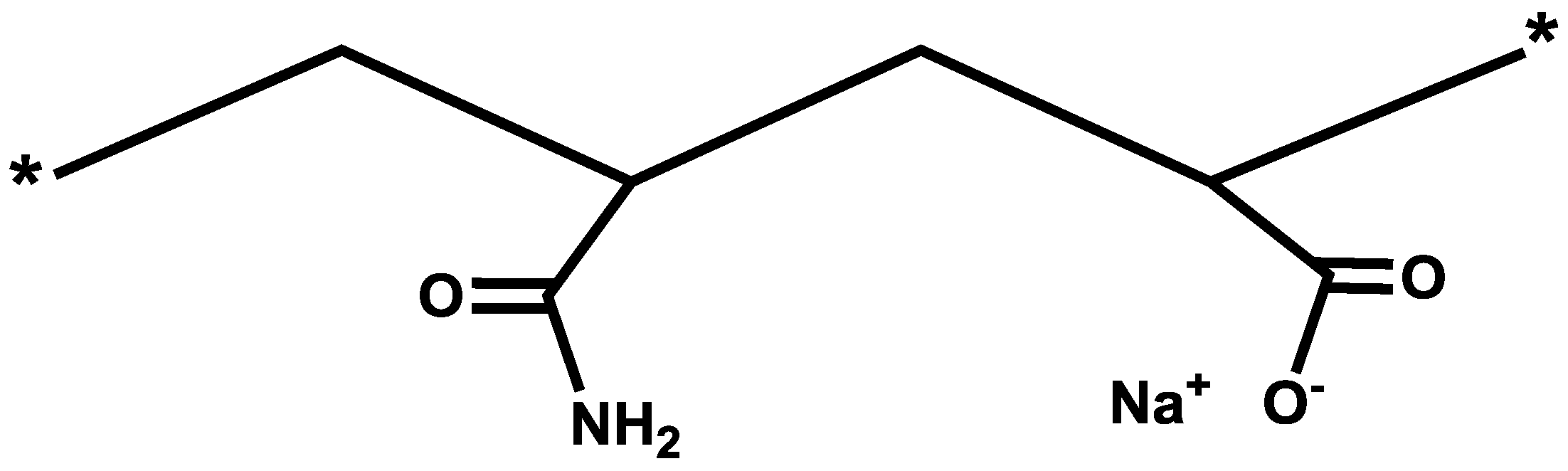
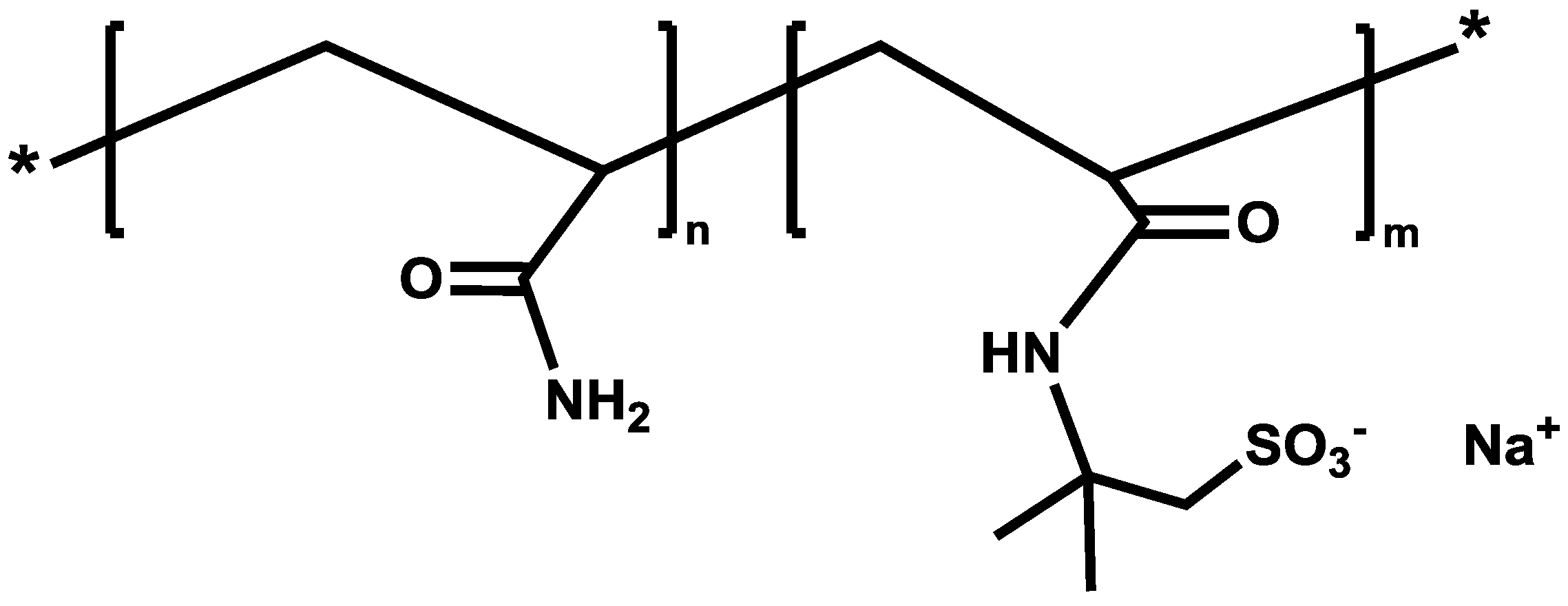
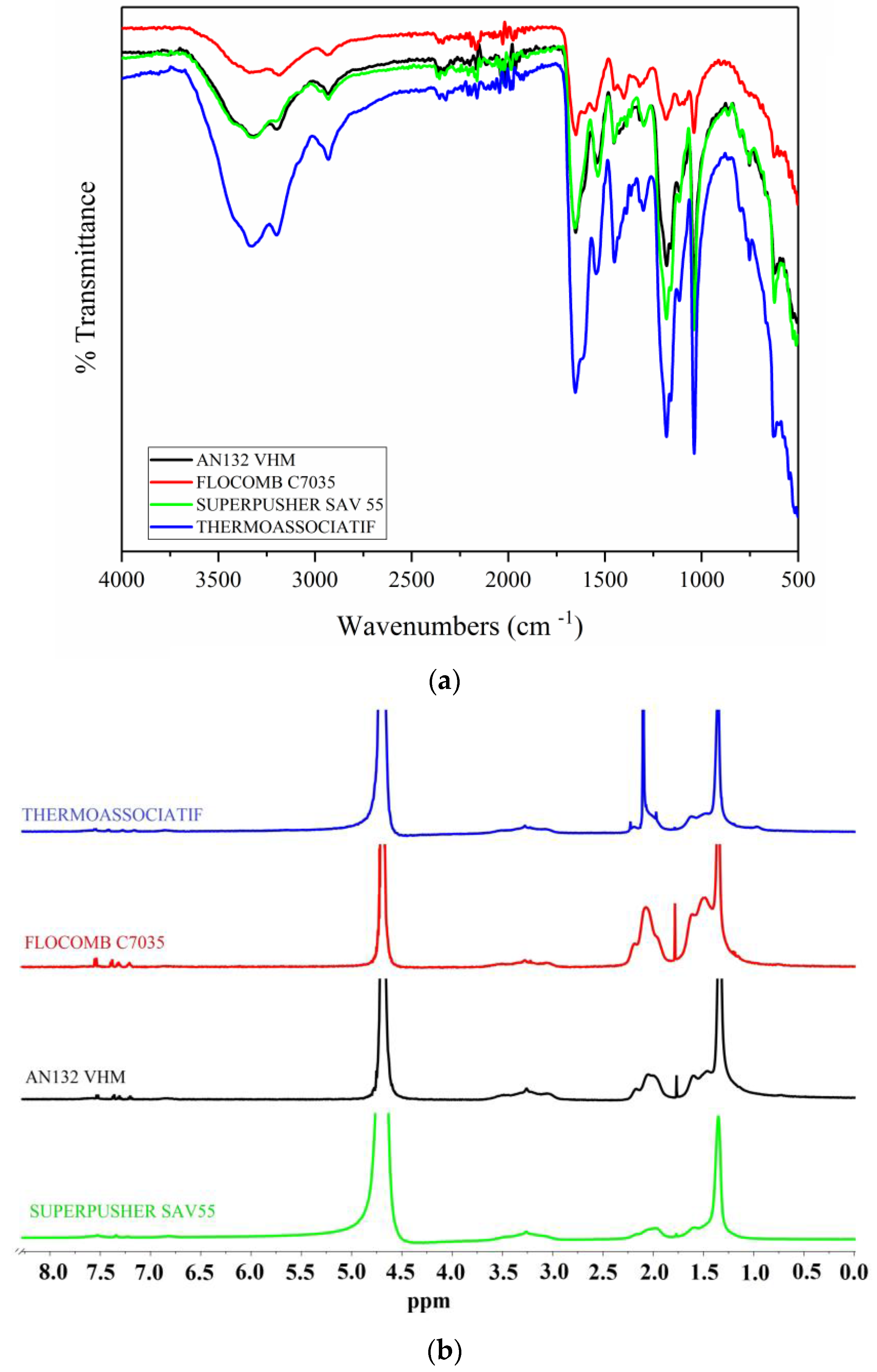

| Polymer product (trade name) | Molecular weight (million daltons) | Sulfonation degree (mol %) | Anionicity |
|---|---|---|---|
| FLOCOMB C7035 | Very High(>18) | low | Medium |
| AN132 VHM | Medium (9–11 1) | Medium (32) | High |
| SUPERPUSHER SAV55 | Low (5–7 1) | high | High |
| THERMOASSOCIATIF | Medium (<12) | medium | Medium |
| Solvent | Amount in g/kg of solvent |
|---|---|
| NaCl 1 | 20 |
| CaCl2 1 | 19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, S.; Mahmood, S.M.; Tan, I.M.; Ghaedi, H.; Ling, O.L. Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness. Polymers 2017, 9, 647. https://doi.org/10.3390/polym9120647
Akbari S, Mahmood SM, Tan IM, Ghaedi H, Ling OL. Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness. Polymers. 2017; 9(12):647. https://doi.org/10.3390/polym9120647
Chicago/Turabian StyleAkbari, Saeed, Syed Mohammad Mahmood, Isa M. Tan, Hosein Ghaedi, and Onn Lin Ling. 2017. "Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness" Polymers 9, no. 12: 647. https://doi.org/10.3390/polym9120647
APA StyleAkbari, S., Mahmood, S. M., Tan, I. M., Ghaedi, H., & Ling, O. L. (2017). Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness. Polymers, 9(12), 647. https://doi.org/10.3390/polym9120647