Transparent Heat-Resistant PMMA Copolymers for Packing Light-Emitting Diode Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PMMA-co-PIBMA and PMMA-co-PMAA-co-PIBMA Copolymer
2.3. Characterization
3. Results and Discussion
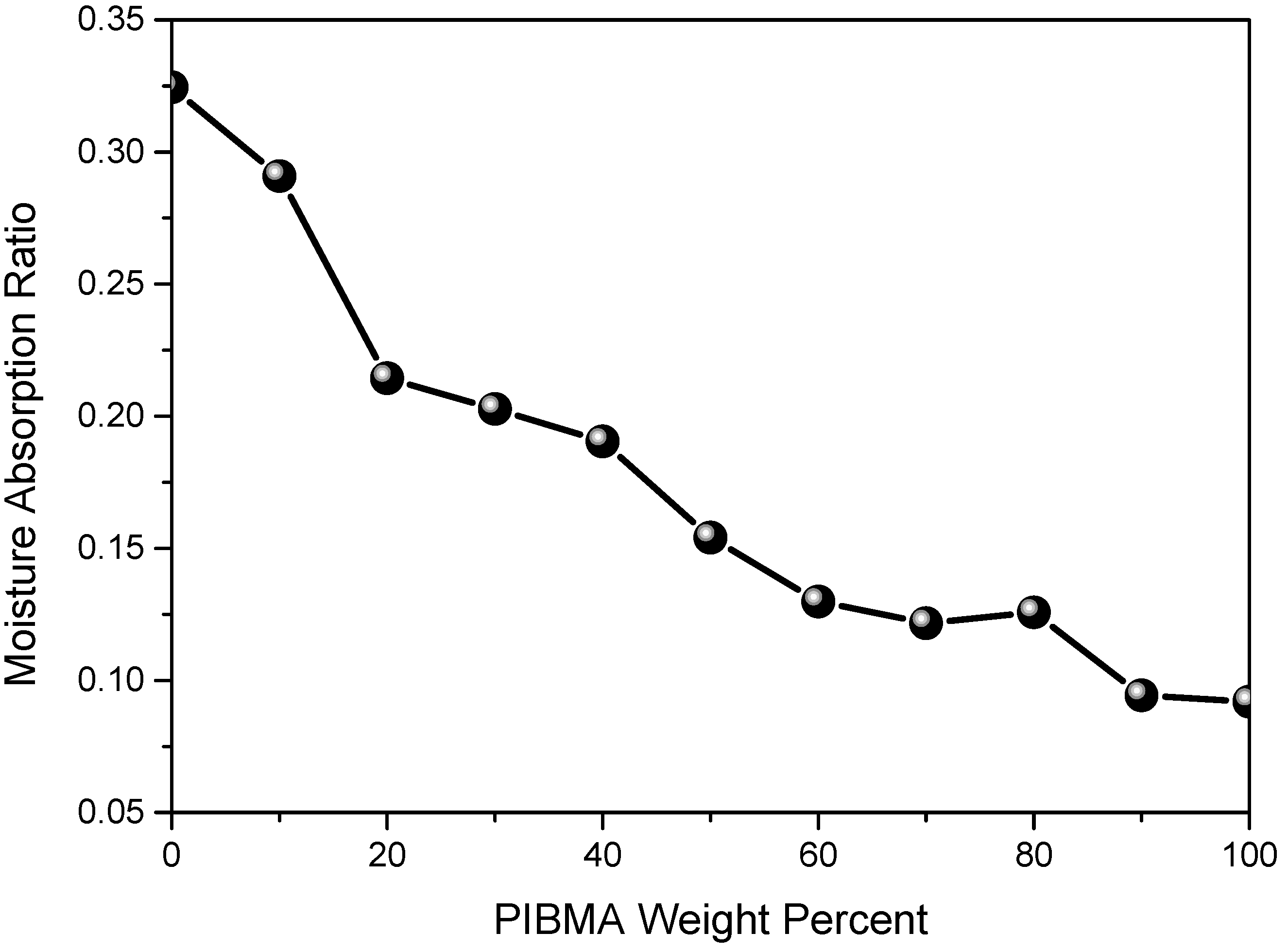
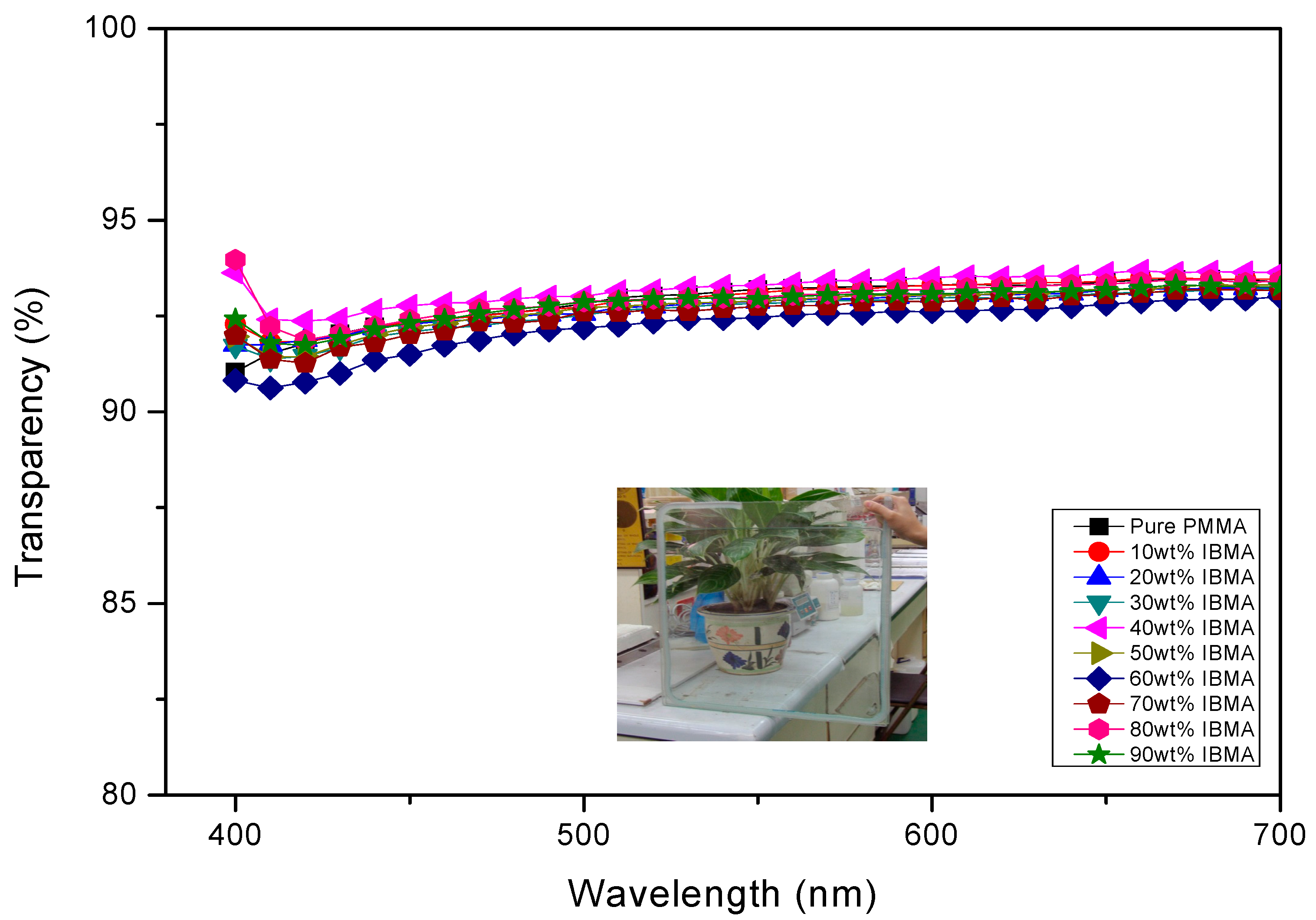
| Copolymers a | MMA (wt%) | IBMA (wt%) | Moisture absorption (%) | Transmission (400–700 nm) | Yellow index | Mnb (×104) | PDI b |
|---|---|---|---|---|---|---|---|
| PMMA | 100 | 0 | 0.32 | 92.6 | 0.98 | 34.6 | 1.20 |
| PMMA-PIBMA10 | 90 | 10 | 0.29 | 92.9 | 1.03 | 30.6 | 1.25 |
| PMMA-PIBMA20 | 80 | 20 | 0.21 | 92.4 | 0.74 | 29.3 | 1.26 |
| PMMA-PIBMA30 | 70 | 30 | 0.24 | 91.8 | 0.95 | 34.5 | 1.23 |
| PMMA-PIBMA50 | 50 | 50 | 0.15 | 92.4 | 0.84 | 29.5 | 1.22 |
| PMMA-PIBMA60 | 40 | 60 | 0.13 | 92.2 | 1.05 | 34.4 | 1.24 |
| PMMA-PIBMA70 | 30 | 70 | 0.12 | 92.1 | 0.86 | 33.0 | 1.28 |
| PMMA-PIBMA80 | 20 | 80 | 0.13 | 93.1 | 0.71 | 29.0 | 1.25 |
| PMMA-PIBMA90 | 10 | 90 | 0.09 | 92.1 | 0.93 | 29.8 | 1.24 |
| PIBMA | 0 | 100 | 0.08 | 92.3 | 0.92 | 28.1 | 1.24 |
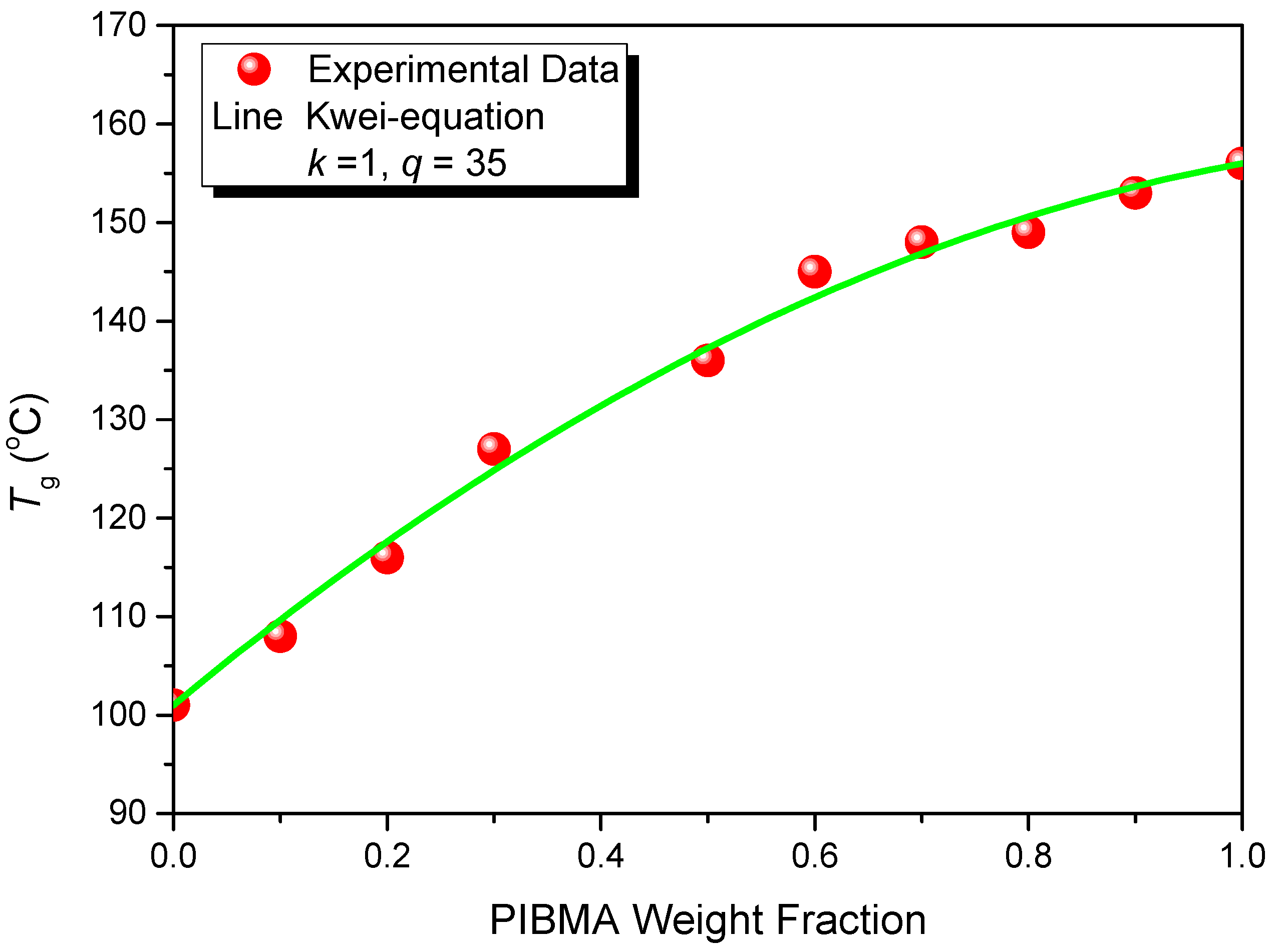
| Copolymers | MMA (wt%) | MAA (wt%) | IBMA (wt%) | Mw (×104) | Mn (×104) | PDI | Tg (°C) |
|---|---|---|---|---|---|---|---|
| PMMA-PMAA | 92 | 8 | 0 | 17.8 | 11.8 | 1.50 | 107 |
| PMMA-PMAA-PIBMA30 | 62 | 8 | 30 | 18.4 | 12.1 | 1.52 | 128 |
| PMMA-PMAA-PIBMA35 | 57 | 8 | 35 | 16.7 | 10.9 | 1.53 | 138 |
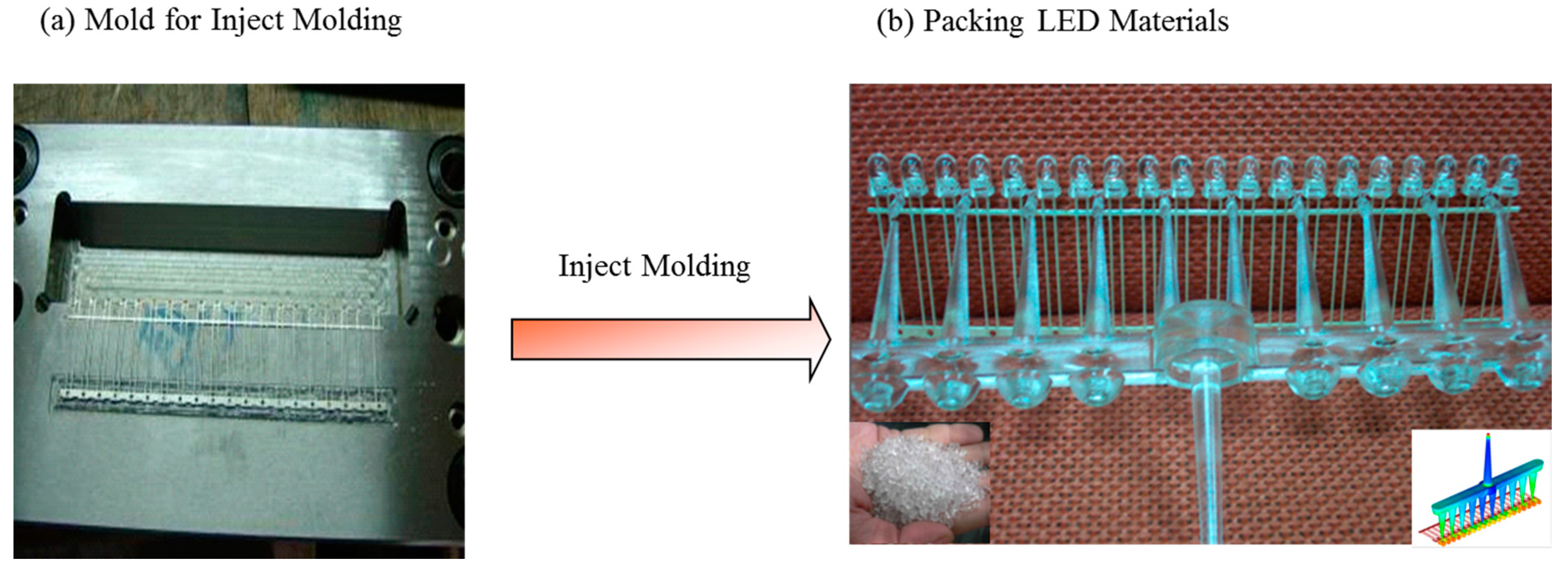
4. Conclusions
Acknowledgments
Author Contributions
References
- Otsu, T.; Motsumoto, T. Reactivity in radical polymerization of N-substituted maleimides and thermal stability of the resulting polymers. Polym. Bull. 1990, 23, 43–50. [Google Scholar] [CrossRef]
- Kim, G.A. PMMA composite as an optical diffuser in a liquid crystal display backlighting unit (BLU). Eur. Polym. J. 2005, 41, 1729–1737. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, Y.K.; Sohn, S.H. Optical properties of neodymium-containing polymethylmethacrylate films for the organic light emitting diode color filter. Appl. Phys. Lett. 2006, 89, 051102. [Google Scholar] [CrossRef]
- Braun, D.; Czerwinski, W.K. Kinetische analyse der copolymerisationsgeschwindigkeit von N-vinyl-2-pyrrolidon mit styrol und methylmethacrylat. Die Makromol. Chemie 1987, 88, 2389–2401. (In Germany) [Google Scholar] [CrossRef]
- Yuichi, K. Synthesis of N-cyclohexylmaleimide for heat-resistant transparent methacrylic resin. J. Appl. Polym. Sci. 1997, 63, 363–368. [Google Scholar]
- Syronmyatnikov, V.G.; Paskal, L.P.; Savchenko, I.A. Aryl (meth)acrylates and polymers based on them. Russ. Chem. Rev. 1999, 68, 781–799. [Google Scholar] [CrossRef]
- Teng, H.; Koike, K.; Zhou, D.; Satoh, Z.; Koike, Y.; Okamoto, Y. High glass transition temperatures of poly(methyl methacrylate) prepared by free radical initiators. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 315–317. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Effect of copolymer composition on the miscibility of poly(styrene-co-acetoxystyrene) with phenolic resin. Polymer 2001, 42, 9843–9848. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Effects of copolymer composition and free volume change on the miscibility of poly(styrene-co-vinylphenol) with poly(ε-caprolactone). Macromolecules 2001, 34, 7737–7743. [Google Scholar] [CrossRef]
- Li, G.; Cowie, J.M.G.; Arrighi, V. Miscibility of polymer blends of poly(styrene-co-4-hydroxystyrene) with bisphenol-A polycarbonate. J. Appl. Polym. Sci. 1999, 74, 639–646. [Google Scholar] [CrossRef]
- Mishra, A.; Sinha, T.M.J.; Choudhary, V. Methyl methacrylate–N-chlorophenyl maleimide copolymers: Effect of structure on properties. J. Appl. Polym. Sci. 1998, 68, 527–534. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Q.; Wei, Y.; Zhang, Z. Study on the synthesis of heat-resistant PMMA. J. Appl. Polym. Sci. 1999, 72, 1335–1339. [Google Scholar]
- Tagaya, A.; Harada, T.; Koike, K.; Koike, Y.; Okamoto, Y.; Teng, H.; Yang, L. Improvement of the physical properties of poly(methyl methacrylate) by copolymerization with pentafluorophenyl methacrylate. J. Appl. Polym. Sci. 2007, 106, 4219–4224. [Google Scholar] [CrossRef]
- Xu, U.; Graf, J.; Painter, P.C.; Coleman, M.M. Miscibility windows for poly(styrene-co-vinyl phenol) blends with poly(n-butyl methacrylate) and poly(n-hexyl methacrylate): A comparison of theoretical predictions with Fourier transform infra-red experimental data. Polymer 1991, 32, 3103–3118. [Google Scholar] [CrossRef]
- Lin, C.T.; Kuo, S.W.; Huang, C.F.; Chang, F.C. Glass transition temperature enhancement of PMMA through copolymerization with PMAAM and PTCM mediated by hydrogen bonding. Polymer 2010, 51, 883–889. [Google Scholar] [CrossRef]
- Kuo, S.W.; Kao, H.C.; Chang, F.C. Thermal behavior and specific interaction in high glass transition temperature PMMA copolymer. Polymer 2003, 44, 6873–6882. [Google Scholar] [CrossRef]
- Chen, J.K.; Kuo, S.W.; Kao, H.C.; Chang, F.C. Thermal properties, specific interactions, and surface energies of PMMA terpolymers having high glass transition temperatures and low moisture absorptions. Polymer 2005, 46, 2354–2364. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tsai, H.T. Complementary multiple hydrogen-bonding interactions increase the glass transition temperatures to PMMA copolymer mixtures. Macromolecules 2009, 42, 4701–4711. [Google Scholar] [CrossRef]
- Maria de, F.A.; vanessa de, F.L.; Vânya, M.D.P.; Cíntia, G.F. Infrared spectroscopy study of photodegradation of polymer modified asphalt binder. J. Appl. Polym. Sci. 2012, 123, 3275–3281. [Google Scholar]
- Tang, B.; Wu, C.; Lin, T.; Zhang, S. Heat-resistant PMMA photonic crystal films with bright structural color. Dyes Pigment. 2013, 99, 1022–1028. [Google Scholar] [CrossRef]
- Wang, D.J.; Gu, C.B.; Chen, P.L.; Liu, S.X.; Zhen, Z.; Zhang, J.C.; Liu, X.H. Preparation of heat-resistant gradient-index polymer optical fiber rods based on poly(N-isopropylmaleimide-co-methyl methacrylate). J. Appl. Polym. Sci. 2003, 87, 280–283. [Google Scholar] [CrossRef]
- Teresa, M.; Luguna, R.; Gallego, J.; Mendicuti, F.; Saiz, E.; Tarazona, M.P. Solution properties of poly(N-vinylcarbazole) and its copolymers with methyl methacrylate. Macromolecules 2002, 35, 7782–7790. [Google Scholar]
- Takasu, A.; Yamamoto, H.; Inai, Y.; Hirabayashi, T.; Nagata, K.; Takahashi, K. Synthesis of head-to-tail and head-to-tead poly(propylene-alt-methyl methacrylate)s via anionic polymerization of methyl 2,4-alkadienoates. 2.1 Synthesis and thermal properties. Macromolecules 2001, 34, 6235–6242. [Google Scholar] [CrossRef]
- Matsumoto, A.; Mizuta, K.; Otsu, T. Radical polymerization of 4-tert-butylcyclohexyl methacrylate: Polymerization kinetics and polymer properties. Macromolecules 1993, 26, 1659–1665. [Google Scholar] [CrossRef]
- Mayo, F.R.; Lewis, F.M. Copolymerization. I. A basis for comparing the behavior of monomers in copolymerization; the copolymerization of styrene and methyl methacrylate. J. Am. Chem. Soc. 1944, 66, 1594–1601. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Hirano, C.; Suzuki, Y.; Hayakawa, T.; Kuo, S.W. Strong screening effect of polyhedral oligomeric silsesquioxanes (POSS) nanoparticles on hydrogen bonded polymer blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef]
- Kao, H.C.; Kuo, S.W.; Chang, F.C. Effects of inert diluent segment and hydrogen bonding in poly(styrene-co-methacrylamide) copolymers. J. Polym. Res. 2003, 10, 111–117. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhao, D.M.; Li, L.X.; Wang, H.S.; Liu, G.D. Preparation of blends of poly(methyl methacrylate) copolymers with high glass transition temperatures and low hydrophilicity. Polym. Eng. Sci. 2013, 53, 2370–2377. [Google Scholar] [CrossRef]
- Kwei, T. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, S.-L.; Zhu, C.-Y.; Kuo, S.-W. Transparent Heat-Resistant PMMA Copolymers for Packing Light-Emitting Diode Materials. Polymers 2015, 7, 1379-1388. https://doi.org/10.3390/polym7081379
Yeh S-L, Zhu C-Y, Kuo S-W. Transparent Heat-Resistant PMMA Copolymers for Packing Light-Emitting Diode Materials. Polymers. 2015; 7(8):1379-1388. https://doi.org/10.3390/polym7081379
Chicago/Turabian StyleYeh, Shu-Ling, Chao-Yuan Zhu, and Shiao-Wei Kuo. 2015. "Transparent Heat-Resistant PMMA Copolymers for Packing Light-Emitting Diode Materials" Polymers 7, no. 8: 1379-1388. https://doi.org/10.3390/polym7081379
APA StyleYeh, S.-L., Zhu, C.-Y., & Kuo, S.-W. (2015). Transparent Heat-Resistant PMMA Copolymers for Packing Light-Emitting Diode Materials. Polymers, 7(8), 1379-1388. https://doi.org/10.3390/polym7081379







