Facile Synthesis of Electroactive and Electrochromic Triptycene Poly(ether-imide)s Containing Triarylamine Units via Oxidative Electro-Coupling
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
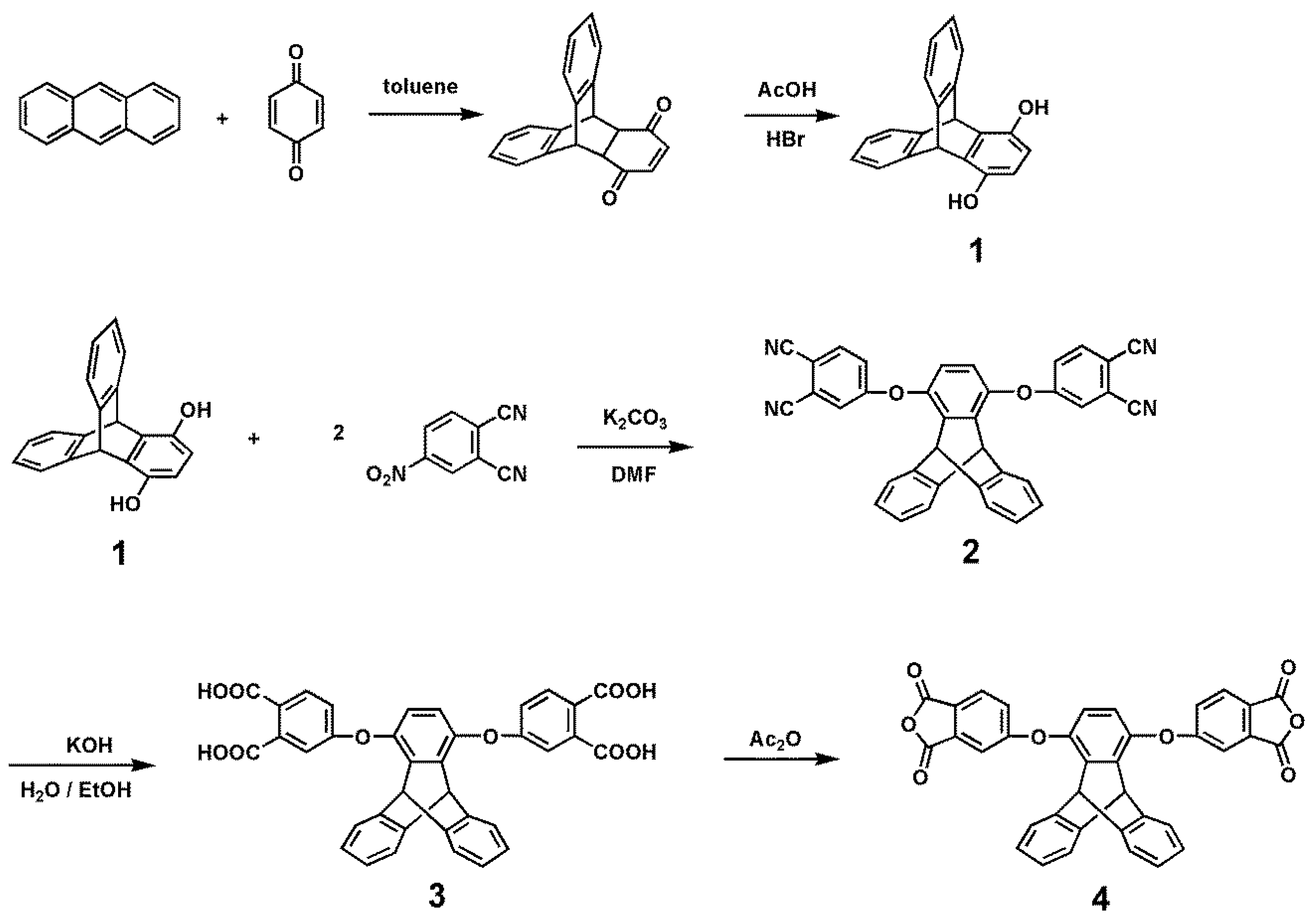
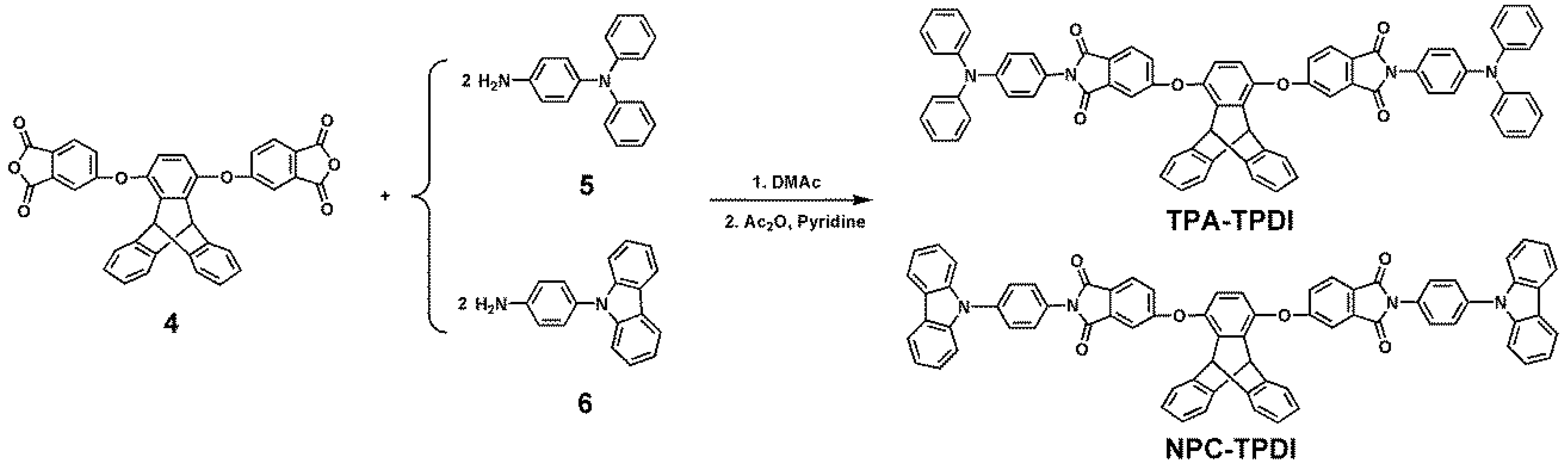
2.2. Monomer Synthesis
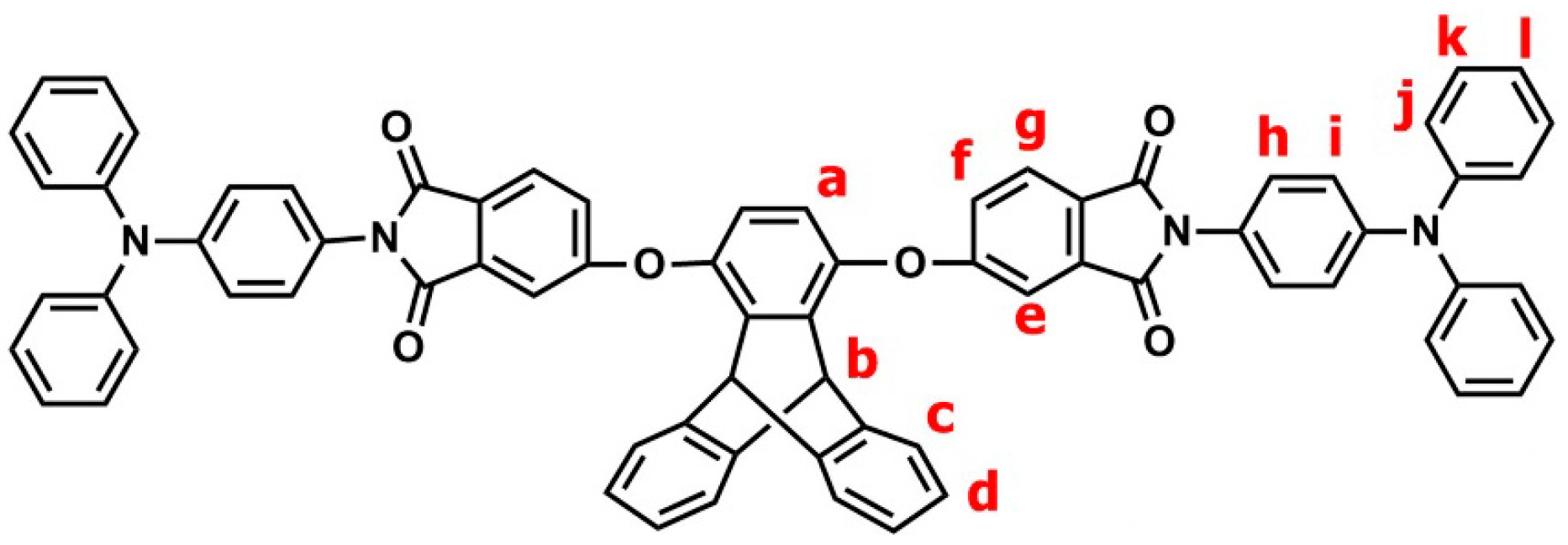
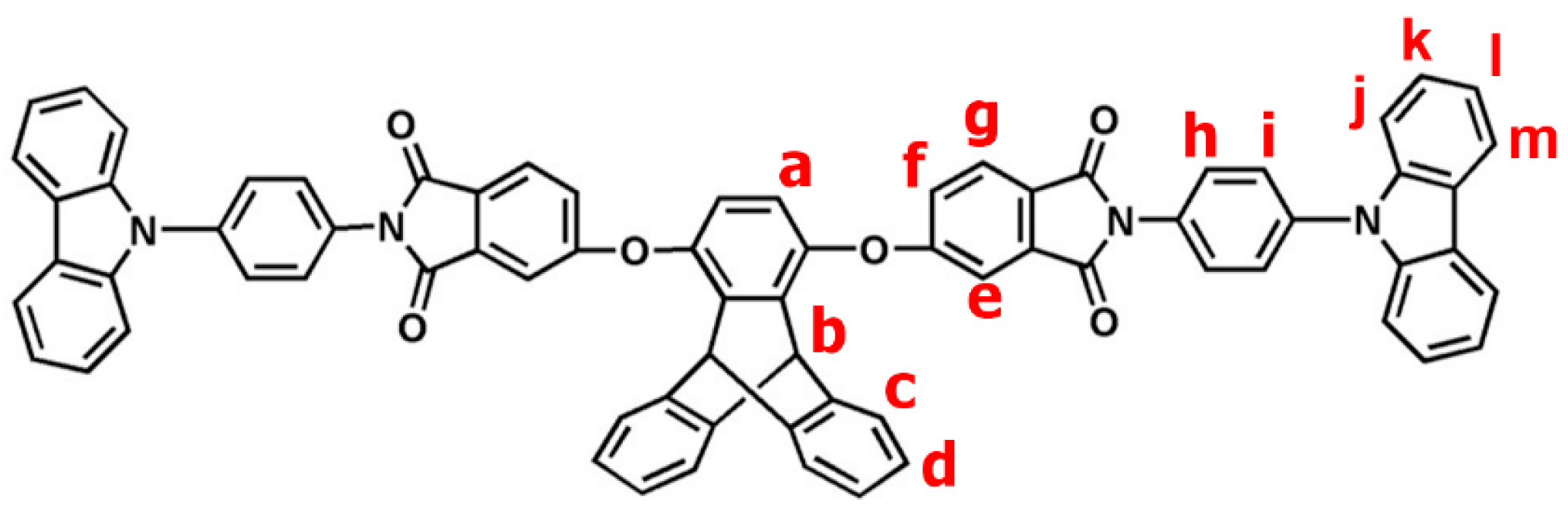
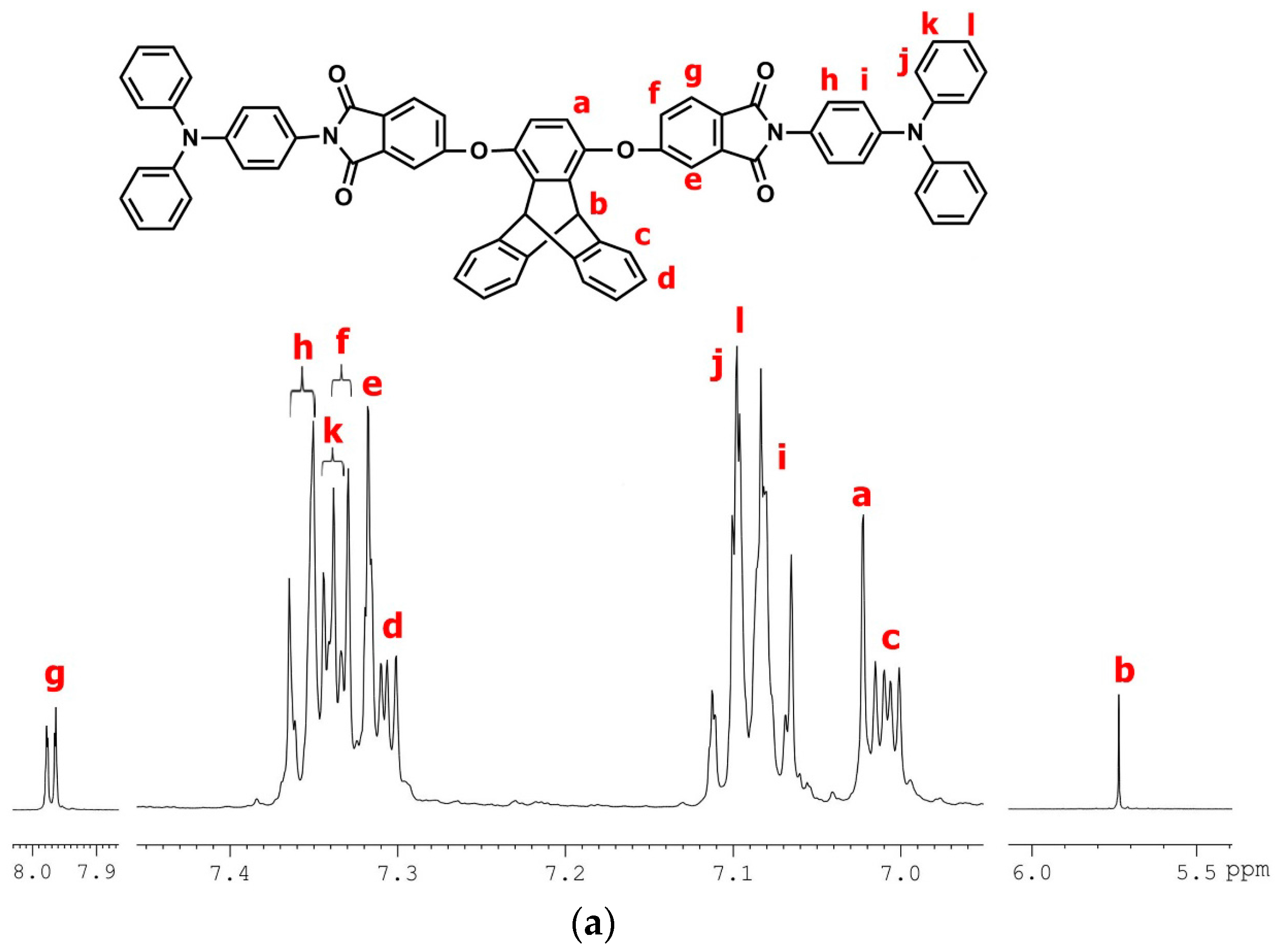
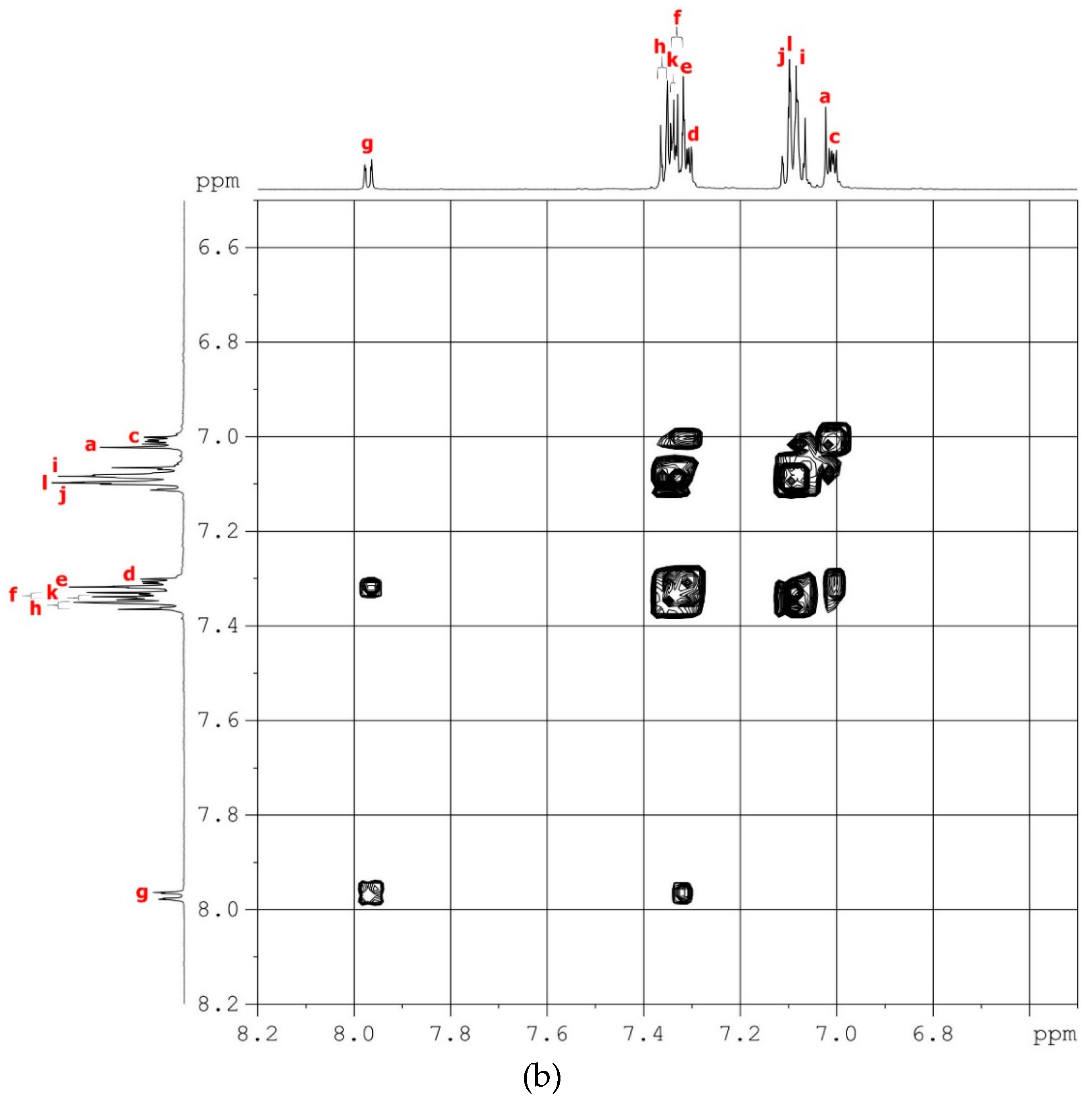
2.2.1. TPA–TPDI
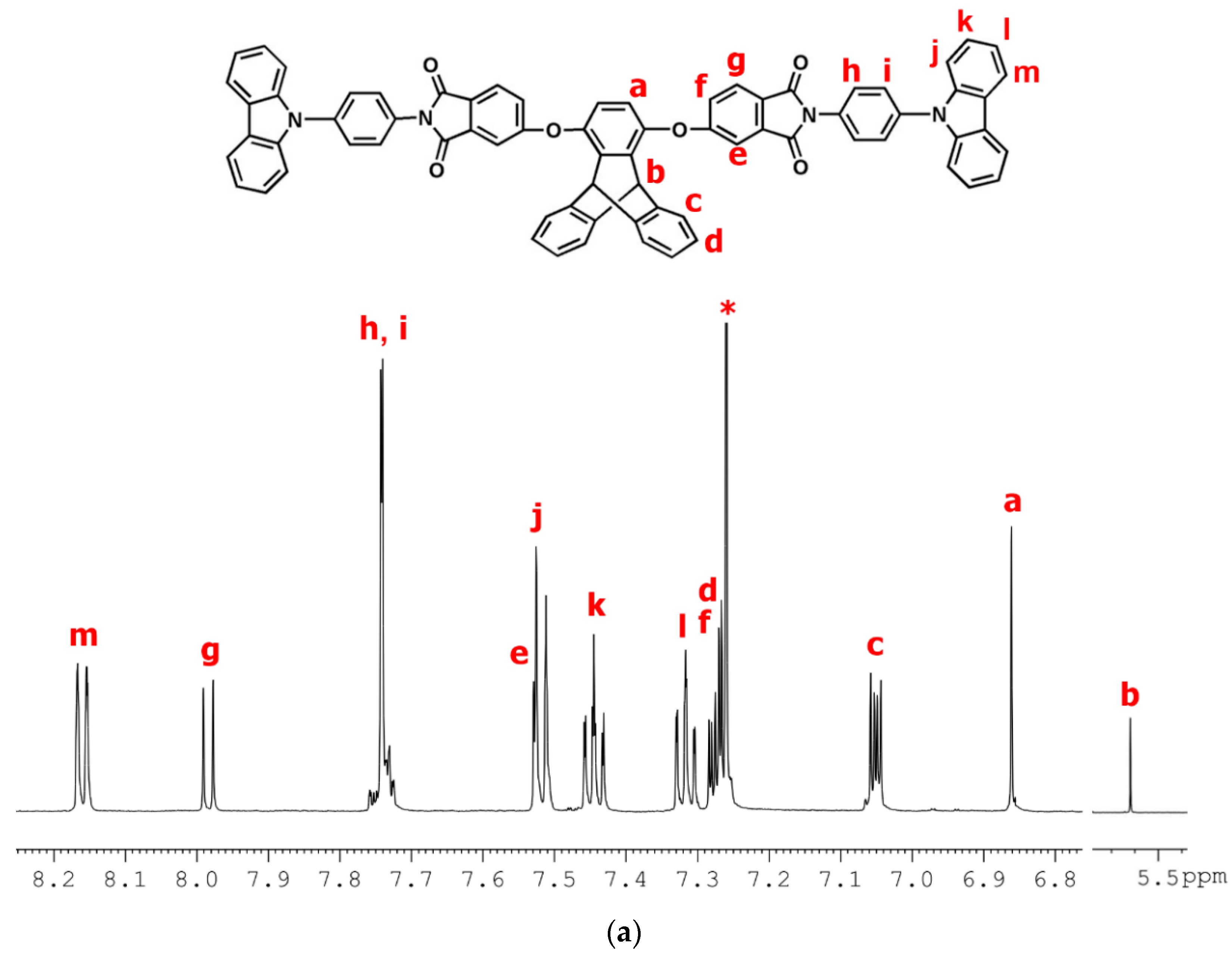
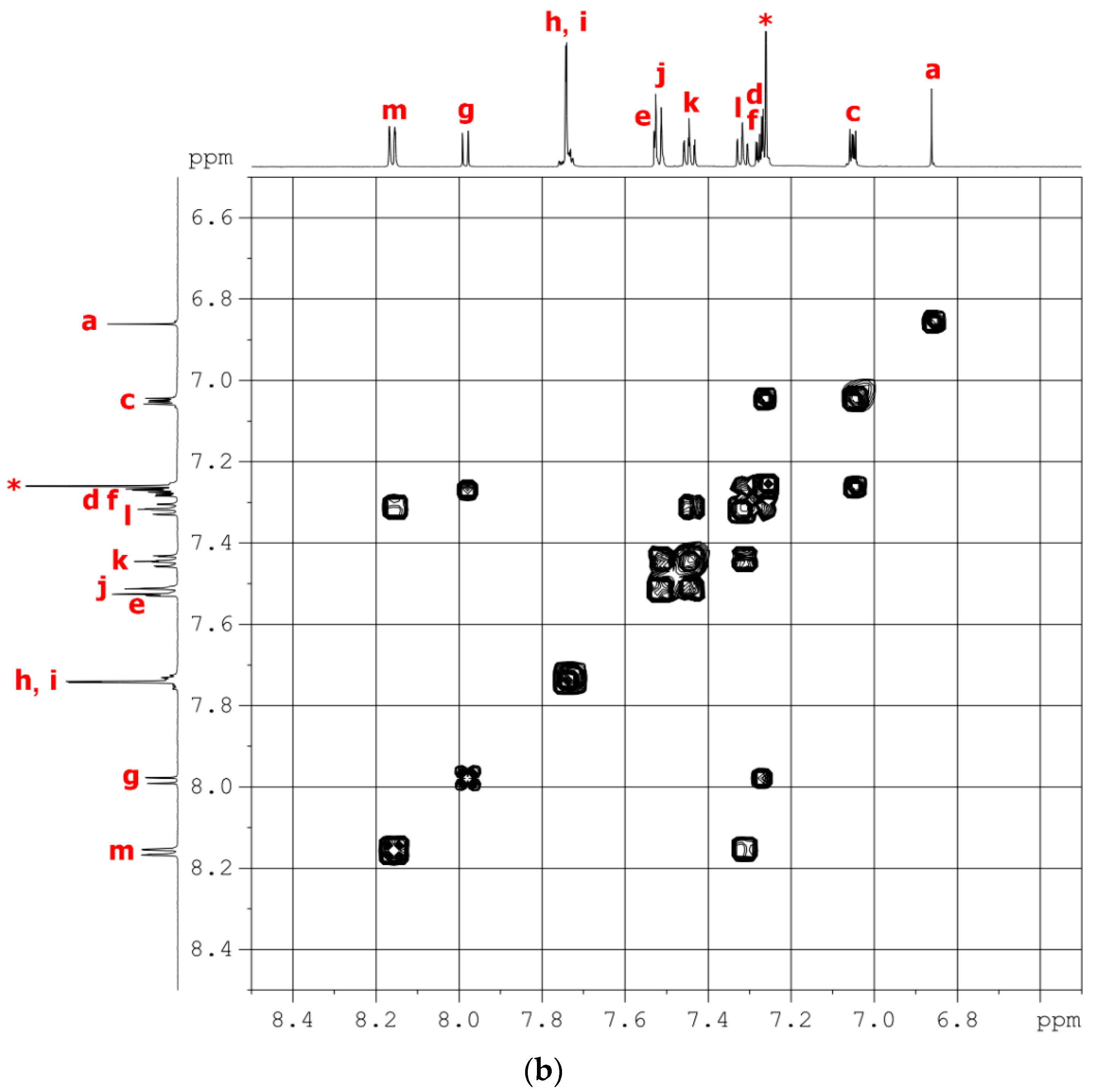
2.2.2. NPC–TPDI
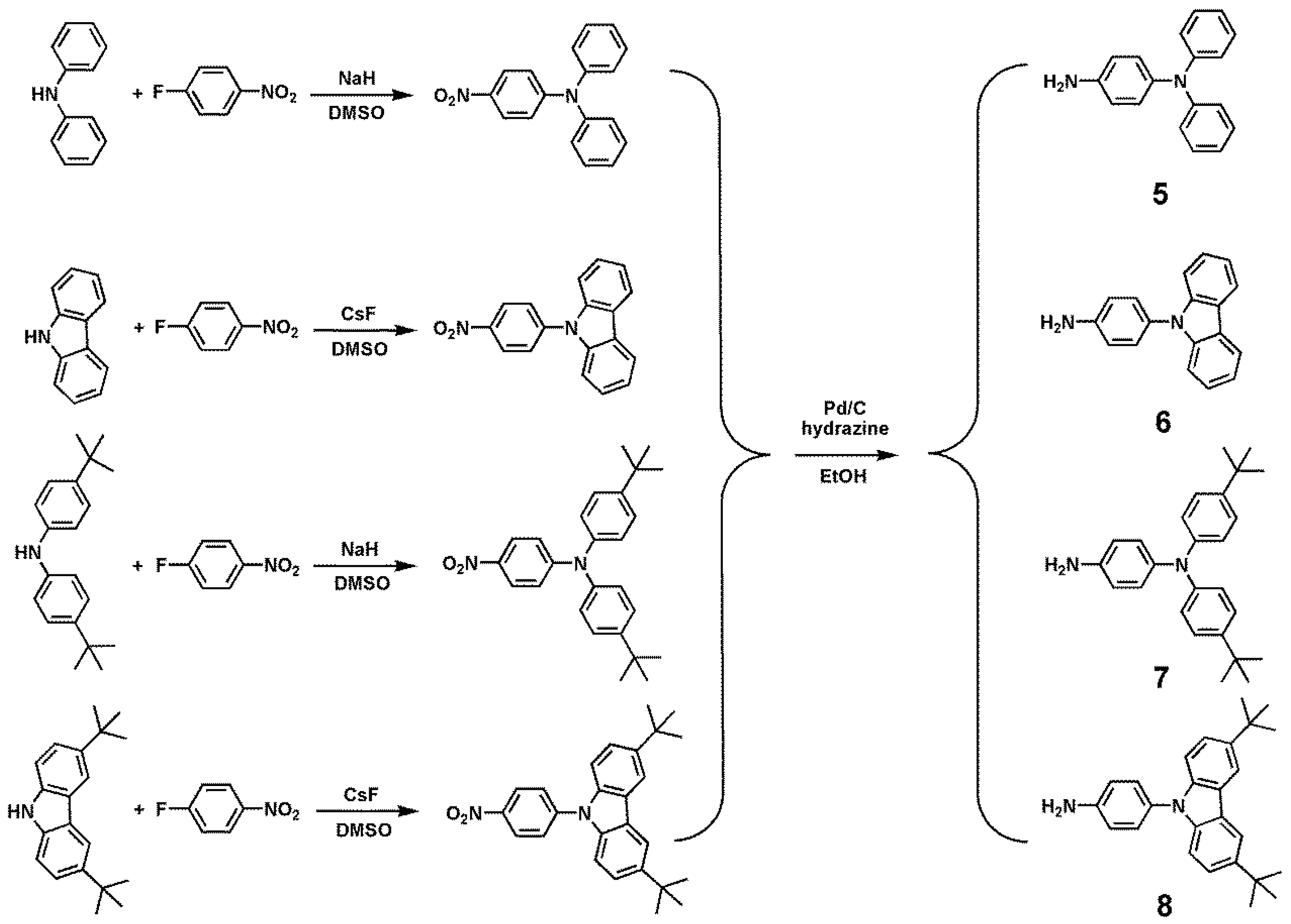
2.3. Synthesis of Model Compounds
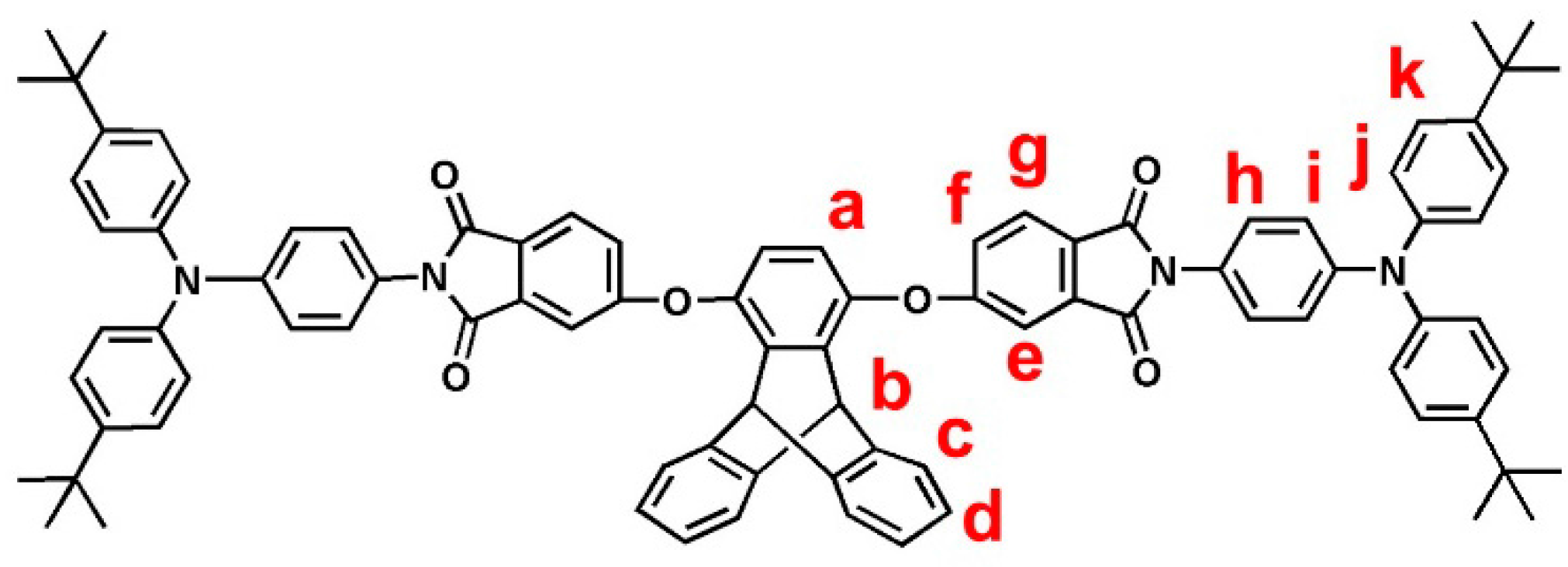
2.3.1. tBuTPA–TPDI (M1)
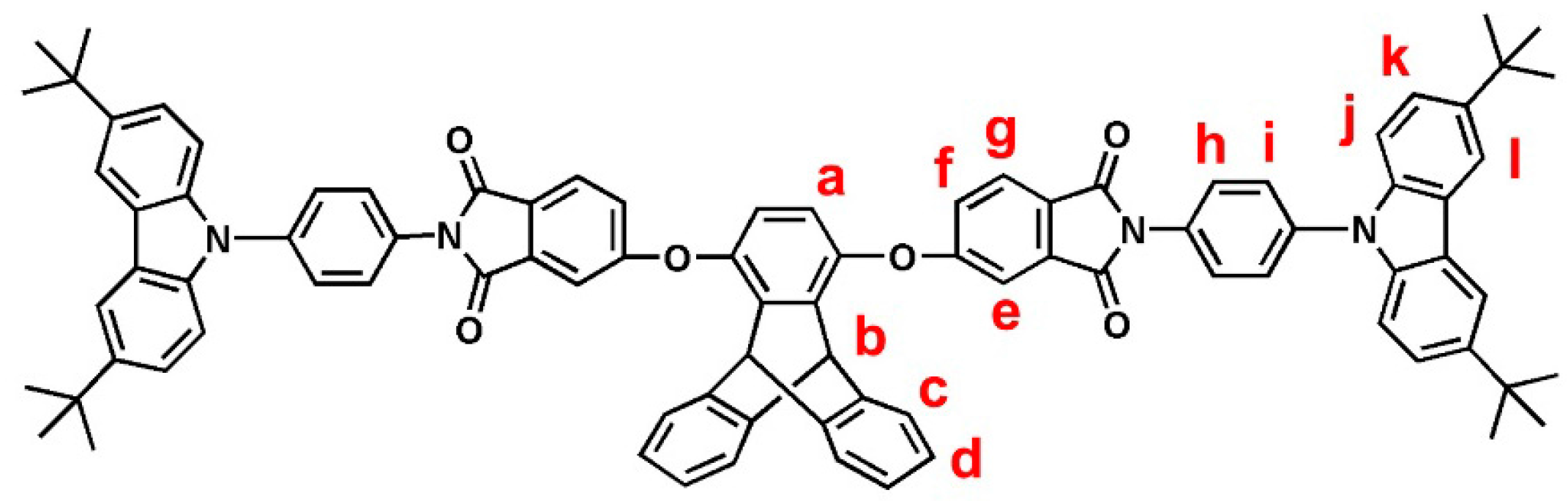
2.3.2. tBuNPC–TPDI (M2)
2.4. Electrochemical Polymerization
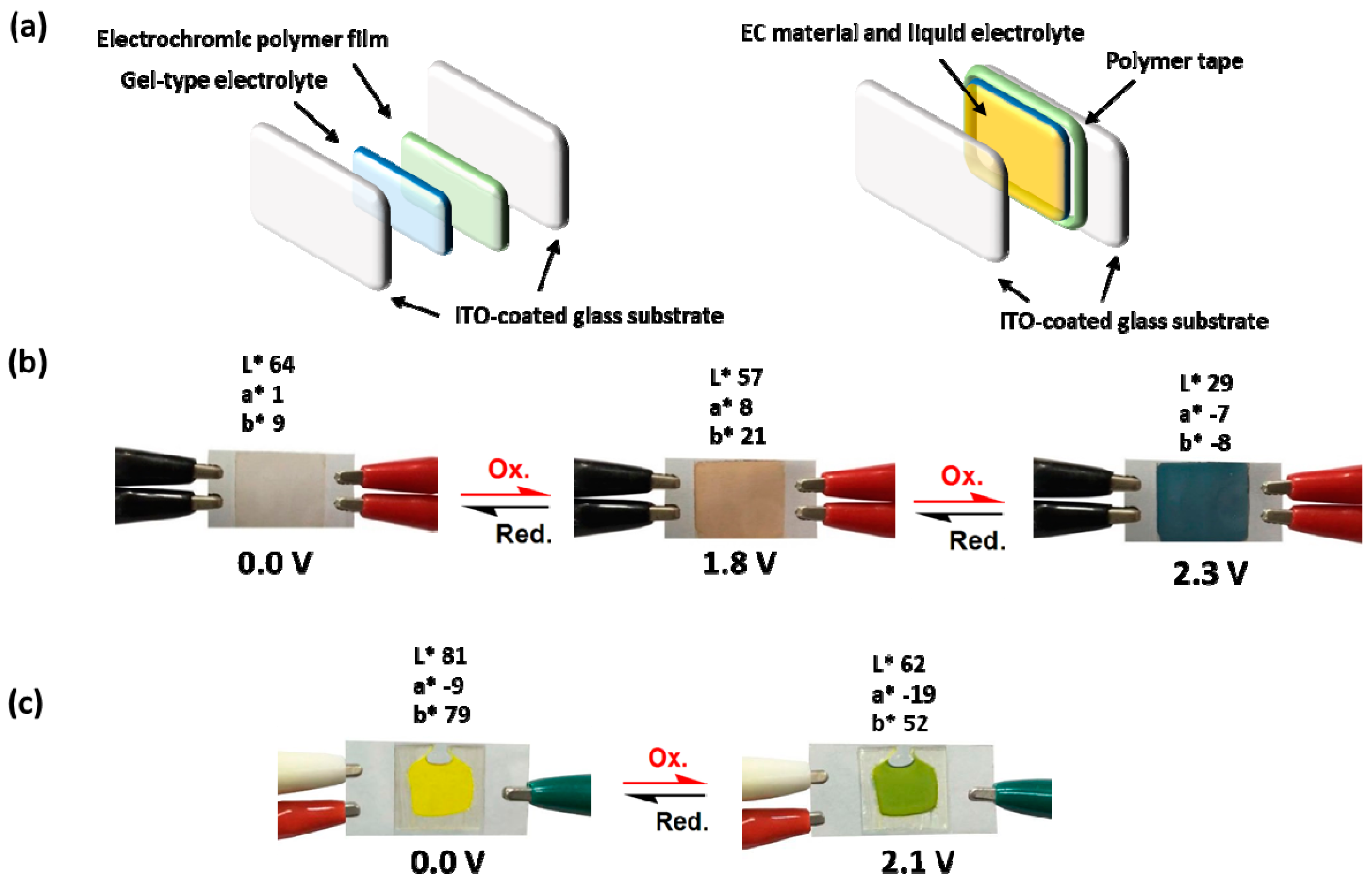
2.5. Fabrication of the Electrochromic Devices (ECDs)
2.6. Instrumentation and Measurements
3. Results and Discussion
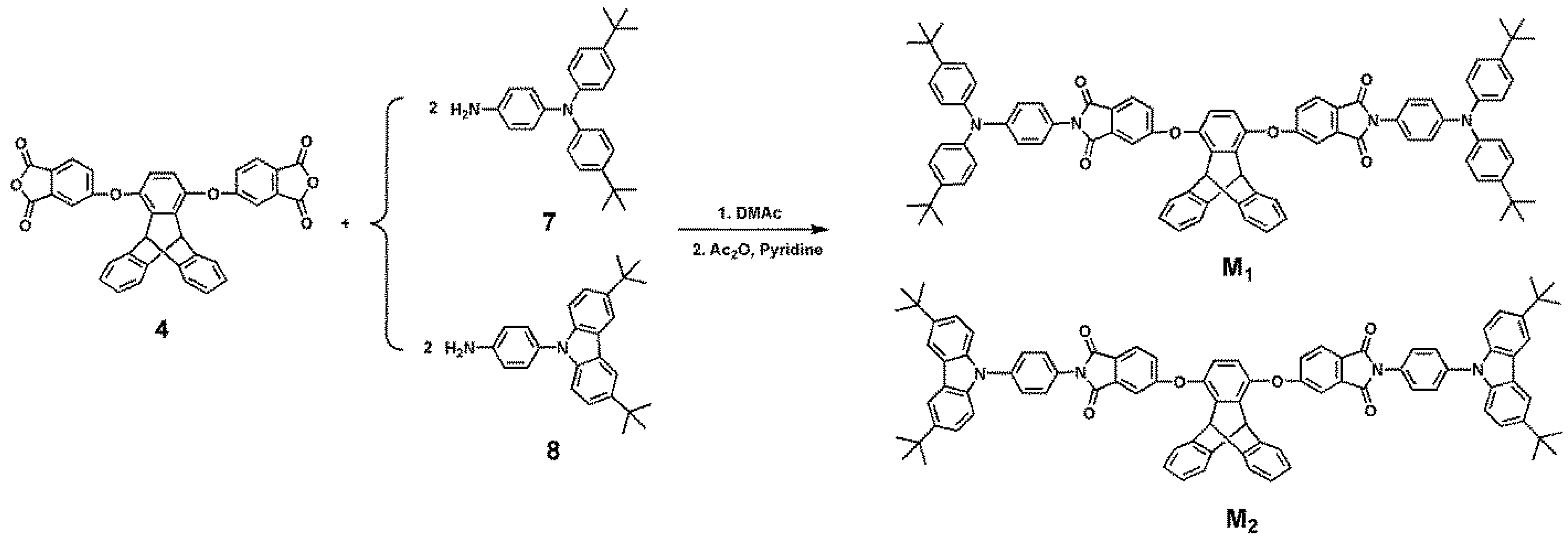
3.1. Synthesis of Monomers and Model Compounds
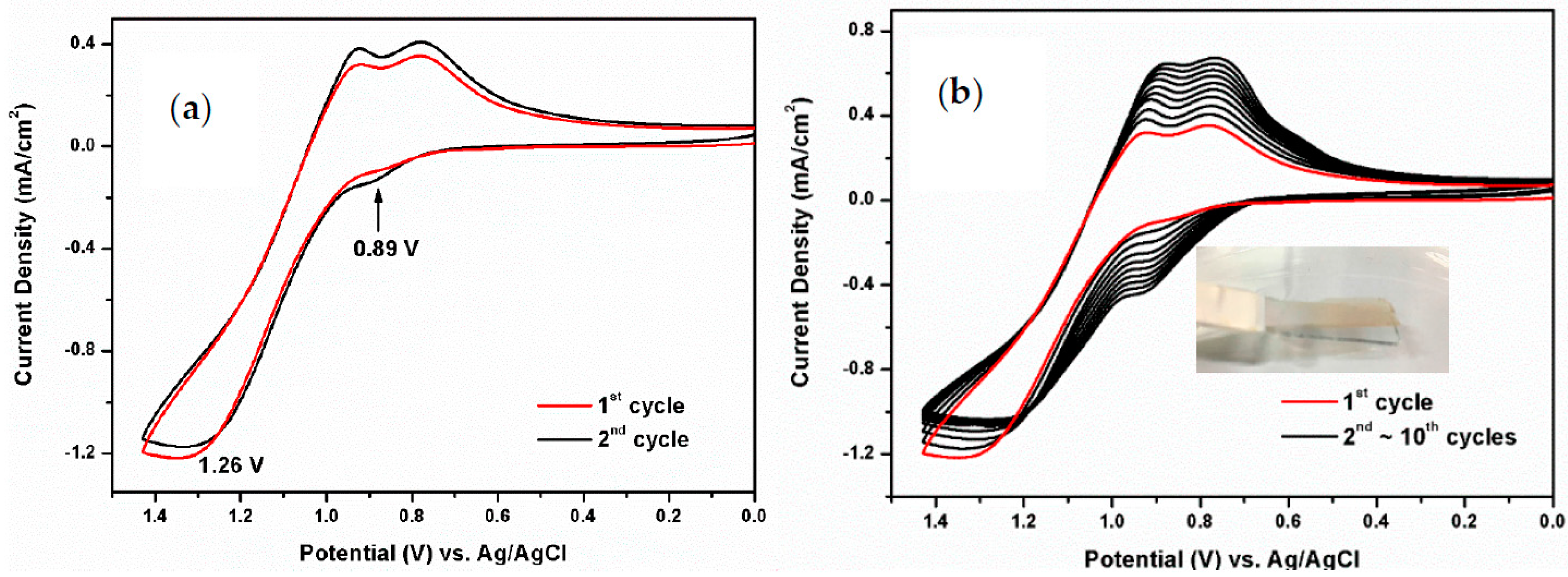
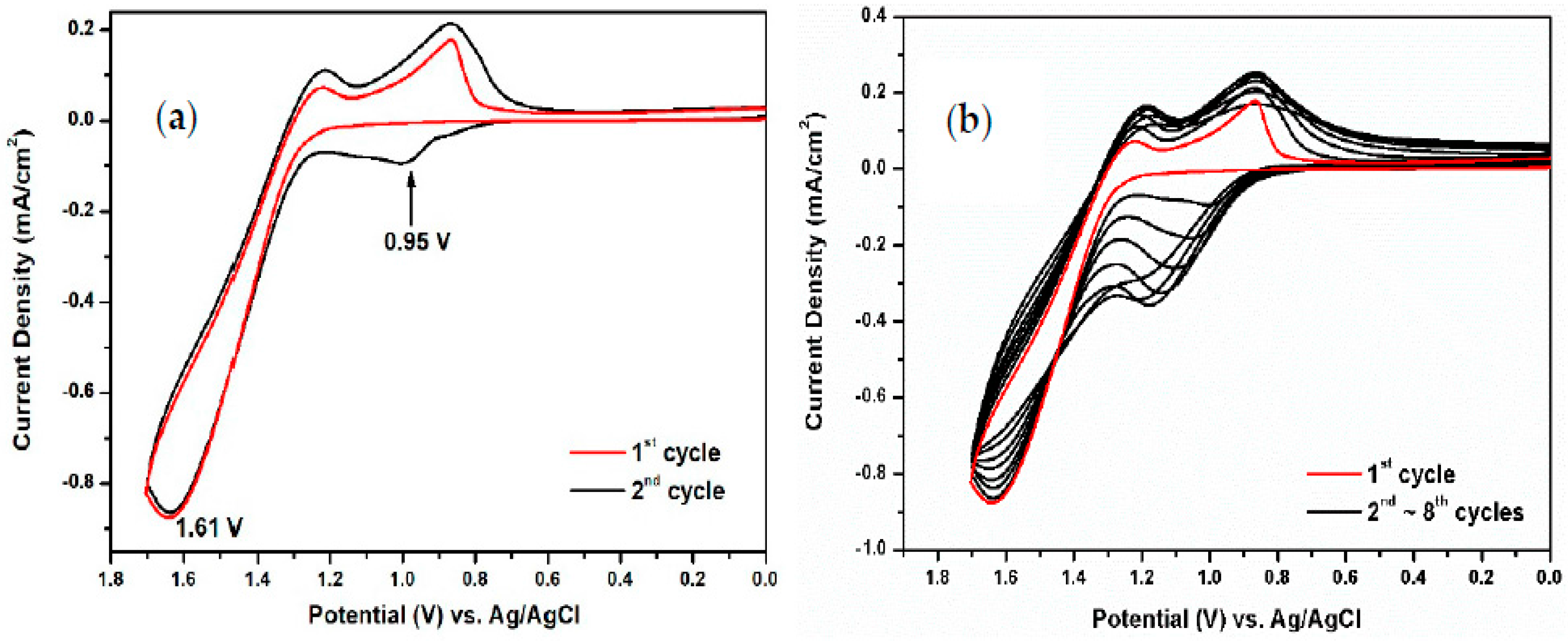
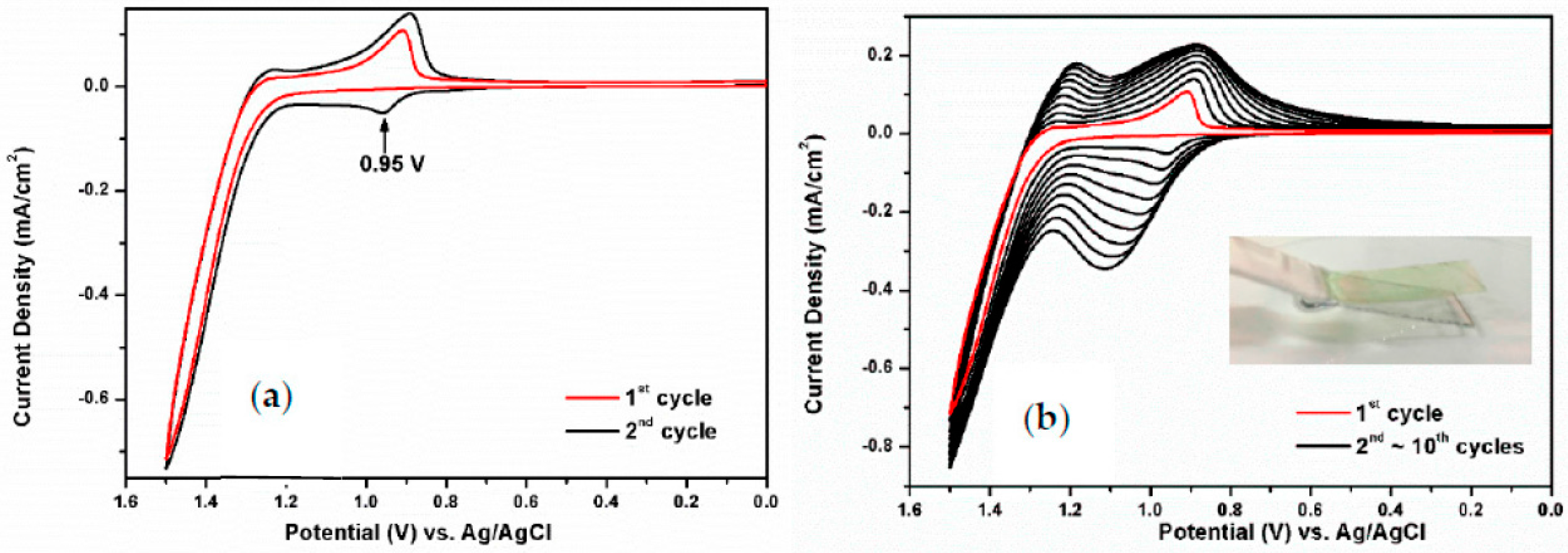
3.2. Electrochemical Activity of Monomers and Model Compounds
3.3. Optical Properties
3.4. Redox Response of Polymers
3.5. Electro-Optical Properties
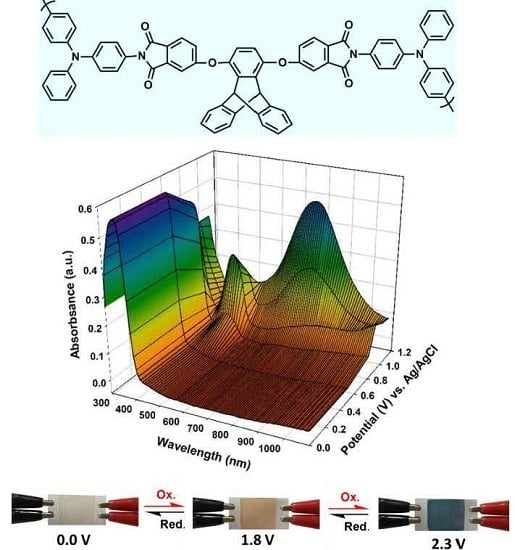
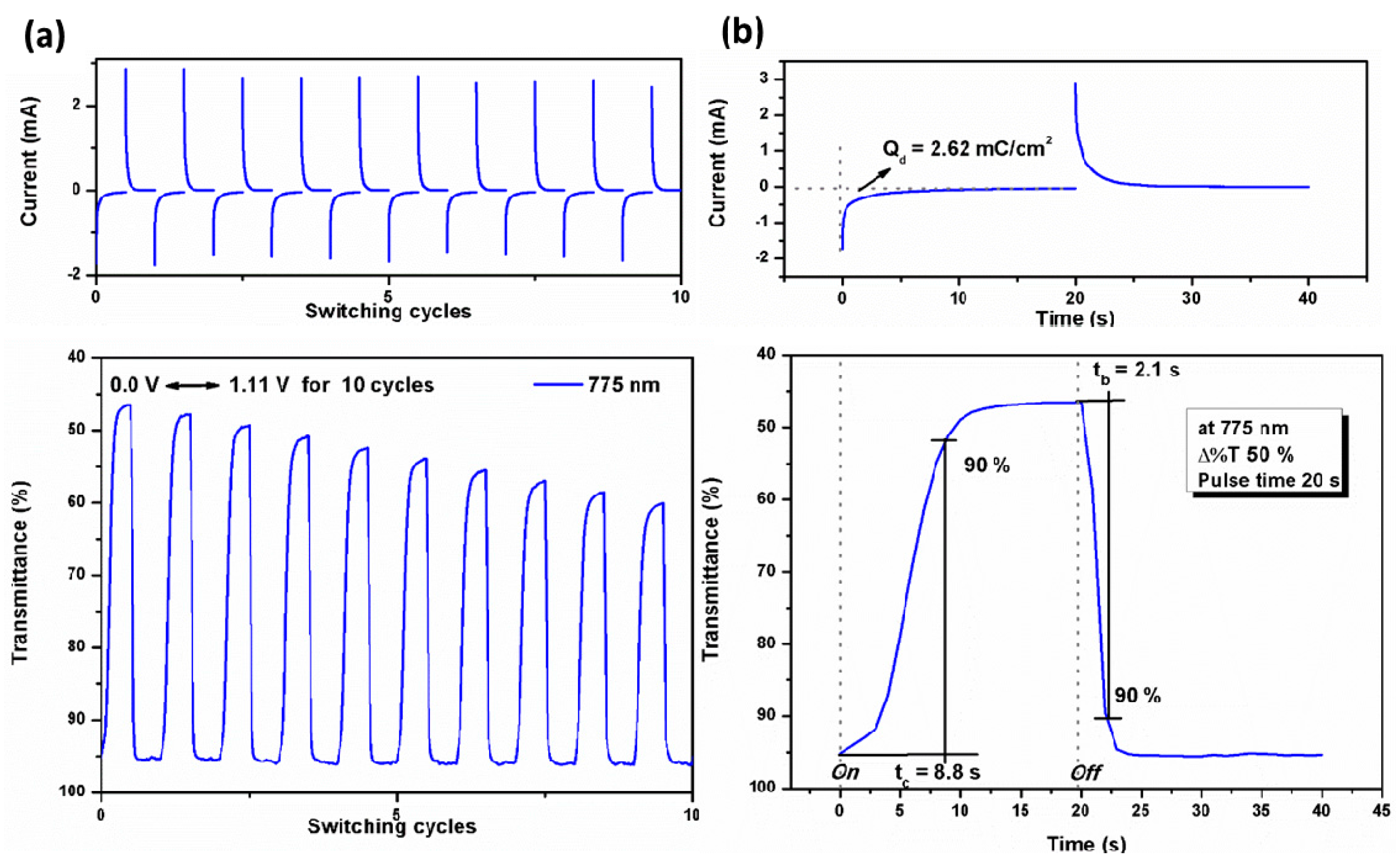
3.6. Electrochromic Switching
3.7. Electrochromic Devices
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartlett, P.D.; Ryan, M.J.; Cohen, S.G. Triptycene (9,10-o-Benzoanthracene). J. Am. Chem. Soc. 1942, 64, 2649–2953. [Google Scholar] [CrossRef]
- Skvarchenko, V.R.; Shalaev, V.K.; Klabunovskii, E.I. Advances in the chemistry of triptycene. Russ. Chem. Rev. 1974, 43, 951–966. [Google Scholar] [CrossRef]
- Hart, H.; Shamouilian, S.; Takehira, Y. Generalization of the triptycene concept. Use of diaryne equivalents in the synthesis of iptycenes. J. Org. Chem. 1981, 46, 4427–7732. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, C.-F. Synthesis and structure of 2,6,14- and 2,7,14-trisubstituted triptycene derivatives. J. Org. Chem. 2006, 71, 6626–6629. [Google Scholar]
- Zhu, C.; Chen, C.-F. Triptycene-based expanded oxacalixarenes: Synthesis, structure, and tubular assemblies in the solid state. J. Org. Chem. 2007, 72, 3880–3888. [Google Scholar]
- Yang, J.-S.; Yan, J.-L. Central-ring functionalization and application of the rigid, aromatic, and H-shaped pentiptycene scaffold. Chem. Commun. 2008, 39, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-H.; Shih, H.-H.; Cheng, C.-H. Triptycene derivatives as high-Tg host materials for various electrophosphorescent devices. J. Mater. Chem. 2010, 20, 798–805. [Google Scholar] [CrossRef]
- Long, T.M.; Swager, T.M. Molecular design of free volume as a route to low-k dielectric materials. J. Am. Chem. Soc. 2003, 125, 14113–14119. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.T.; Paraskos, A.J.; Torun, L.; Swager, T.M.; Thomas, E.L. Minimization of internal molecular free volume: A mechanism for the simultaneous enhancement of polymer stiffness, strength, and ductility. Macromolecules 2006, 39, 3350–3358. [Google Scholar] [CrossRef]
- Tsui, N.T.; Torun, L.; Pate, B.D.; Paraskos, A.J.; Swager, T.M.; Thomas, E.L. Molecular barbed wire: Threading and interlocking for the mechanical reinforcement of polymers. Adv. Funct. Mater. 2007, 17, 1595–1602. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Li, W.; Zhang, S. Synthesis and properties of novel organosoluble polyimides derived from 1,4-bis(3,4-dicarboxyphenoxy)triptycene dianhydride and various aromatic diamines. Polymer 2007, 48, 6246–6253. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chen, W.-J.; Lee, T.-M.; Leu, C.-M. Synthesis and properties of novel triptycene-based polyimides. J. Polym. Sci. A 2011, 49, 3109–3120. [Google Scholar] [CrossRef]
- Sydlik, S.A.; Chen, Z.; Swager, T.M. Triptycene polyimides: Soluble polymers with high thermal stability and low refractive indices. Macromolecules 2011, 44, 976–980. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, H.B. High performance polyimide with high internal free volume elements. Macromol. Rapid Commun. 2011, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, J.R.; Smith, Z.P.; Liu, Q.; Patterson, C.T.; Freeman, B.D.; Guo, R. Synthesis and characterization of triptycene-based polyimides with tunable high fractional free volume for gas separation membranes. J. Mater. Chem. A 2014, 2, 13309–13320. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-shaped, dendrimeric and polymeric triarylamines as photoconductors and hole transport materials for electro-optical applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials. Chem. Commun. 2009, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, P.-L.T.; Beaupre, S.; Leclerc, M. Polycarbazoles for plastic electronics. Polym. Chem. 2010, 1, 127–136. [Google Scholar] [CrossRef]
- Iwan, A.; Sek, D. Polymers with triphenylamine units: Photonic and electroactive materials. Prog. Polym. Sci. 2011, 36, 1277–1325. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, T.; Higashihara, T.; Ueda, M. Polyimide memory: A pithy guideline for future applications. Polym. Chem. 2013, 4, 16–30. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable triarylamine-based high-performance polymers for resistive switching memory devices. Polym. J. 2016, 48, 117–138. [Google Scholar] [CrossRef]
- Yen, H.-J.; Wu, J.-H.; Huang, Y.-H.; Chen, W.-C.; Lee, K.-R.; Liou, G.-S. Novel thermally stable and soluble triarylamine functionalized polyimides for gas separation. Polym. Chem. 2014, 5, 4219–4226. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, S. Synthesis, characterization and gas transport properties of novel poly(amine-imide)s containing tetraphenylmethane pendant groups. J. Mater. Chem. A 2014, 2, 9835–9843. [Google Scholar] [CrossRef]
- Bera, D.; Bandyopadhyay, P.; Ghosh, S.; Banerjee, S.; Padmanabhan, V. Highly gas permeable polyamides containing adamantine substituted triphenylamine. J. Membr. Sci. 2015, 474, 20–31. [Google Scholar] [CrossRef]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic oxidation pathways of aromatic amines. Electrochemical and electron paramagnetic resonance studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic oxidation pathways of carbazoles I. Carbazole and N-substituted derivatives. J. Electrochem. Soc. 1968, 115, 1159–1164. [Google Scholar] [CrossRef]
- Leung, M.-K.; Chou, M.-Y.; Su, Y.-O.; Chiang, C.-L.; Chen, H.-L.; Yang, C.-F.; Yang, C.-C.; Lin, C.-C.; Chen, H.-T. Diphenylamino group as an effective handle to conjugated donor-acceptor polymers through electropolymerization. Org. Lett. 2003, 5, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-C.; Chen, H.-C.; Lee, C.-S.; Leung, M.-K.; Lin, K.-R.; Hsieh, K.-H. Electrochemical deposition of bis(N,N′-diphenylaminoaryl) substituted ferrocenes, and their application as a hole-injection layer on polymeric light-emitting diodes. Chem. Mater. 2008, 20, 540–552. [Google Scholar] [CrossRef]
- Natera, J.; Otero, L.; D’Eramo, F.; Sereno, L.; Fungo, F.; Wang, N.-S.; Tsai, Y.-M.; Wong, K.-T. Synthesis and properties of a novel cross-linked electroactive polymer formed from a bipolar starburst monomer. Macromolecules 2009, 42, 626–635. [Google Scholar] [CrossRef]
- Mangione, M.I.; Spanevello, R.A.; Rumbero, A.; Heredia, D.; Marzari, G.; Fernandez, L.; Otero, L.; Fungo, F. Electrogenerated conductive polymers from triphenylamine end-capped dendrimers. Macromolecules 2013, 46, 4754–4763. [Google Scholar] [CrossRef]
- Usluer, O.; Koyuncu, S.; Demic, S.; Janssen, R.A. A novel high-contrast ratio electrochromic material from spiro(cyclododecane-1,9′-fluorene)bicarbazole. J. Polym. Sci. B 2011, 49, 333–341. [Google Scholar] [CrossRef]
- Mangione, M.I.; Spanevello, R.A.; Minudri, D.; Heredia, D.; Fernandez, L.; Otero, L.; Fungo, F. Electropolimerization of functionalized carbazole end-capped dendrimers. Formation of conductive films. Electrochim. Acta 2016, 207, 143–151. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, L.-C. Fluorescent and electrochromic polymers from 2,8-di(carbazol-9-yl)-dibenzothiophene and its S,S-dioxide derivative. Dyes Pigments. 2016, 134, 51–63. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrochemically generated thin films of microporous polymer networks: Synthesis, properties, and applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-W. Facile preparation of electrochromic poly(amine-imide) films from diimide compounds with terminal triphenylamino groups via electrochemical oxidative coupling reactions. Polym. Chem. 2014, 5, 6770–6778. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-W. Facile fabrication of electrochromic poly(amine-amide) and poly(amine-imide) films via carbazole-based oxidative coupling electropolymerization. Macromol. Chem. Phys. 2014, 215, 1525–1532. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chou, J.-S.; Guo, W.; Lee, T.-M.; Leu, C.-M.; Su, C.-W. Triptycene poly(ether-imide)s with high solubility and optical transparency. J. Polym. Res. 2012, 19, 9757. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Substituent effects on electrochemical and electrochromic properties of aromatic polyimides with 4-(carbazol-9-yl)triphenylamine moieties. J. Polym. Sci. A 2014, 52, 1172–1184. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chou, Y.-T. Synthesis and electrochromic properties of aromatic polyimides bearing pendent triphenylamine units. Polymer 2014, 55, 2411–2421. [Google Scholar] [CrossRef]
- Lambert, C.; Noll, G. The class II/III transition in triarylamine redox systems. J. Am. Chem. Soc. 1999, 121, 8434–8442. [Google Scholar] [CrossRef]

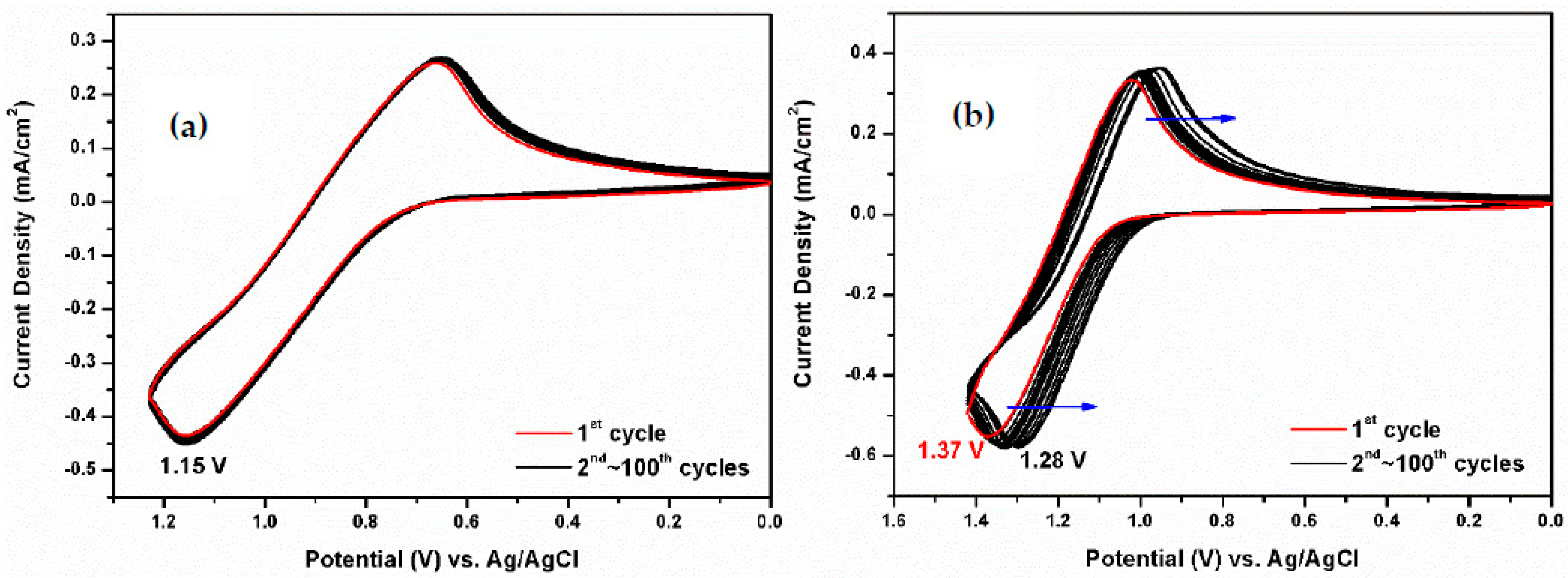
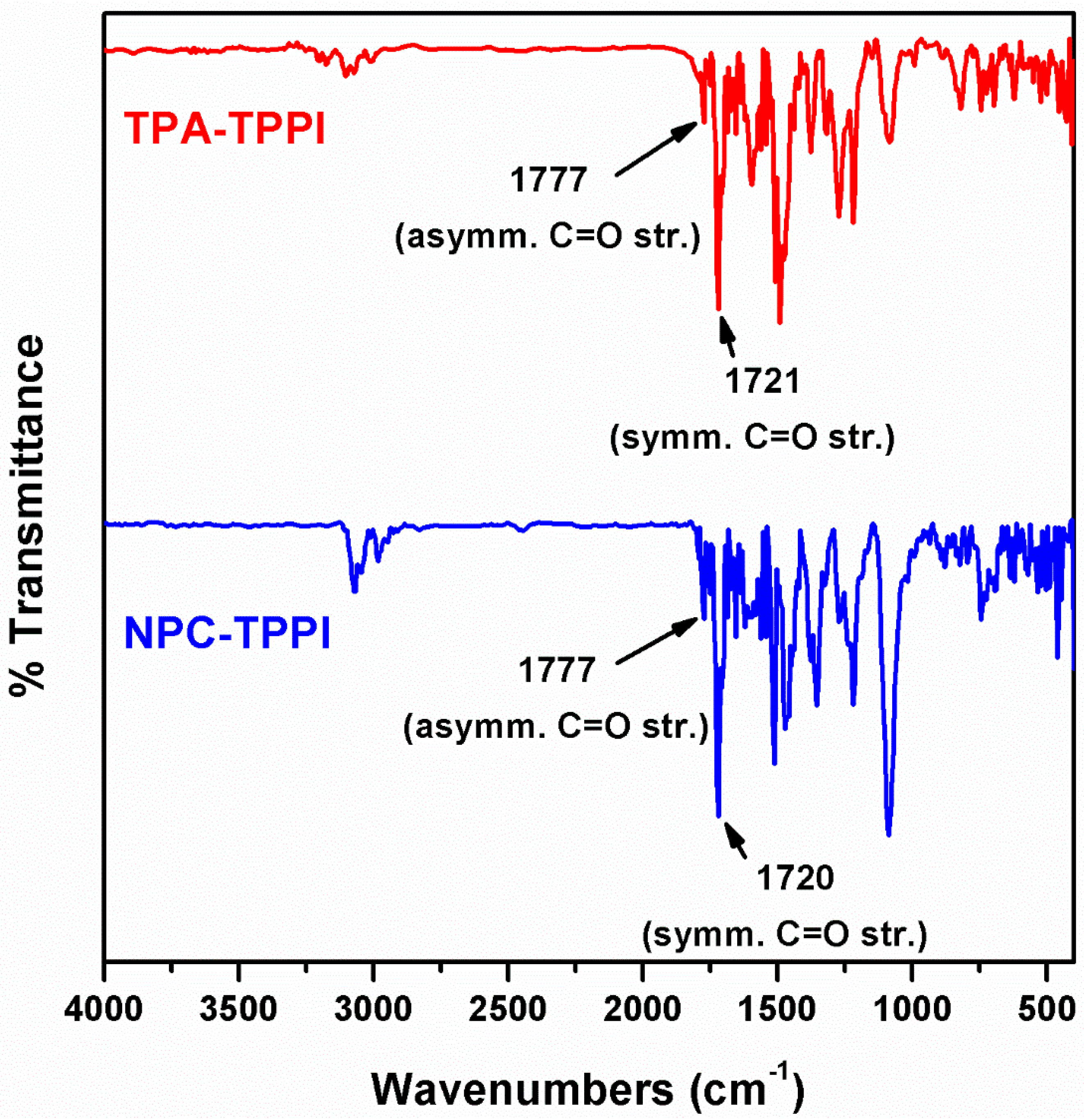
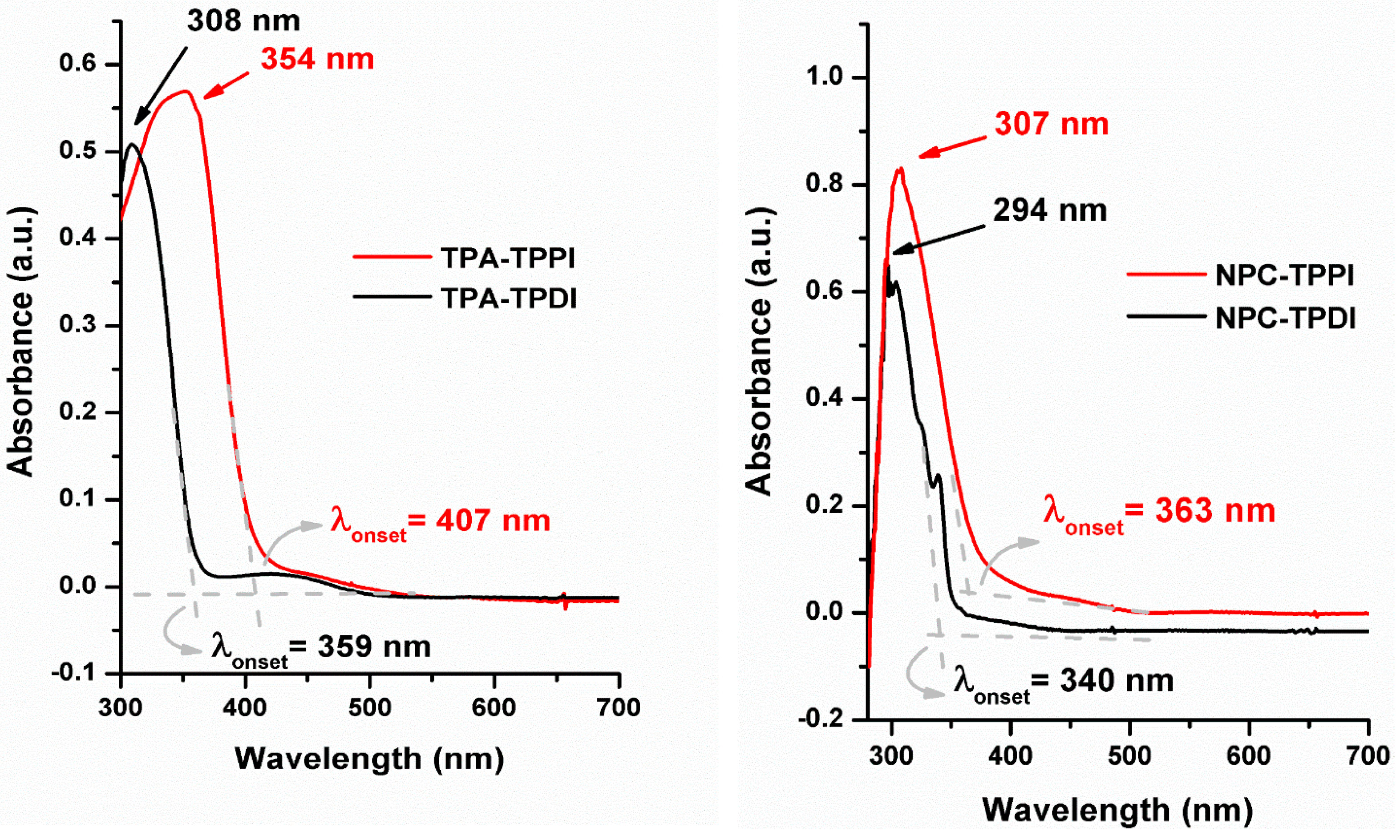
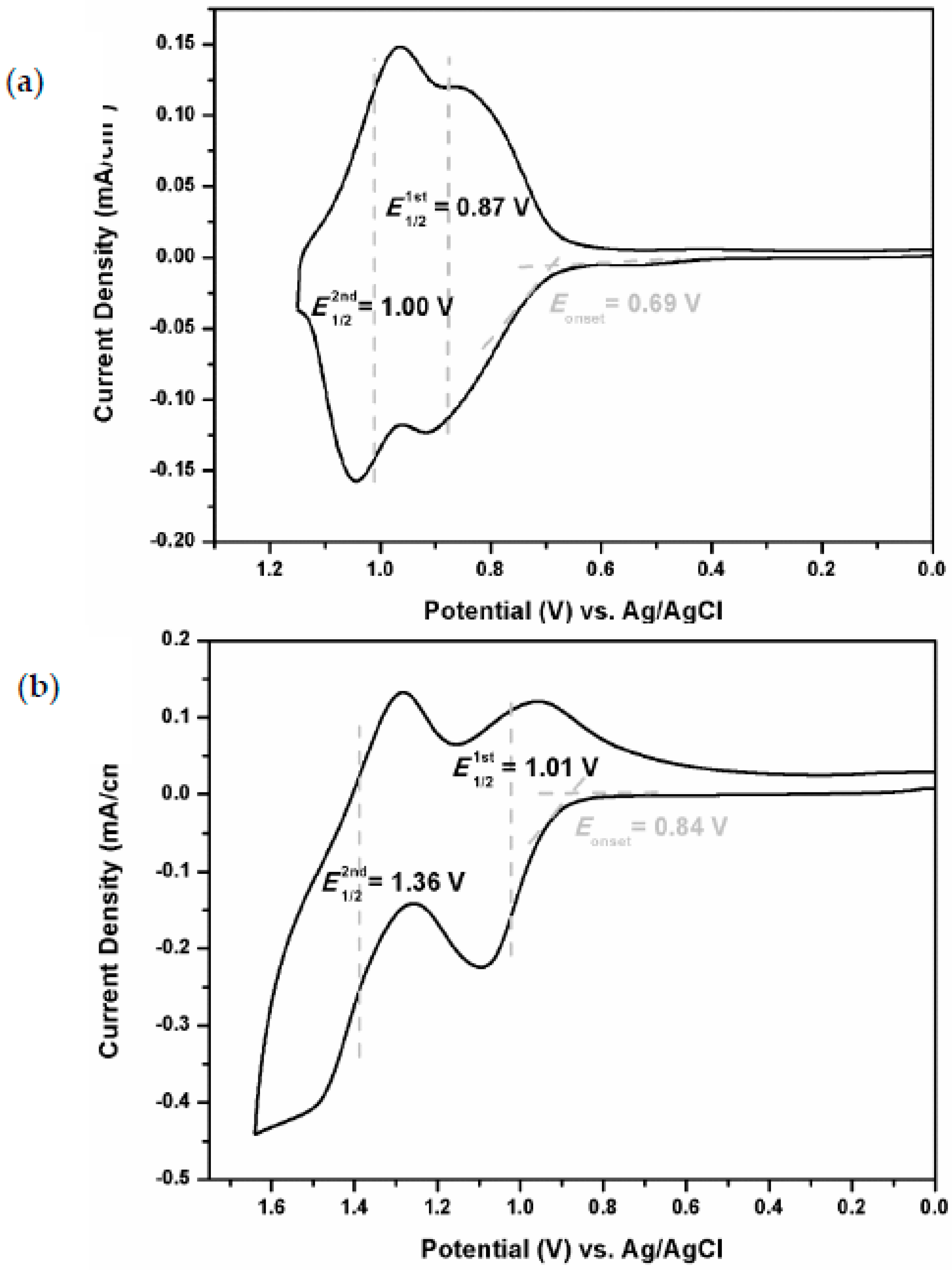

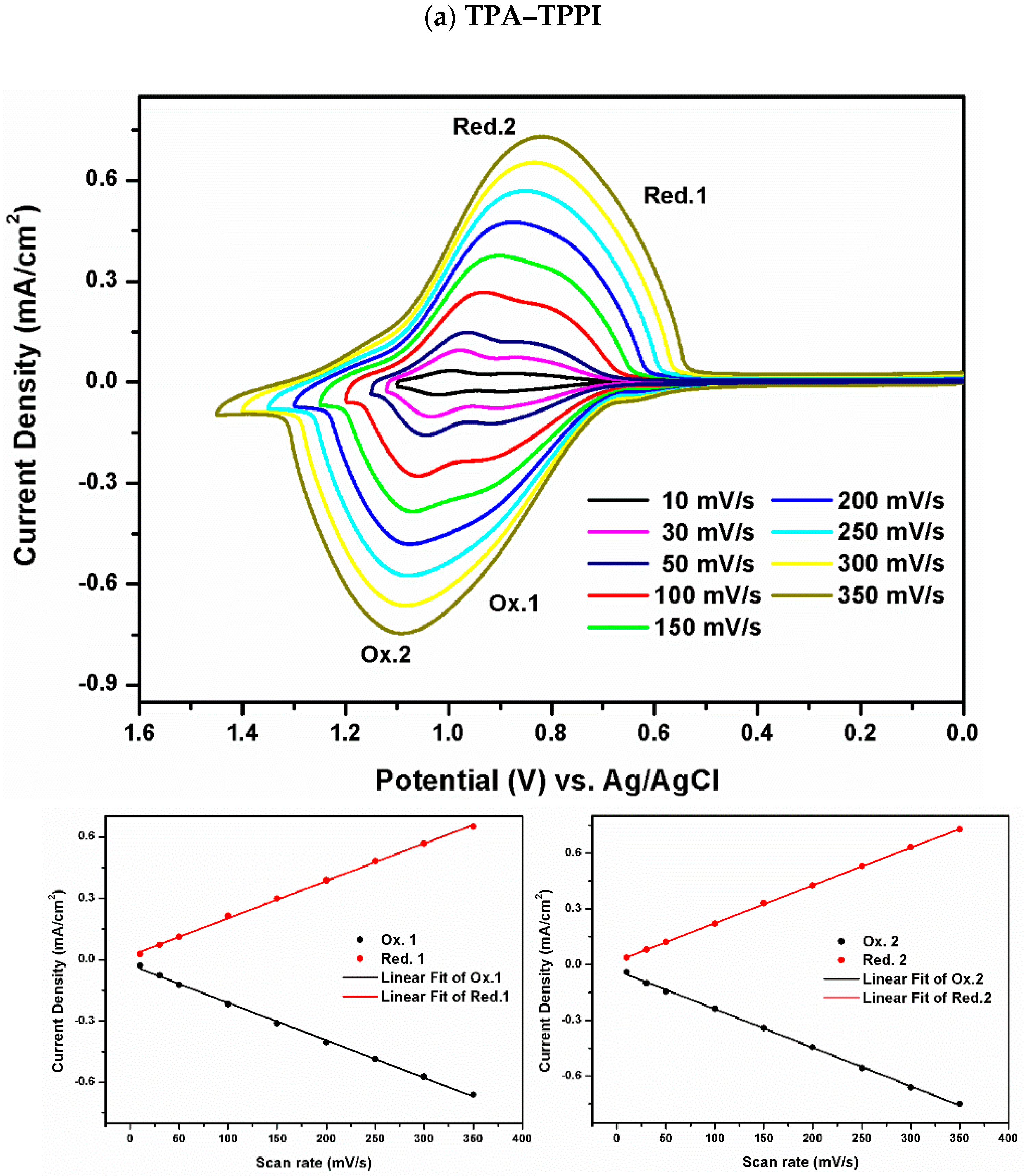
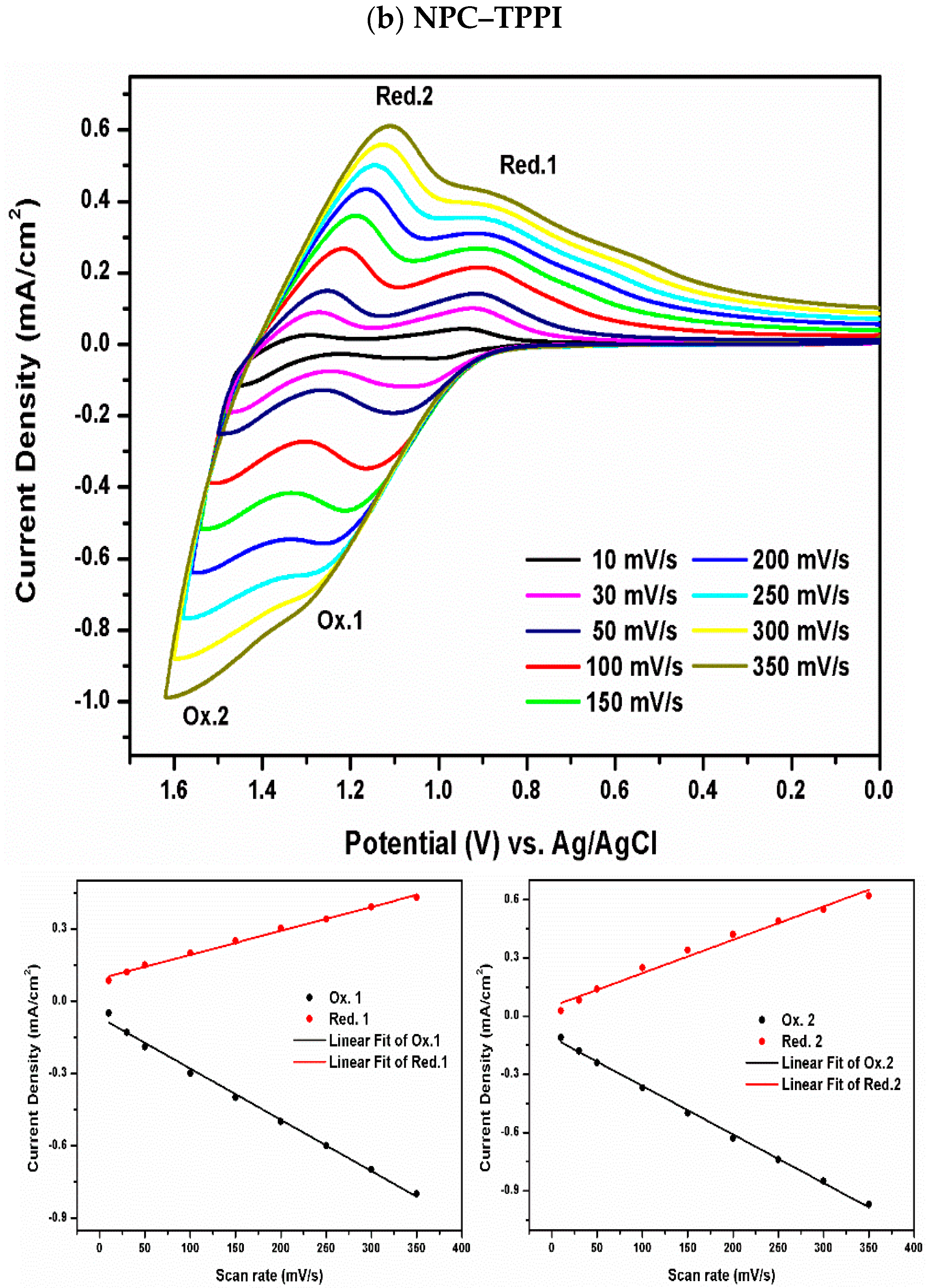
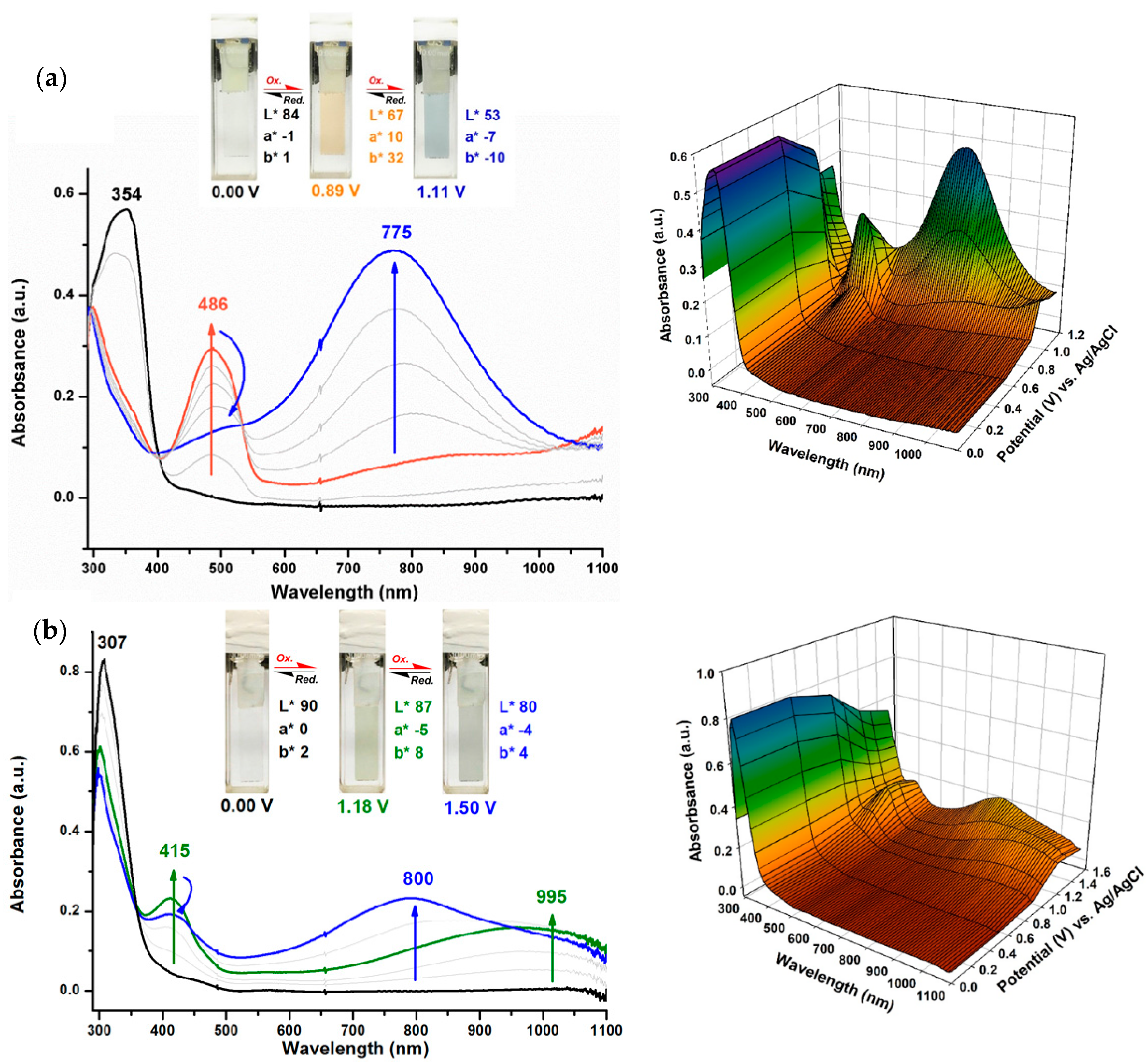


| Polymer | UV–Vis absorption (nm) a | Oxidation potential (V) b | Eg (eV) c | HOMO (eV) d | LUMO (eV) d | |||
|---|---|---|---|---|---|---|---|---|
| λmax | λonset | Eonest | E1/2Ox1 | E1/2Ox2 | ||||
| TPA–TPPI | 354 | 407 | 0.69 | 0.87 | 1.00 | 3.05 | 5.05 | 2.00 |
| NPC–TPPI | 307 | 363 | 0.84 | 1.01 | 1.36 | 3.42 | 5.20 | 1.78 |
| Polymer | λmax a (nm) | Δ%T | Response time b | ΔOD c | Qd d (mC cm−2) | CE e (cm2 C−1) | |
|---|---|---|---|---|---|---|---|
| tc (s) | tb (s) | ||||||
| TPA-TPPI | 486 | 30 | 11.8 | 1.2 | 0.17 | 1.62 | 105 |
| 775 | 50 | 8.8 | 2.1 | 0.31 | 2.62 | 118 | |
| NPC-TPPI | 415 | 30 | 6.7 | 5.0 | 0.21 | 2.68 | 78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Liao, Y.-C. Facile Synthesis of Electroactive and Electrochromic Triptycene Poly(ether-imide)s Containing Triarylamine Units via Oxidative Electro-Coupling. Polymers 2017, 9, 497. https://doi.org/10.3390/polym9100497
Hsiao S-H, Liao Y-C. Facile Synthesis of Electroactive and Electrochromic Triptycene Poly(ether-imide)s Containing Triarylamine Units via Oxidative Electro-Coupling. Polymers. 2017; 9(10):497. https://doi.org/10.3390/polym9100497
Chicago/Turabian StyleHsiao, Sheng-Huei, and Yu-Chuan Liao. 2017. "Facile Synthesis of Electroactive and Electrochromic Triptycene Poly(ether-imide)s Containing Triarylamine Units via Oxidative Electro-Coupling" Polymers 9, no. 10: 497. https://doi.org/10.3390/polym9100497
APA StyleHsiao, S.-H., & Liao, Y.-C. (2017). Facile Synthesis of Electroactive and Electrochromic Triptycene Poly(ether-imide)s Containing Triarylamine Units via Oxidative Electro-Coupling. Polymers, 9(10), 497. https://doi.org/10.3390/polym9100497