Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
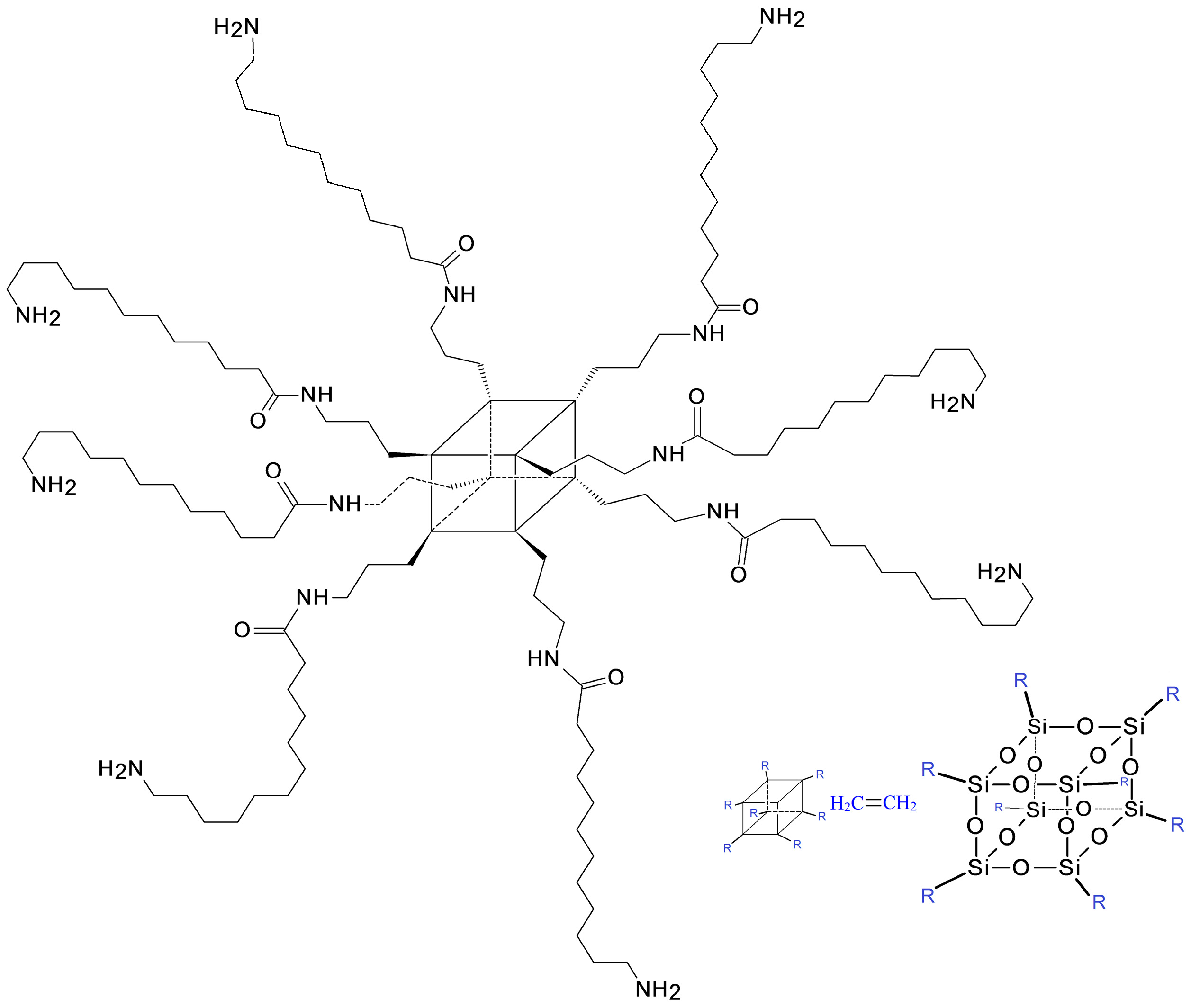
2.1.1. POSS Compound
2.1.2. Paperboard

2.1.3. Amine Masterbatches
2.2. Sample Preparation
2.2.1. Preparation of PE and PP Film Containing Iron/Stearyl Amine Proxidant
| Sample Mixture | Iron Masterbatch [%] | Amine Masterbatch [%] | Polyolefin [%] | Iron [ppm] | Amine [%] |
|---|---|---|---|---|---|
| PP-1 | A [2%] | 0 | PP [98%] | 72 | 0 |
| PP-2 | A [5%] | 0 | PP [5%] | 180 | 0 |
| PP-4 | A [2%] | D [10%] | PP [88%] | 72 | 1 |
| PP-8 | B [2%] | 0 | PP [98%] | 76 | 0 |
| PE-9 | B [2%] | 0 | PE [98%] | 76 | 0 |
| PE-12 | B [2%] | D [10%] | PE [98%] | 76 | 1 |
2.2.2. Preparation of PE and PE/Amino-POSS/FeSt3 Film
| Sample Code | PE (pellet) (wt%) | mb A (wt%) | mb C (wt%) | Composition | ||
|---|---|---|---|---|---|---|
| PE [%] | Fe [ppm] | amino-POSS [%] | ||||
| PE | 100 | 0 | 0 | 100 | 0 | 0 |
| 90505 | 90 | 5 | 5 | 99 | 180 | 0.5 |
| 85510 | 85 | 5 | 10 | 98.5 | 180 | 1 |
| 80515 | 80 | 5 | 15 | 98 | 180 | 1.5 |
2.2.3. Thermal Ageing of the Film
2.2.4. Coating on Paperboard
2.3. Characterization
2.3.1. Tensile Testing of Dumbbell Shape Samples and Respective Thermal Ageing
2.3.2. ATR-FTIR and Carbonyl Index (CI)
2.3.3. Adhesion Measurement
2.3.4. Melt Flow Index
2.3.5. Mechanical Properties
3. Results and Discussion
3.1. Polyolefin Samples with Iron Pro-Oxidant and Stearyl Amine Accelerator
Tensile Properties after Different Periods of Ageing
| Sample Mixture | 0 Days | 5 Days | 10 Days |
|---|---|---|---|
| PP-1 | 652 ± 113 | 210 ± 165 | 8 ± 5 |
| PP-2 | 536 ± 261 | 426 ± 367 | 13 ± 13 |
| PP-4 | 868 ± 84 | 270 ± 286 | <1 (extremely brittle) |
| PP-8 | 669 ± 242 | 720 ± 155 | 569 ± 291 |
| PE-9 | 614 ± 311 | 355 ± 361 | 610 ± 331 |
| PE-12 | 797 ± 103 | 765 ± 166 | 11 ± 10 |
| Sample Mixture | 0 Days | 5 Days | 10 Days |
|---|---|---|---|
| PP-1 | 27.1 ± 2.1 | 29.7 ± 0.7 | 22.2 ± 2.0 |
| PP-2 | 27.0 ± 1.2 | 27.3 ± 2.7 | 21.7 ± 5.1 |
| PP-4 | 33.5 ± 2.4 | 28.7 ± 2.6 | <2 (extremely brittle) |
| PP-8 | 30.3 ± 1.6 | 32.7 ± 3.1 | 28.5 ± 1.5 |
| PP-9 | 28.7 ± 3.6 | 30.1 ± 2.5 | 28.4 ± 1.3 |
| PP-12 | 29.7 ± 6.9 | 27.9 ± 7.7 | 12.7 ± 3.0 |
3.2. PE Samples with Iron Pro-Oxidant and Amin-POSS Accelerator
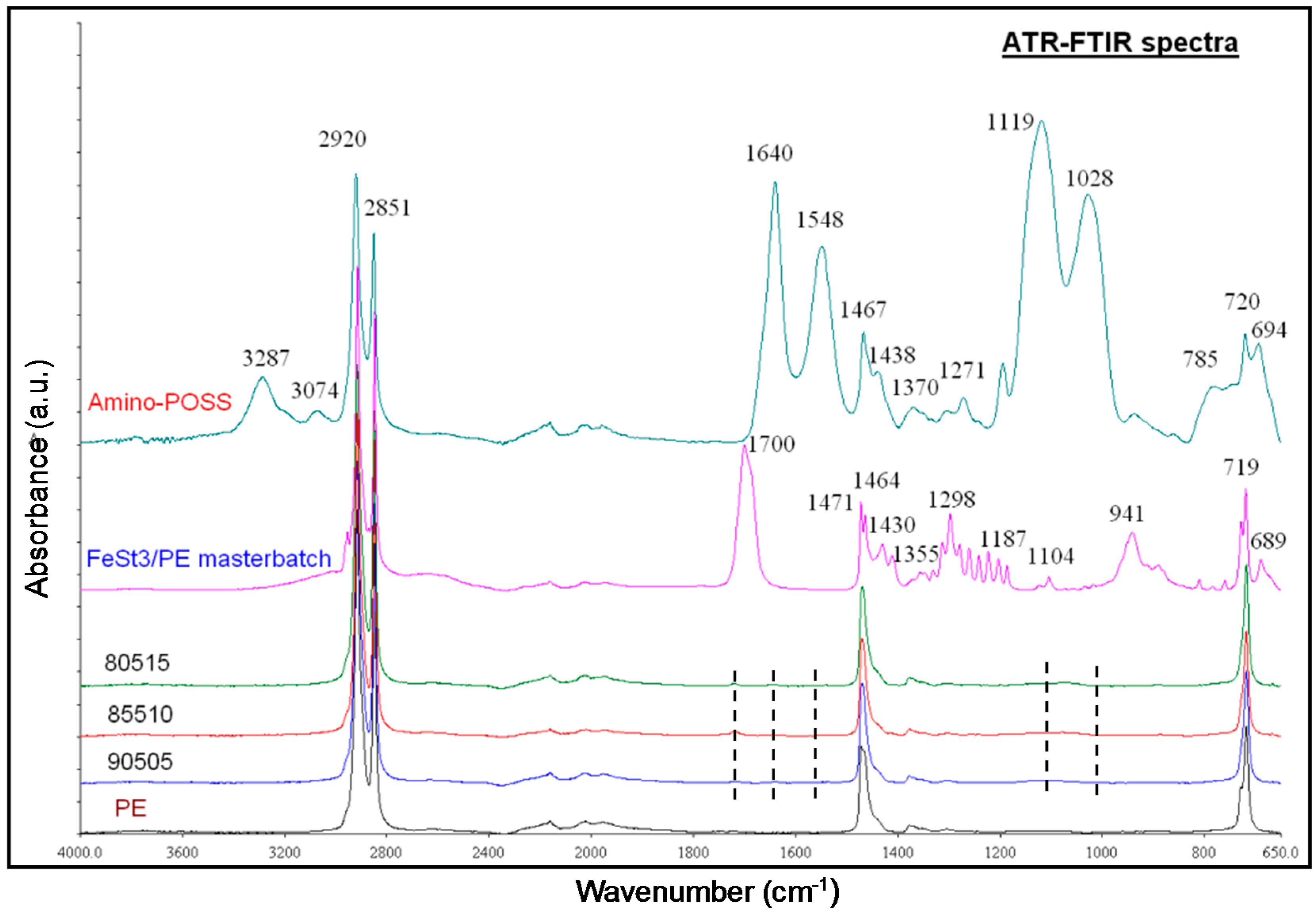
3.2.1. ATR-FTIR Studies
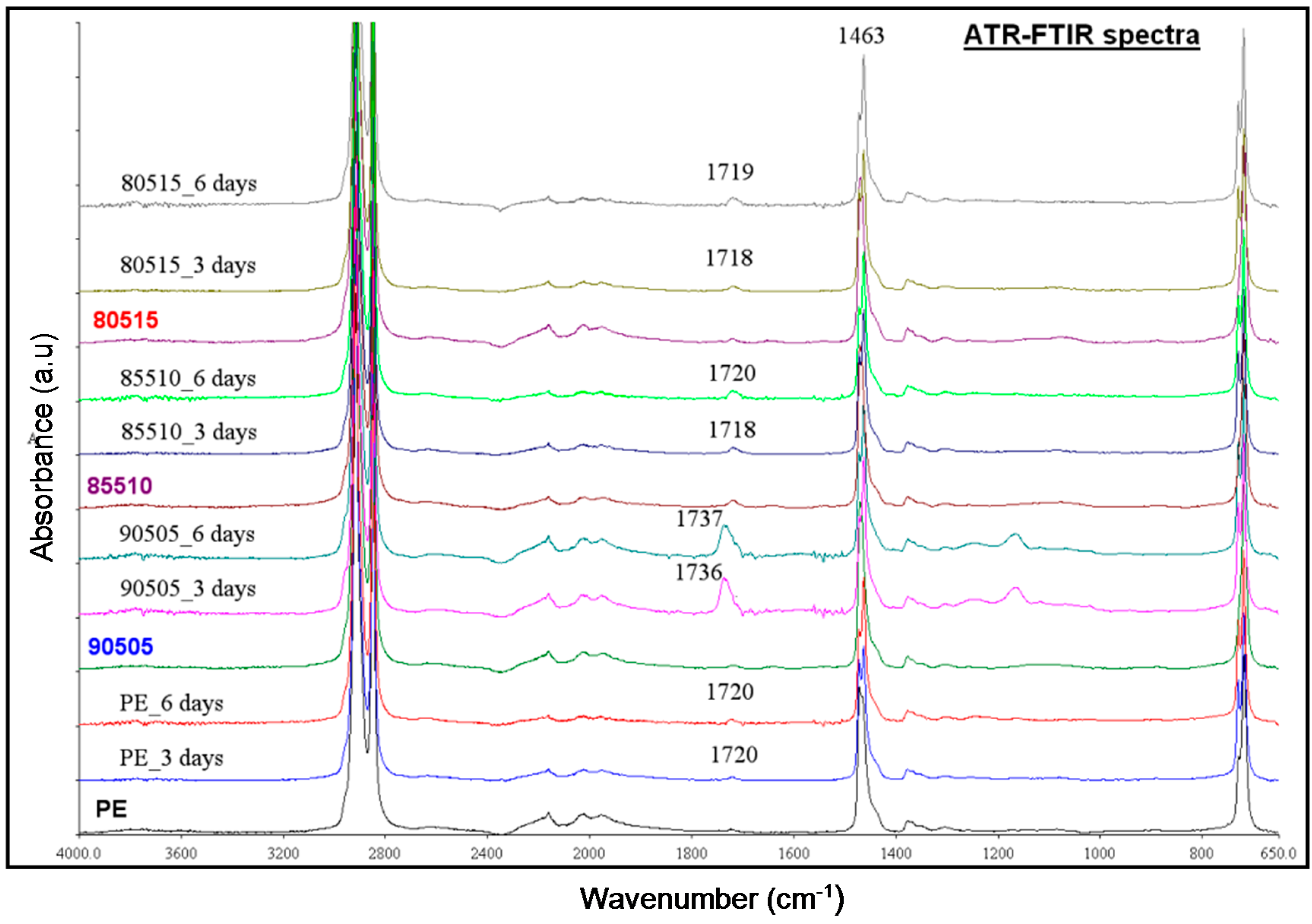
3.2.2. Thermal Ageing
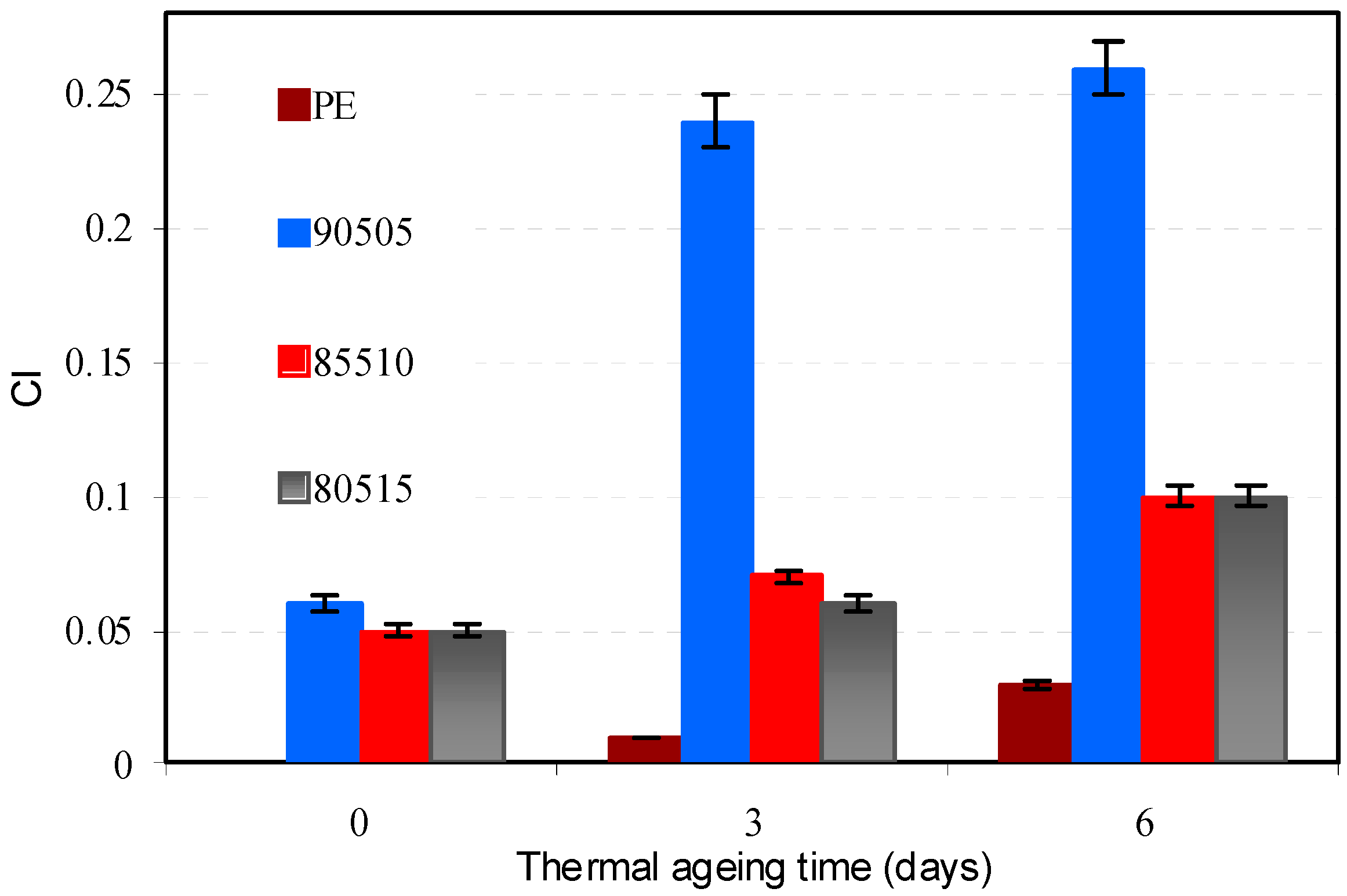
3.2.3. Melt Flow Index (MFI)
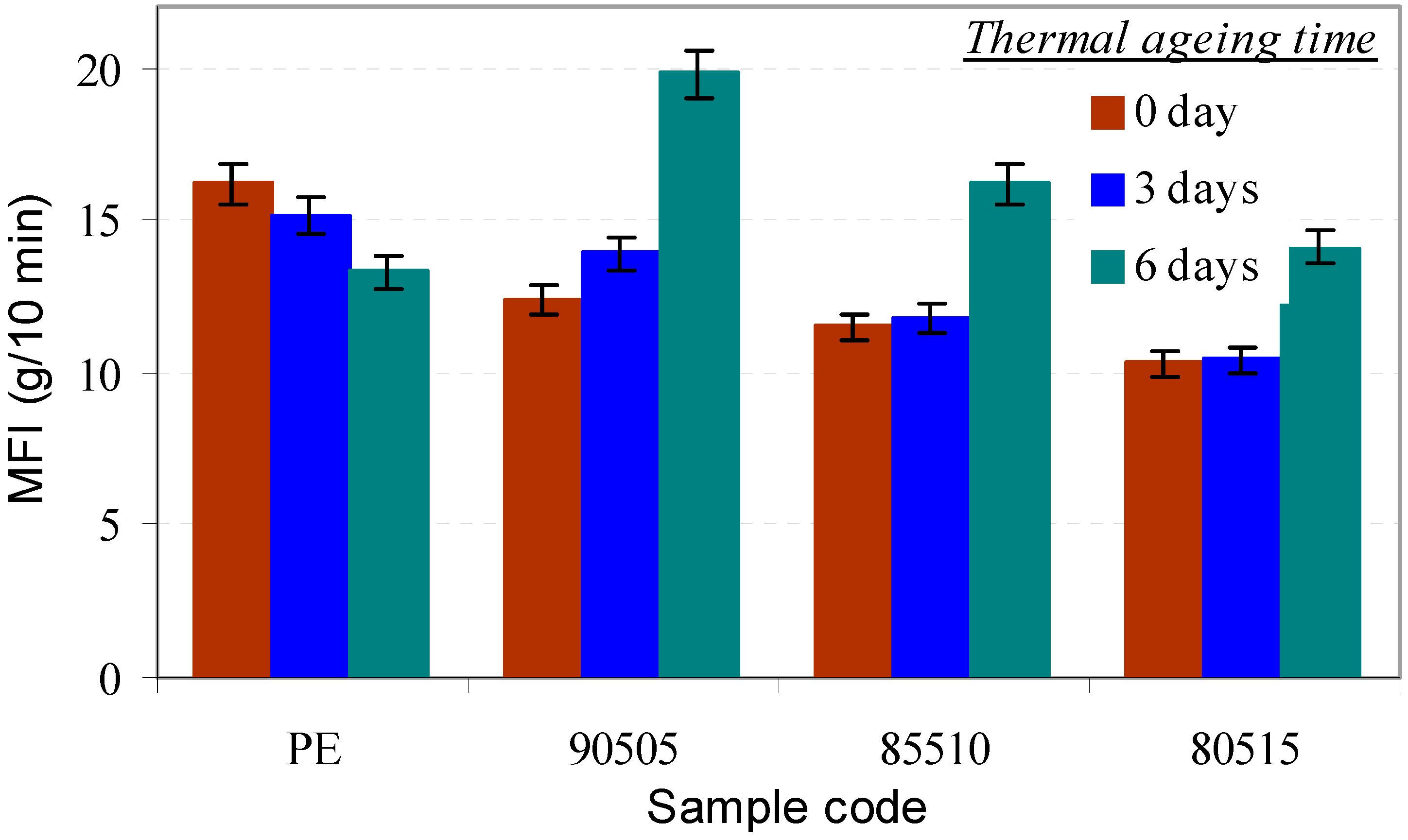
3.2.4. Mechanical Properties
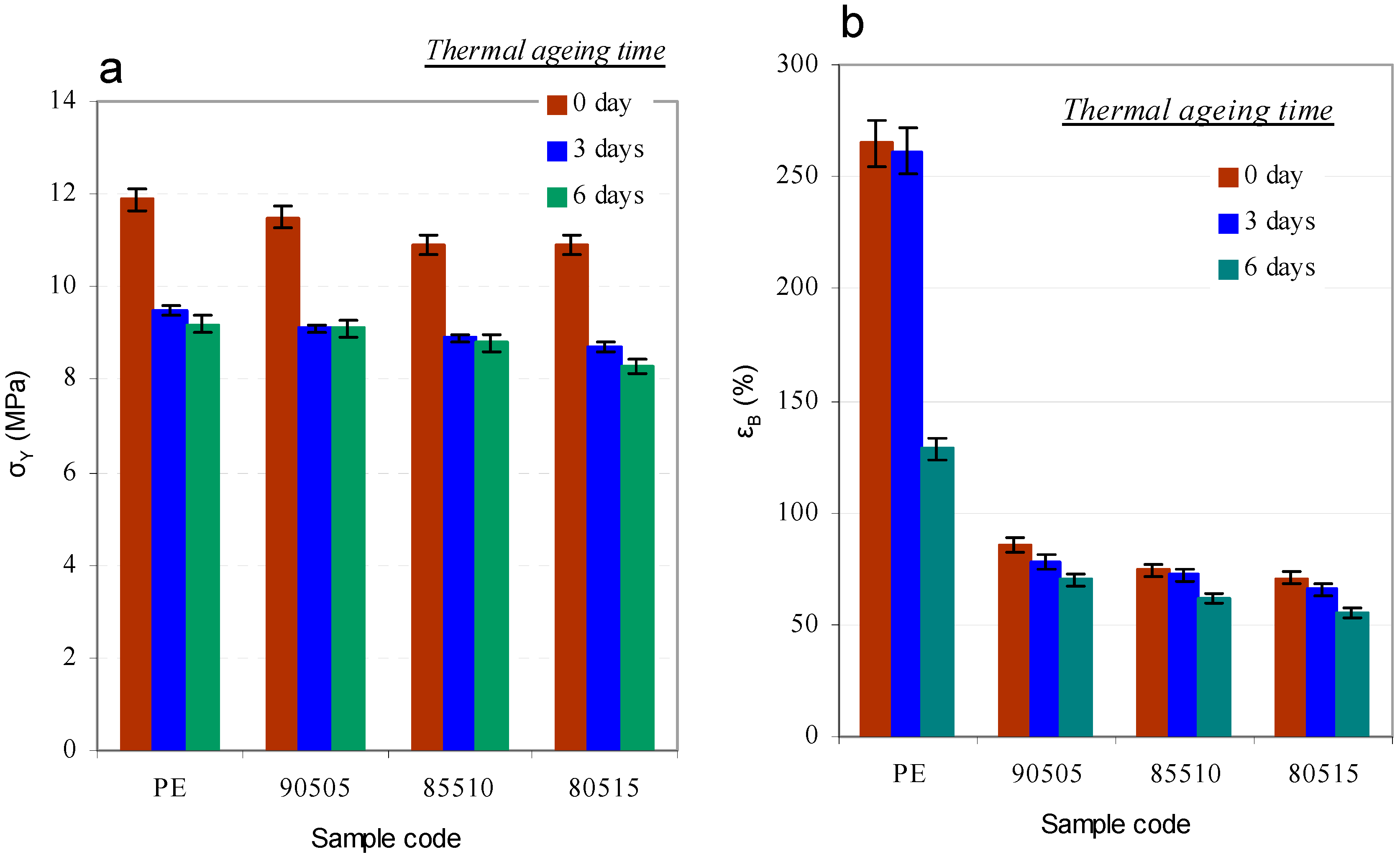
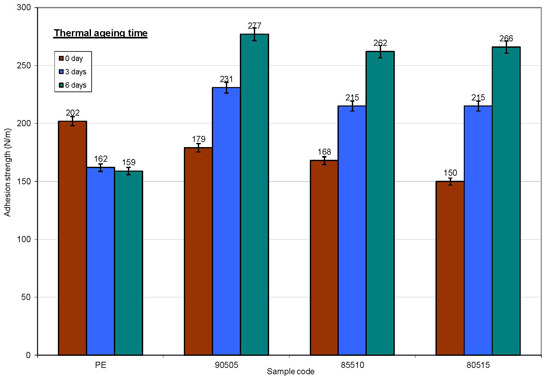
3.2.5. Adhesion to Paperboard
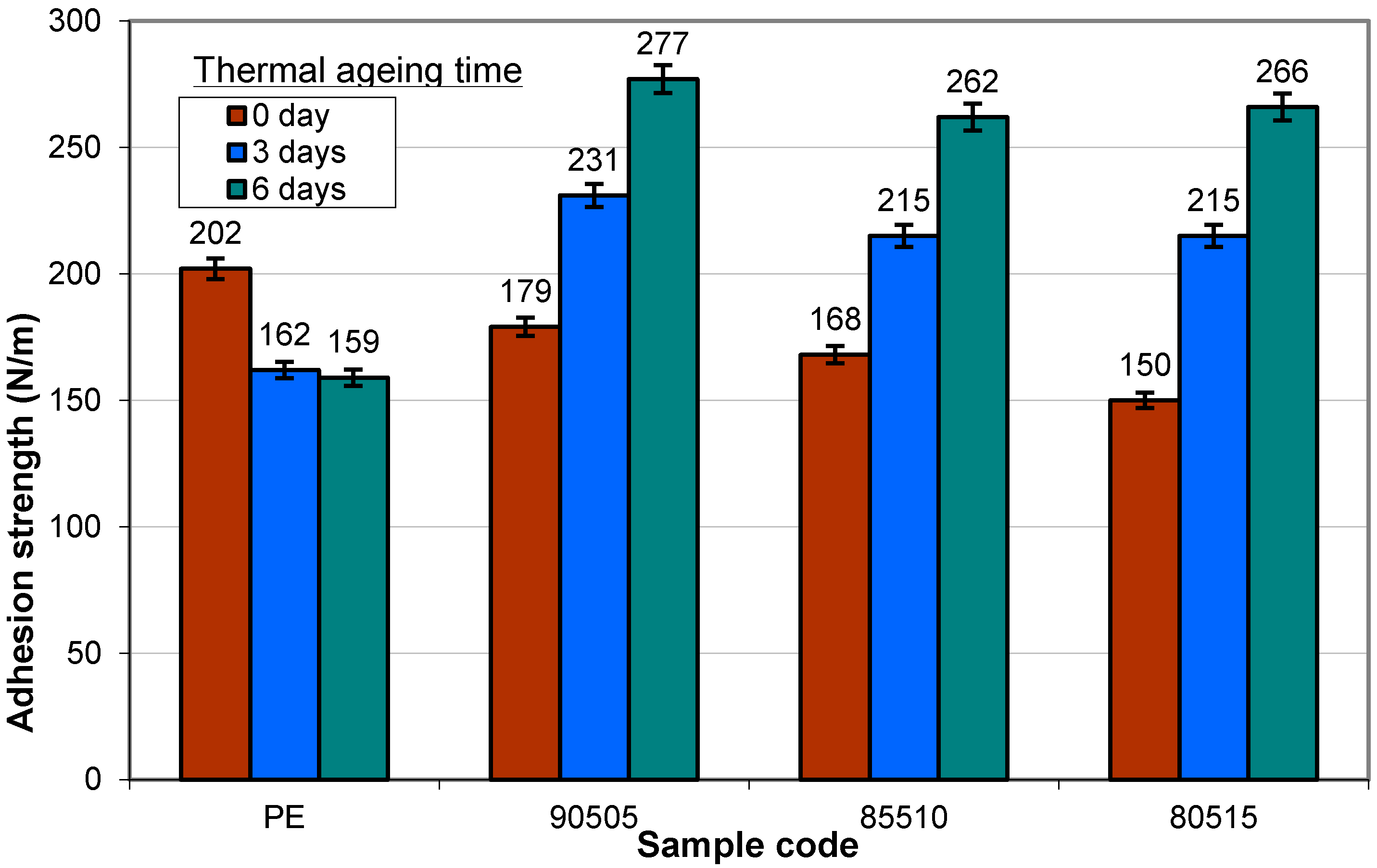
3.2.6. Proposed Mechanism for Oxidation of PE by Iron Pro-Oxidant and Amine Accelerator
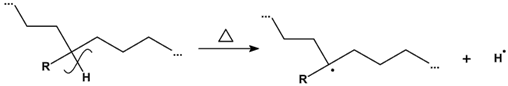
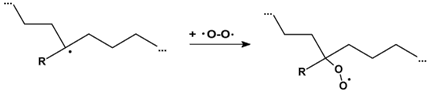
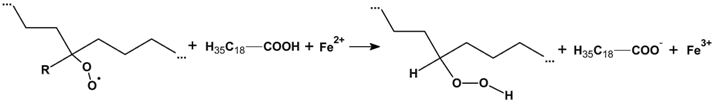
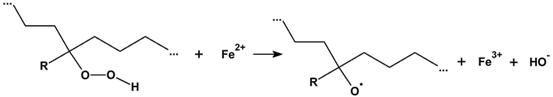
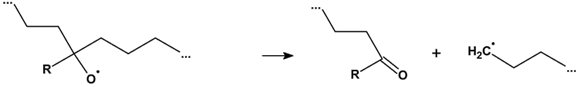
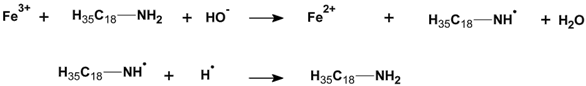
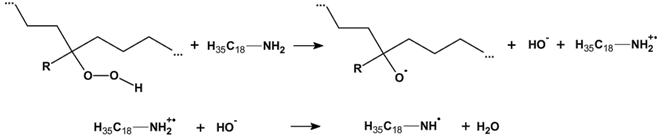
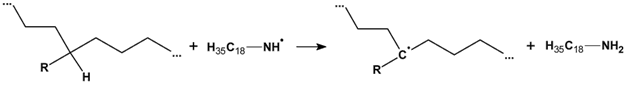
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Kemppi, A. Studies on the Adhesion between Paper and Low Density Polyethylene; Åbo Akademi University: Åbo, Finland, 1997; p. 39. [Google Scholar]
- Westerlind, B.; Larsson, A.; Rigdahl, M. Determination of the degree of adhesion in plasma-treated polyethylene/paper laminates. Int. J. Adhes. Adhes. 1987, 7, 141–146. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Rose, P.W. Plasma surface treatment of plastics to enhance adhesion. Int. J. Adhes. Adhes. 1991, 11, 109–113. [Google Scholar] [CrossRef]
- Nikos, K. Oxobiodegradable Polyolefins: Their Biodegradation and Recycling. Available online: http://eurasia12.uoi.gr/Abstracts_pdf/(4)%20Environmental%20and%20Green%20chemistry/S4%20ORAL/OP20_Abstract_Katsaros.pdf (accessed on 11 August 2015).
- Scott, G. Photo-biodegradable plastics: Their role in the protection of the environment. Polym. Degrad. Stable 1990, 29, 135–154. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Raman, R.; Rajagopal, C. Investigating the role of metal oxidation state on the degradation behaviour of LDPE. Polym. Degrad. Stable 2009, 94, 1033–1039. [Google Scholar] [CrossRef]
- Männle, F.; Jørgensen, J.K.; Tanem, B.S. Increased performance of thermoplastic packaging materials by using a mild oxidizing biobased additive. Int. J. Polym. Sci. 2012, 2012, 297923. [Google Scholar] [CrossRef]
- Sipinen, A.J.; Rutherford, D.R. A study of the oxidative degradation of polyolefins. J. Environ. Polym. Degr. 1993, 3, 193–202. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Nozaki, K. The kinetics of decomposition of benzoyl peroxide in solvents. J. Am. Chem. Soc. 1946, 68, 1686–1692. [Google Scholar]
- Tobolsky, A.V.; Mesrobian, R.B. Organic Peroxides—Their Chemistry, Decomposition and Role in Polymerization; Interscience Publishers, Inc.: New York, NY, USA, 1954; p. 55. [Google Scholar]
- Salem, I.A. Role of aliphatic diamine ligands in hydrogen peroxide decomposition with Dowex-50W resin as transition metal complex ions. J. Mol. Catal. 1993, 80, 11–19. [Google Scholar] [CrossRef]
- Eyler, G.N.; Canizo, A.I.; Alvarez, E.E. Thermal decomposition of cyclic organic peroxides in aliphatic amines solution. Afinidad Barcelona 2007, 64, 538–542. [Google Scholar]
- Tidjani, A. Comparison of formation of oxidation products during photo-oxidation of linear low density polyethylene under different natural and accelerated weathering conditions. Polym. Degrad. Stabil. 2000, 68, 465–469. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; Swift, G. Biodegradation of thermally-oxidized, fragmented low-density polyethylenes. Polym. Degrad. Stabil. 2003, 81, 341–351. [Google Scholar] [CrossRef]
- Koutny, M.; Sancelme, M.; Dabin, C.; Pichon, N.; Delort, A.-M.; Lemaire, J. Acquired biodegradability of polyethylene containing pro-oxidant additives. Polym. Degrad. Stabil. 2006, 9, 1495–1503. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Baciu, R. Oxo-biodegradable carbon backbone polymers—Oxidative degradation of polyethylene under accelerated test conditions. Polym. Degrad. Stabil. 2006, 91, 2739–2747. [Google Scholar] [CrossRef]
- Kumanayaka, T.O.; Parthasarathy, R.; Jollands, M. Accelerating effect of montmorillonite on oxidative degradation of polyethylene nanocomposites. Polym. Degrad. Stabil. 2010, 95, 672–676. [Google Scholar] [CrossRef]
- Corti, A.; Muniyasamy, S.; Vitali, M.; Imam, S.H.; Chiellini, E. Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: Synergistic effects of sunlight exposure, thermal ageing and fungal biodegradation. Polym. Degrad. Stabil. 2010, 95, 1106–1114. [Google Scholar] [CrossRef]
- Yashchuk, O.; Portillo, F.S.; Hermida, E.B. Degradation of polyethylene film samples containing oxo-degradable additives. Procedia Mater. Sci. 2012, 1, 439–445. [Google Scholar] [CrossRef]
- Briggs, D.; Brewis, D.M.; Konieczko, M.B. X-ray photoelectron spectroscopy studies of polymer surfaces. J. Mater. Sci. 1979, 14, 1344–1348. [Google Scholar] [CrossRef]
- Junnila, J.; Savolainen, A.; Forsberg, D. Adhesion Improvements between Paper and Polyethylene by Pre-Treatment of Substrate. In Polymers, Laminations and Coatings Conference, Orlando, FL, USA, 5–8 September 1989; pp. 353–360.
- Savolainen, A.; Kuusipalo, J. The Optimization of Corona and Flame Pre-Treatment in Multilayer Coating. In Proceedings from the 1991 Tappi Extrusion Coating Short Course, Düsseldorf, Germany; 1991; pp. 897–904. [Google Scholar]
- Li, G.Z.; Wang, L.C.; Ni, H.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Markovic, E.; Constantopolous, K.; Matisons, J.G. Polyhedral Oligomeric Silsesquioxanes: From Early and Strategic Development through to Materials Application. In Application of Polyhedral Ologomeric Silsequioxanes; Hartmann-Thompson, C., Ed.; Advances in Silicon Science; Springer Netherlands: Dordrecht, The Netherlands, 2011; Volume 3, pp. 1–46. [Google Scholar]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene-polyhedral oligomeric silsesquioxanes (POSS) nanocomposites. Polymer 2005, 46, 7855–7866. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene–polysilsesquioxane blends. Eur. Polym. J. 2010, 46, 14–23. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Simon, G.; Kukaleva, N. Rheological and viscoelastic behavior of HDPE/octamethyl-POSS nanocomposites. Macromolecules 2006, 39, 1839–1849. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S. Isothermal crystallization of HDPE/octamethyl polyhedral oligomeric silsesquioxane nanocomposites: role of POSS as a nanofiller. J. Appl. Polym. Sci. 2007, 105, 978–985. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, Y.; Yin, N. Rheological behavior of polypropylene/octavinyl polyhedral oligomeric silsesquioxane composites. J. Polym. Sci. Polym. Phys. 2008, 46, 526–533. [Google Scholar] [CrossRef]
- Misra, R.; Fu, B.X.; Morgan, S.E. Surface energetics, dispersion and nanotribomechanical behavior of POSS/PP hybrid nanocomposites. J. Polym. Sci. Polym. Phys. 2007, 45, 2441–2455. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.; Schiraldi, D.A.; Illescas, S. Study of the morphology and properties of melt-mixed polycarbonate–POSS nanocomposites. Eur. Polym. J. 2009, 45, 341–352. [Google Scholar] [CrossRef]
- Bourbigot, S.; Turf, T.; Bellayer, S.; Duquesne, S. Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym. Degrad. Stabil. 2009, 94, 1230–1237. [Google Scholar] [CrossRef]
- Lim, S.K.; Hong, E.P.; Choi, H.; Chin, I.J. Polyhedral oligomeric silsesquioxane and polyethylene nanocomposites and their physical characteristics. J. Ind. Eng. Chem. 2010, 16, 189–192. [Google Scholar] [CrossRef]
- Nguyen, T.-A.; Männle, F.; Gregersen, Ø.W. Polyethylene/octa-(ethyl octadeca-10,13-dienoamide) silsesquioxane blends and the adhesion strength to paperboard. Int. J. Adhes. Adhes. 2012, 38, 117–124. [Google Scholar] [CrossRef]
- Männle, F.; Tofteberg, T.R.; Skaugen, M.; Bu, H.; Peters, T.; Dietzel, P.D.C.; Pilz, M. Polymer nanocomposite coatings based on polyhedral oligosilsesquioxanes: Route for industrial manufacturing and barrier propreties. J. Nanopart. Res. 2011, 13, 4691–4701. [Google Scholar] [CrossRef]
- Rao, C.N.R. Chemical Applications of Infrared Spectroscopy; Academic Press: Waltham, MA, USA, 1963; pp. 125–263. [Google Scholar]
- Abrahamson, H.B.; Lukaski, H.C. Synthesis and characterization of iron stearate compounds. J. Inorg. Biochem. 1994, 54, 115–130. [Google Scholar] [CrossRef]
- Zweifel, H. Plastic Additives Handbook, 5th ed.; Hanser Gardner Publications: Munich, Germany, 2001; pp. 8–9. [Google Scholar]
- Eyenga, I.I.; Focke, W.W.; Prinsloo, L.C.; Tolmay, A.T. Photodegradation: A solution for the shopping bag “visual pollution” problem? Macromol. Symp. 2002, 178, 139–152. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-A.; Gregersen, Ø.W.; Männle, F. Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard. Polymers 2015, 7, 1522-1540. https://doi.org/10.3390/polym7081468
Nguyen T-A, Gregersen ØW, Männle F. Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard. Polymers. 2015; 7(8):1522-1540. https://doi.org/10.3390/polym7081468
Chicago/Turabian StyleNguyen, Tuan-Anh, Øyvind Weiby Gregersen, and Ferdinand Männle. 2015. "Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard" Polymers 7, no. 8: 1522-1540. https://doi.org/10.3390/polym7081468
APA StyleNguyen, T.-A., Gregersen, Ø. W., & Männle, F. (2015). Thermal Oxidation of Polyolefins by Mild Pro-Oxidant Additives Based on Iron Carboxylates and Lipophilic Amines: Degradability in the Absence of Light and Effect on the Adhesion to Paperboard. Polymers, 7(8), 1522-1540. https://doi.org/10.3390/polym7081468