Interactions of Biocidal Polyhexamethylene Guanidine Hydrochloride and Its Analogs with POPC Model Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
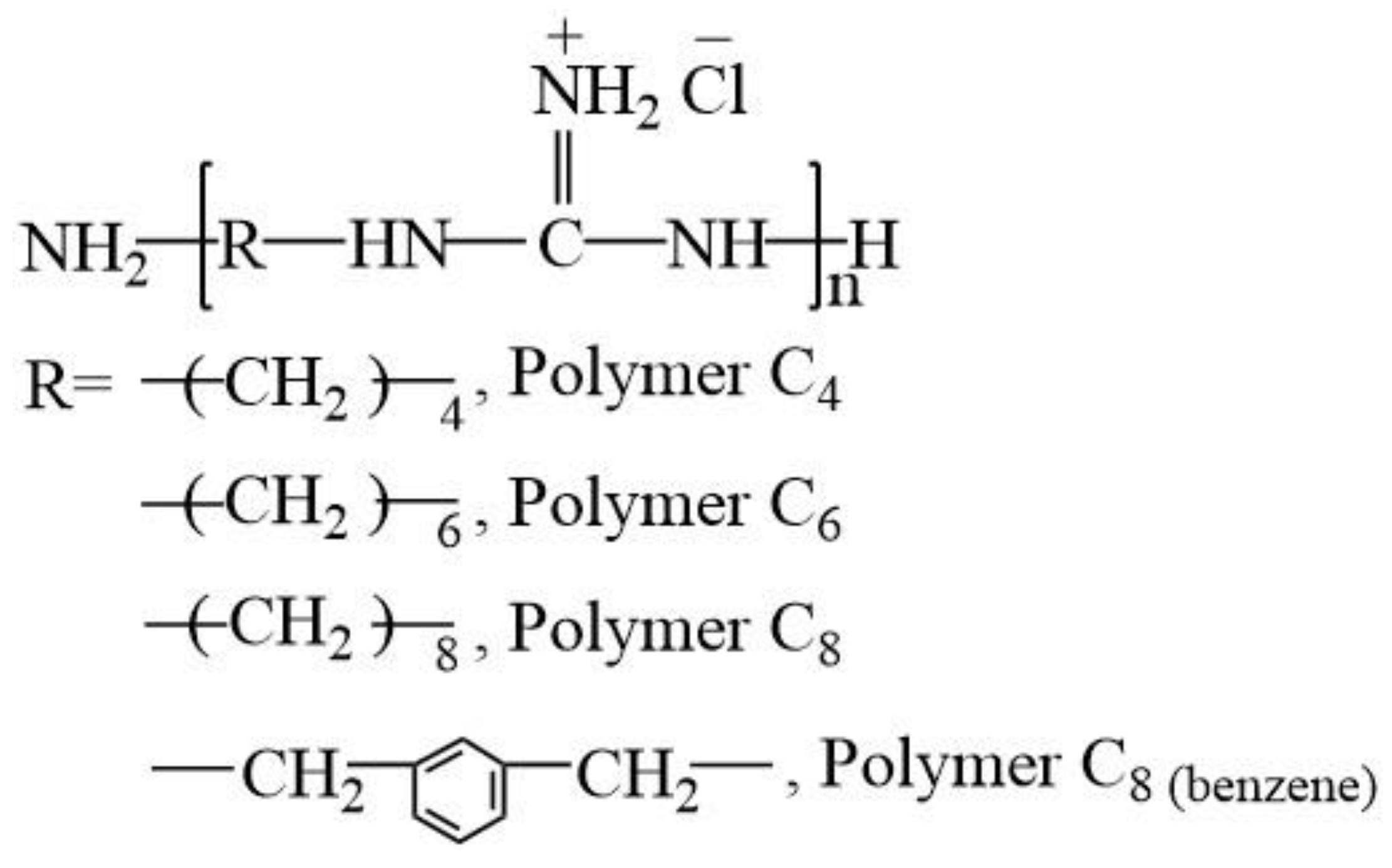
2.2. Oligoguanidine Polymers
2.3. Bacterial Strains
2.4. Bactericidal Dynamics
2.5. Hemolysis Testing
2.6. Polymer-Induced Leakage Assays of Fluorescence Dye
2.7. Isothermal Titration Calorimetry
2.8. Toxicological Studies
3. Results
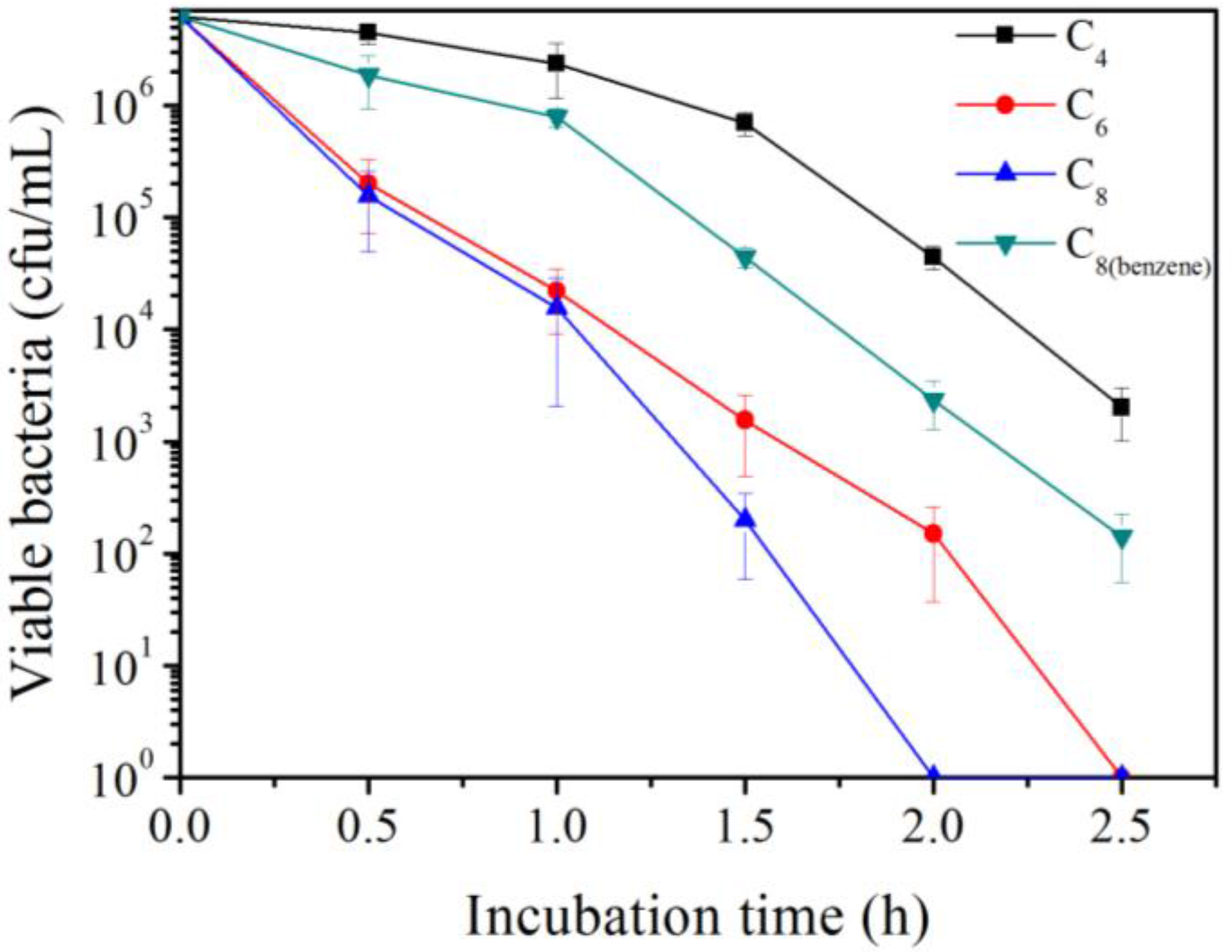
3.1. Polymer C4, C6, C8 Had Systematically Increased Antimicrobial Activity with an Increase in the Alkyl Chain Length of the Polymer
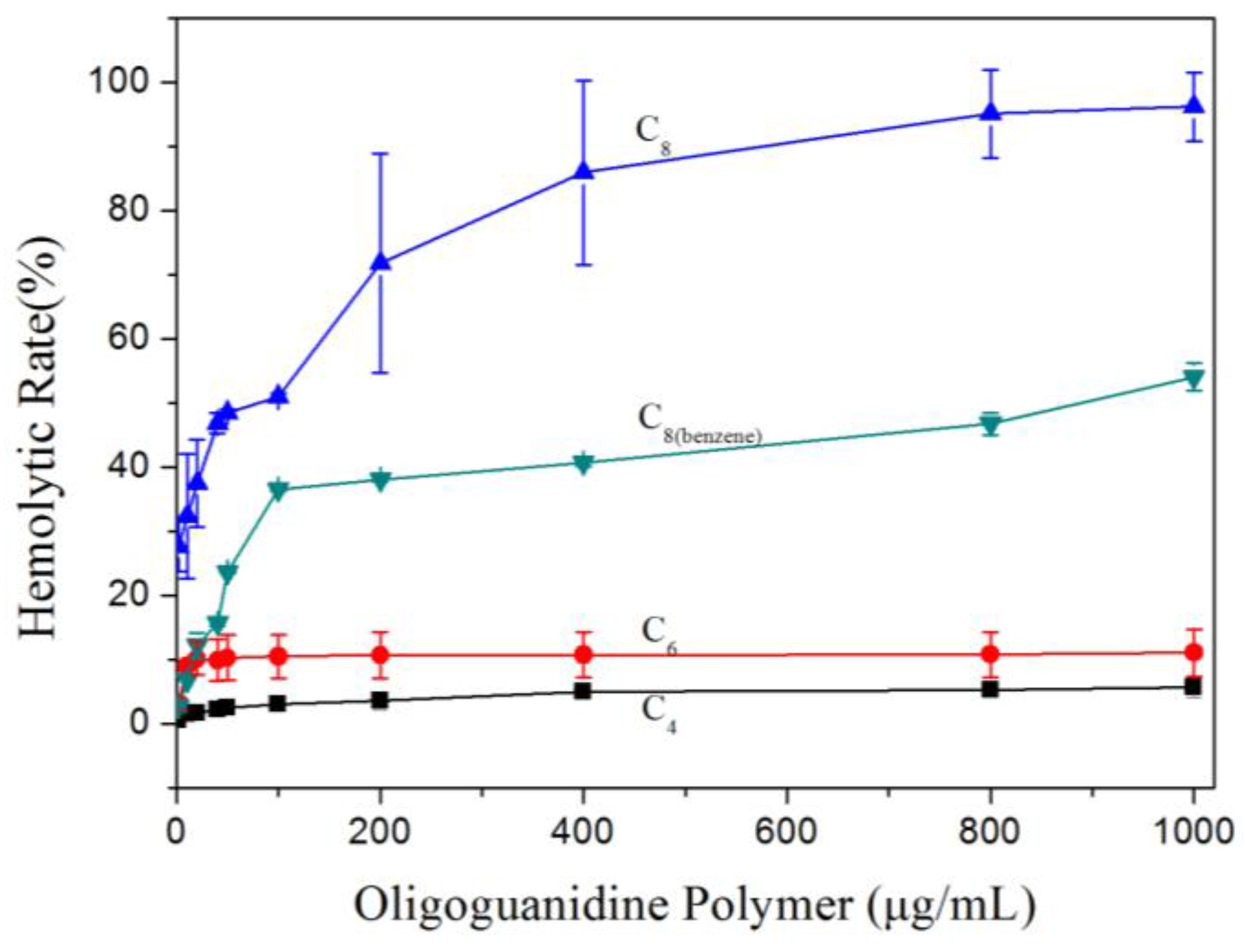
3.2. Hemolytic Property
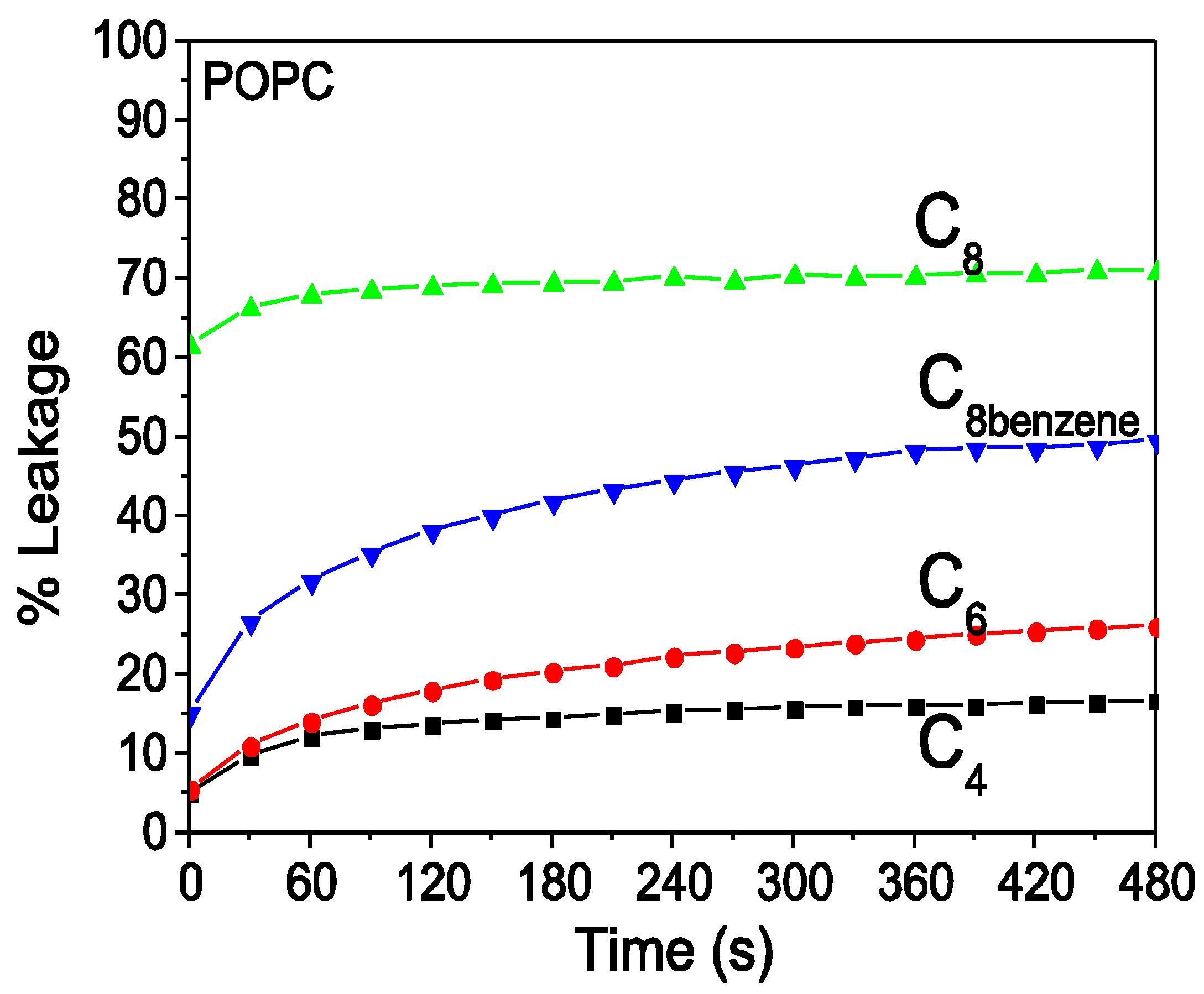
3.3. Dye Leakage of Model POPC Lipid Vesicles Mimicking the Eukaryotic Membrane
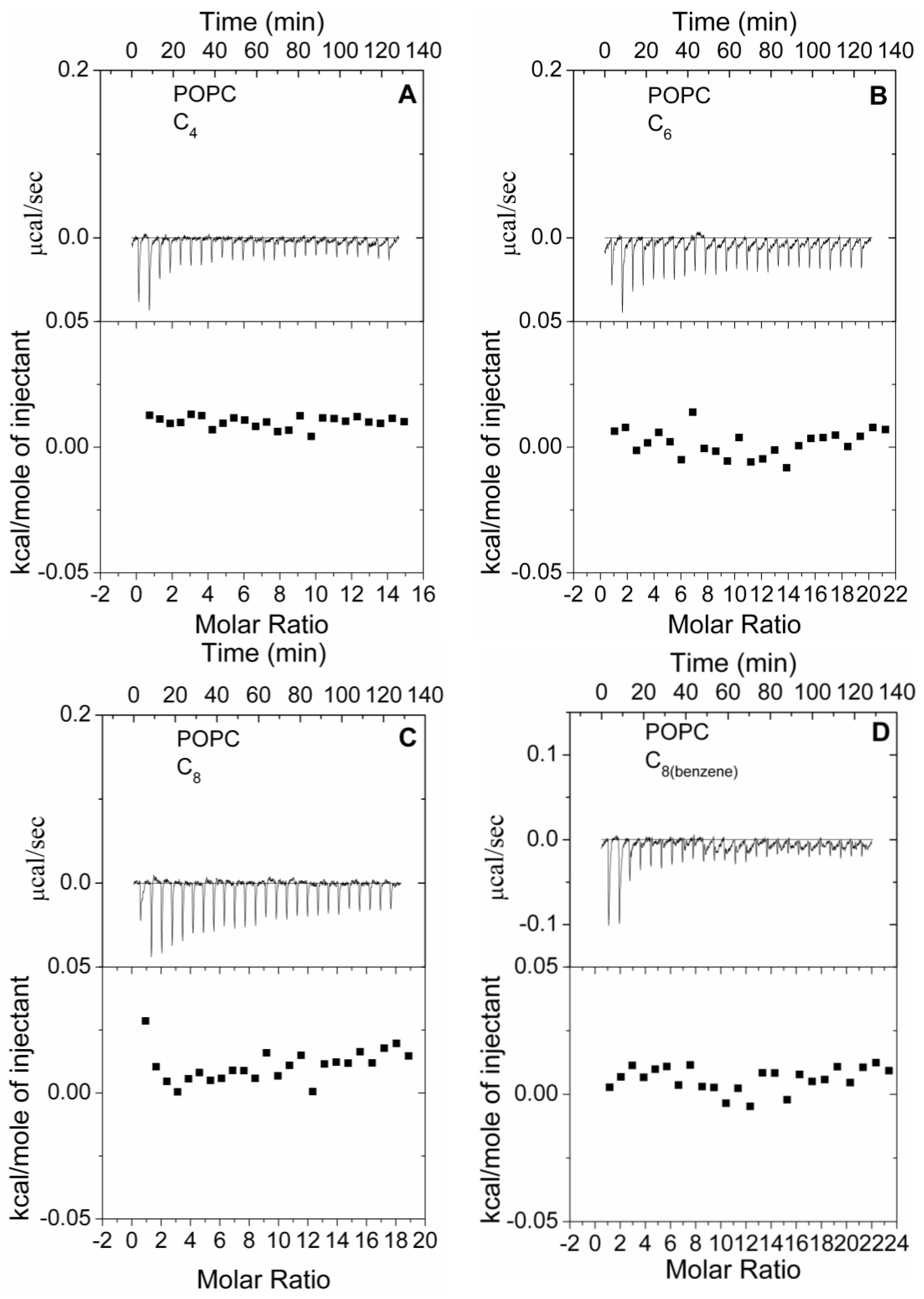
3.4. Thermodynamics of Polymer-Model Membrane Interactions
3.5. Toxicological Studies
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Polymer C4 | Polybutamethylene guanidine hydrochloride |
| Polymer C6 (PHGH) | Polyhexamethylene guanidine hydrochloride |
| Polymer C8 (POGH) | Polyoctamethylene guanidine hydrochloride |
| Polymer C8(benzene) | Poly(m-xylylene guanidine hydrochloride) |
| MDR-PA | Multidrug resistant Pseudomonas aeruginosa |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| ITC | Isothermal titration calorimetry |
References
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Yang, Y.Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Carrasco, L.D.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O'Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Bolla, J.M.; Alibert-Franco, S.; Handzlik, J.; Chevalier, J.; Mahamoud, A.; Boyer, G. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 2011, 585, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.P.; Decout, J.L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. Medchemcomm 2016, 7, 586–611. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Guchhait, G.; Goswami, S.; Fernando, D.M.; Kumar, A.; Zhanel, G.G.; Schweizer, F. Hybrid antibiotic overcomes resistance in P. aeruginosa by enhancing outer membrane penetration and reducing efflux. J. Med. Chem. 2016, 59, 8441–8455. [Google Scholar] [CrossRef] [PubMed]
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother. 2010, 54, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Gonzalez, J.A.; Rodriguez-Castellon, E.; Teves, S.; Copello, G.J. Antimicrobial surface functionalization of PVC by a guanidine based antimicrobial polymer. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.F.; Wang, H.; Ziaee, Z.; Chibante, F.; Zheng, A.N.; Xiao, H.N. Non-leaching antimicrobial biodegradable PBAT films through a facile and novel approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Ghamrawi, S.; Bouchara, J.P.; Tarasyuk, O.; Rogalsky, S.; Lyoshina, L.; Bulko, O.; Bardeau, J.F. Promising silicones modified with cationic biocides for the development of antimicrobial medical devices. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, K.J.; Lee, D.G. Antifungal activity of the cationic antimicrobial polymer-polyhexamethylene guanidine hydrochloride and its mode of action. Fungal Biol. 2017, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Ranucci, E.; Cagno, V.; Civra, A.; Manfredi, A.; Cavalli, R.; Ferruti, P.; Lembo, D. Agmatine-containing poly(amidoamine)s as a novel class of antiviral macromolecules: Structural properties and in vitro evaluation of infectivity inhibition. Antimicrob. Agents Chemother. 2014, 58, 6315–6319. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, N.; Pirrone, V.; Passic, S.; Zhu, W.; Kholodovych, V.; Welsh, W.; Rando, R.F.; Labib, M.E.; Wigdahl, B.; Krebs, F.C. Specific interactions between the viral coreceptor CXCR4 and the biguanide-based compound NB325 mediate inhibition of human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 2009, 53, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Grare, M.; Dibama, H.M.; Lafosse, S.; Ribon, A.; Mourer, M.; Regnouf-de-Vains, J.B.; Finance, C.; Duval, R.E. Cationic compounds with activity against multidrug-resistant bacteria: Interest of a new compound compared with two older antiseptics, hexamidine and chlorhexidine. Clin. Microbiol. Infect. 2010, 16, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Zhanel, G.G.; Schweizer, F. Antibacterial activity of guanidinylated neomycin B- and kanamycin A-derived amphiphilic lipid conjugates. J. Antimicrob. Chemother. 2010, 65, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Wei, D.F.; Guan, Y.; Zheng, A.N.; Zhong, J.J. Extensive in vitro activity of guanidine hydrochloride polymer analogs against antibiotics-resistant clinically isolated strains. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1836–1843. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Wei, D.F.; Lu, Y.H. Polyhexamethylene guanidine hydrochloride shows bactericidal advantages over chlorhexidine digluconate against ESKAPE bacteria. Biotechnol. Appl. Biochem. 2015, 62, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Oule, M.K.; Azinwi, R.; Bernier, A.M.; Kablan, T.; Maupertuis, A.M.; Mauler, S.; Nevry, R.K.; Dembélé, K.; Forbes, L.; Diop, L. Polyhexamethylene guanidine hydrochloride-based disinfectant: A novel tool to fight meticillin-resistant Staphylococcus aureus and nosocomial infections. J. Med. Microbiol. 2008, 57, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, A.; Zhong, J. Interactions of biocidal guanidine hydrochloride polymer analogs with model membranes: A comparative biophysical study. Acta Biochim. Biophys. Sin. 2011, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Wei, D.F.; Guan, Y.; Zheng, A.N.; Zhong, J.J. Damage of Escherichia coli membrane by bactericidal agent polyhexamethylene guanidine hydrochloride: Micrographic evidences. J. Appl. Microbiol. 2010, 108, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.H.; Khin, M.M.; Ravikumar, V.; Si, Z.Y.; Rice, S.A.; Chan-Park, M.B. Modulating antimicrobial activity and mammalian cell biocompatibility with glucosamine-functionalized star polymers. Biomacromolecules 2016, 17, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Horner, I.J.; Kraut, N.D.; Hurst, J.J.; Rook, A.M.; Collado, C.M.; Atilla-Gokcumen, G.E.; Maziarz, E.P.; Liu, X.M.; Merchea, M.M.; Bright, F.V. Effects of polyhexamethylene biguanide and polyquaternium-1 on phospholipid bilayer structure and dynamics. J. Phys. Chem. B 2015, 119, 10531–10542. [Google Scholar] [CrossRef] [PubMed]
- Grau-Campistany, A.; Manresa, A.; Pujol, M.; Rabanal, F.; Cajal, Y. Tryptophan-containing lipopeptide antibiotics derived from polymyxin B with activity against Gram positive and Gram negative bacteria. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, A.; Kratzer, C.; Graninger, W.; Georgopoulos, A. Antimicrobial and toxicological profile of the new biocide Akacid plus®. J. Antimicrob. Chemother. 2006, 58, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; McGowan, J.E.; McAllister, S.; Tenover, F.C. In vitro activity of ceftobiprole against coagulase-negative staphylococci isolated in the USA. Int. J. Antimicrob. AG 2008, 31, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.L.; Huang, J.X.; Cheah, S.E.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Velkov, T.; Whittaker, M.R. Antibacterial low molecular weight cationic polymers: Dissecting the contribution of hydrophobicity, chain length and charge to activity. RSC Adv. 2016, 6, 15469–15477. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; O'Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Reynolds, E.C.; Qiao, G.G.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162–16173. [Google Scholar] [CrossRef] [PubMed]
- Locock, K.E.S.; Michl, T.D.; Valentin, J.D.P.; Vasilev, K.; Hayball, J.D.; Qu, Y.; Traven, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Guanylated polymethacrylates: A class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules 2013, 14, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.L.; Elliott, A.G.; Huang, J.X.; Schneider, E.K.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Velkov, T.; Whittaker, M.R. Cationic acrylate oligomers comprising amino acid mimic moieties demonstrate improved antibacterial killing efficiency. J. Mater. Chem. B 2017, 5, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Tew, G.N. Influence of lipid composition on membrane activity of antimicrobial phenylene ethynylene oligomers. J. Phys. Chem. B 2008, 112, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.F.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Salvio, R. The guanidinium unit in the catalysis of phosphoryl transfer reactions: From molecular spacers to nanostructured supports. Chem. Eur. J. 2015, 21, 10960–10971. [Google Scholar] [CrossRef] [PubMed]
- Savelli, C.; Salvio, R. Guanidine-based polymer brushes grafted onto silica nanoparticles as efficient artificial phosphodiesterases. Chem. Eur. J. 2015, 21, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Uppu, D.; Samaddar, S.; Hoque, J.; Konai, M.M.; Krishnamoorthy, P.; Shome, B.R.; Haldar, J. Side chain degradable cationic-amphiphilic polymers with tunable hydrophobicity show in vivo activity. Biomacromolecules 2016, 17, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Chakrabarty, S.; Zhang, W.; Zeng, X.M.; Ohman, D.E.; Wood, L.F.; Abraham, S.; Rao, R.; Wynne, K.J. High antimicrobial effectiveness with low hemolytic and cytotoxic activity for PEG/Quaternary copolyoxetanes. Biomacromolecules 2014, 15, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Sahraro, M.; Yeganeh, H.; Sorayya, M. Guanidine hydrochloride embedded polyurethanes as antimicrobial and absorptive wound dressing membranes with promising cytocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Kukharenko, O.; Bardeau, J.F.; Zaets, I.; Ovcharenko, L.; Tarasyuk, O.; Porhyn, S.; Mischenko, I.; Vovk, A.; Rogalsky, S.; Kozyrovska, N. Promising low cost antimicrobial composite material based on bacterial cellulose and polyhexamethylene guanidine hydrochloride. Eur. Polym. J. 2014, 60, 247–254. [Google Scholar] [CrossRef]
- Ziaee, Z.; Xiao, H.N.; Guan, Y.; Fatehi, P. Coating PHGH-modified starch on papers to induce antimicrobial properties. BioResources 2014, 9, 3632–3641. [Google Scholar] [CrossRef]
- Ziaee, Z.; Qian, L.Y.; Guan, Y.; Fatehi, P.; Xiao, H.N. Antimicrobial/antimold polymer-grafted starches for recycled cellulose fibers. J. Biomater. Sci. Polym. Ed. 2010, 21, 1359–1370. [Google Scholar]
| Polymer | Median Lethal Dose (LD50) |
|---|---|
| PHGH (Polymer C6) | 500–800 mg/kg |
| POGH (Polymer C8) | 500–800 mg/kg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Jiang, Z.; Zhang, N.; Yang, Z.; Zhou, Z. Interactions of Biocidal Polyhexamethylene Guanidine Hydrochloride and Its Analogs with POPC Model Membranes. Polymers 2017, 9, 517. https://doi.org/10.3390/polym9100517
Luo X, Jiang Z, Zhang N, Yang Z, Zhou Z. Interactions of Biocidal Polyhexamethylene Guanidine Hydrochloride and Its Analogs with POPC Model Membranes. Polymers. 2017; 9(10):517. https://doi.org/10.3390/polym9100517
Chicago/Turabian StyleLuo, Xuliang, Ziran Jiang, Niya Zhang, Zixin Yang, and Zhongxin Zhou. 2017. "Interactions of Biocidal Polyhexamethylene Guanidine Hydrochloride and Its Analogs with POPC Model Membranes" Polymers 9, no. 10: 517. https://doi.org/10.3390/polym9100517
APA StyleLuo, X., Jiang, Z., Zhang, N., Yang, Z., & Zhou, Z. (2017). Interactions of Biocidal Polyhexamethylene Guanidine Hydrochloride and Its Analogs with POPC Model Membranes. Polymers, 9(10), 517. https://doi.org/10.3390/polym9100517




