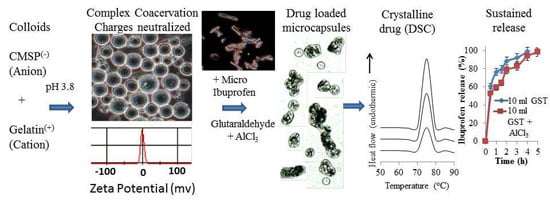
Dual Cross-Linked Carboxymethyl Sago Pulp-Gelatine Complex Coacervates for Sustained Drug Delivery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Carboxymethyl Sago Pulp
2.3. Preparation of Micronized Ibuprofen
2.4. Encapsulation of m-INN Using CMSP/Gelatine Complex Coacervation
| Formulation Code | CMSP (g) | Gelatine (g) | m-INN (g) | GST (mL) | Drug Loading (%) (n = 3 ± s.d.) | Entrapment Efficiency (%) (n = 3 ± s.d.) | |
|---|---|---|---|---|---|---|---|
| Theoretical | Experimental | ||||||
| A | 2 | 2 | 2 | 10 | 33.33 | 29.54 ± 0.71 | 88.62 ± 2.15 |
| B | 2 | 2 | 4 | 10 | 50 | 44.72 ± 4.03 | 89.44 ± 8.06 |
| C | 2 | 2 | 6 | 10 | 60 | 56.09 ± 2.31 | 93.48 ± 3.85 |
| D | 2 | 2 | 2 | 20 | 33.3 | 28.22 ± 0.84 | 84.66 ± 2.53 |
| E | 2 | 2 | 2 | 30 | 33.3 | 30.07 ± 0.55 | 90.21 ± 1.66 |
2.5. Drug Loading and Entrapment Efficiency
2.6. Particle Size Analysis and Zeta Potential Measurement
2.7. Fourier Transform Infrared Spectroscopy
2.8. Optical and Field Emission Scanning Electron Microscopy
2.9. X-Ray Diffractometry
2.10. Differential Scanning Calorimetry
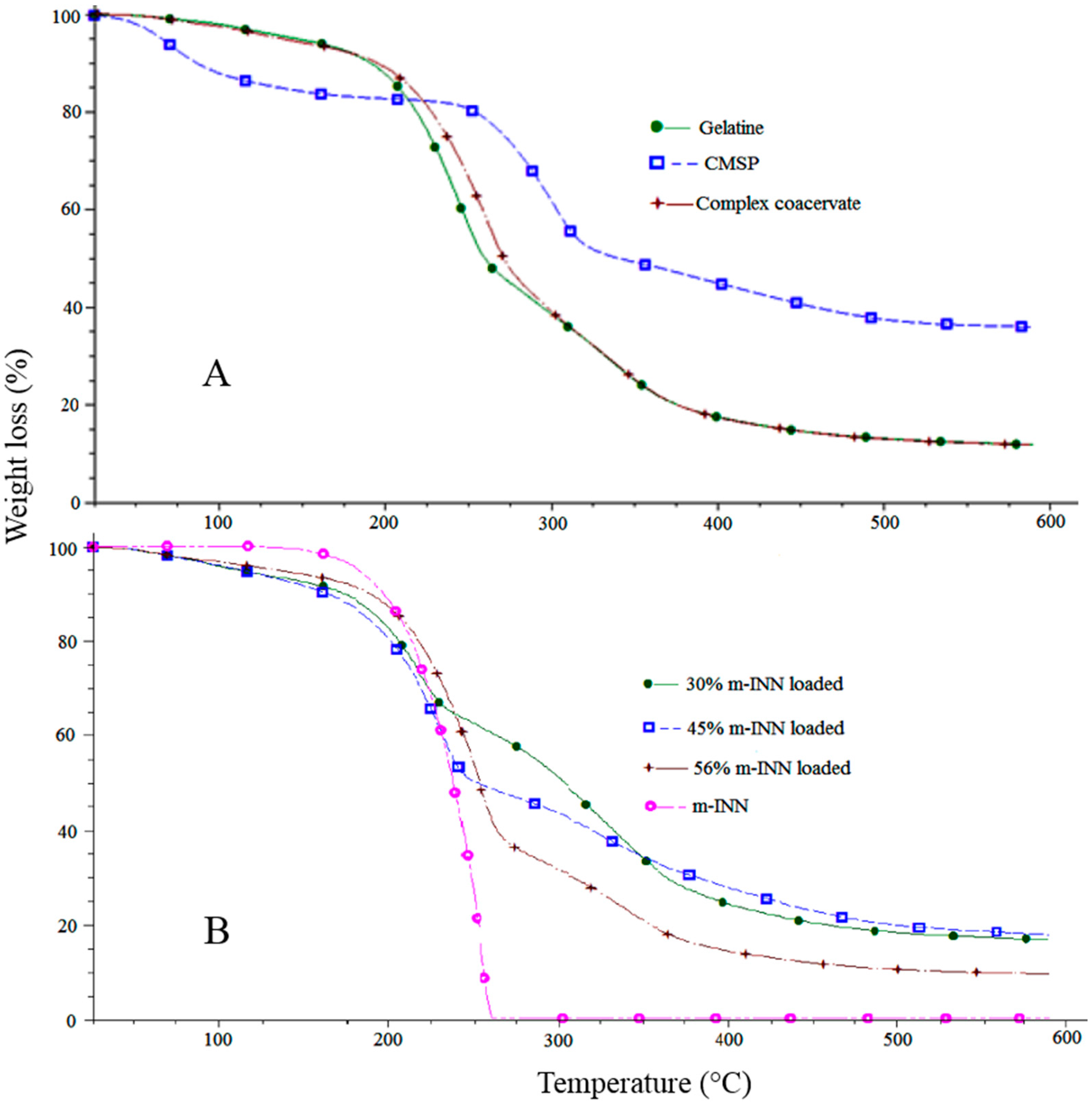
2.11. Thermogravimetric Analysis
2.12. In Vitro Release
2.13. Test for Residual Glutaraldehyde
3. Results and Discussion
3.1. Complex Coacervation and m-INN Encapsulation
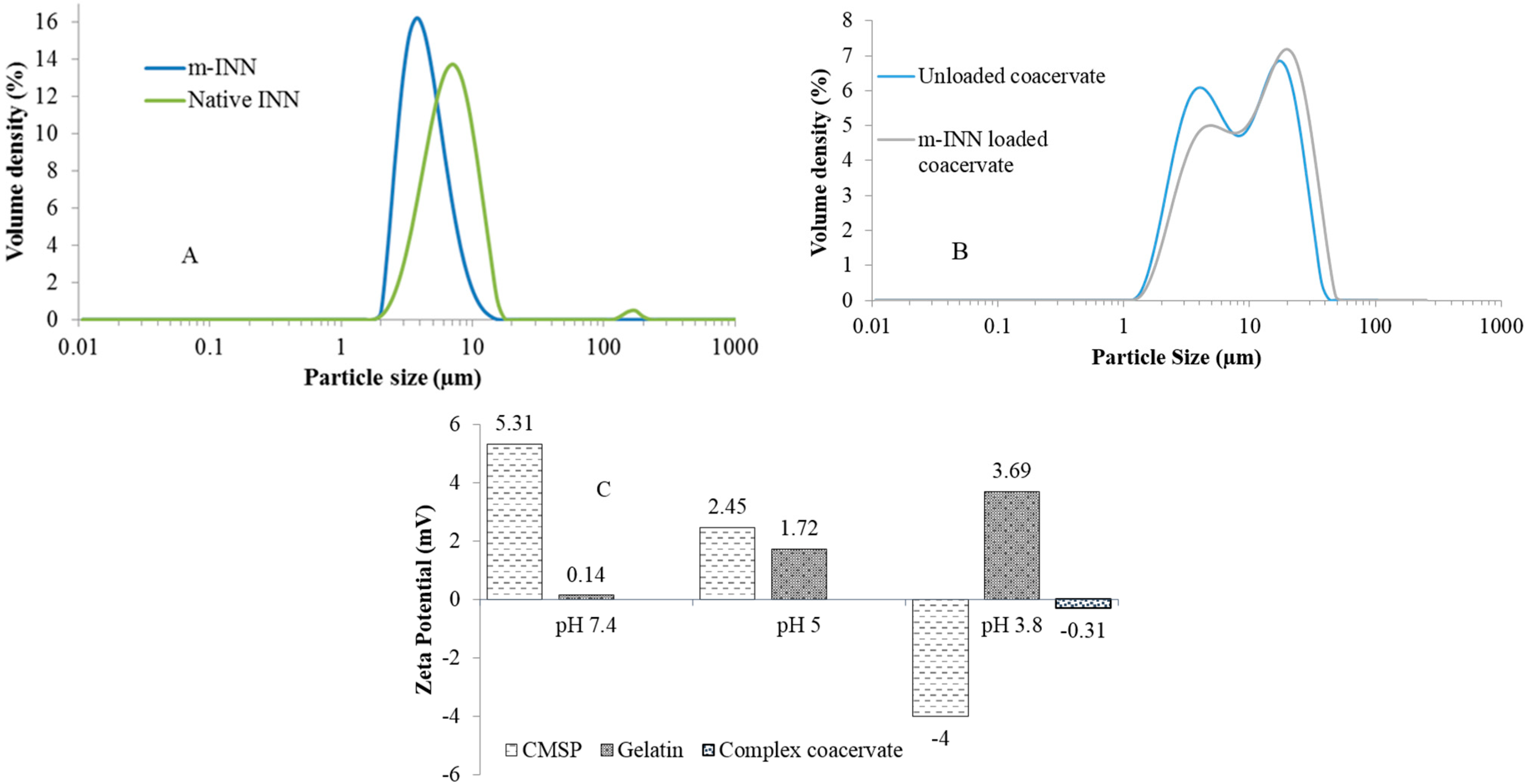
3.2. Particle Size and Zeta Potential Analysis
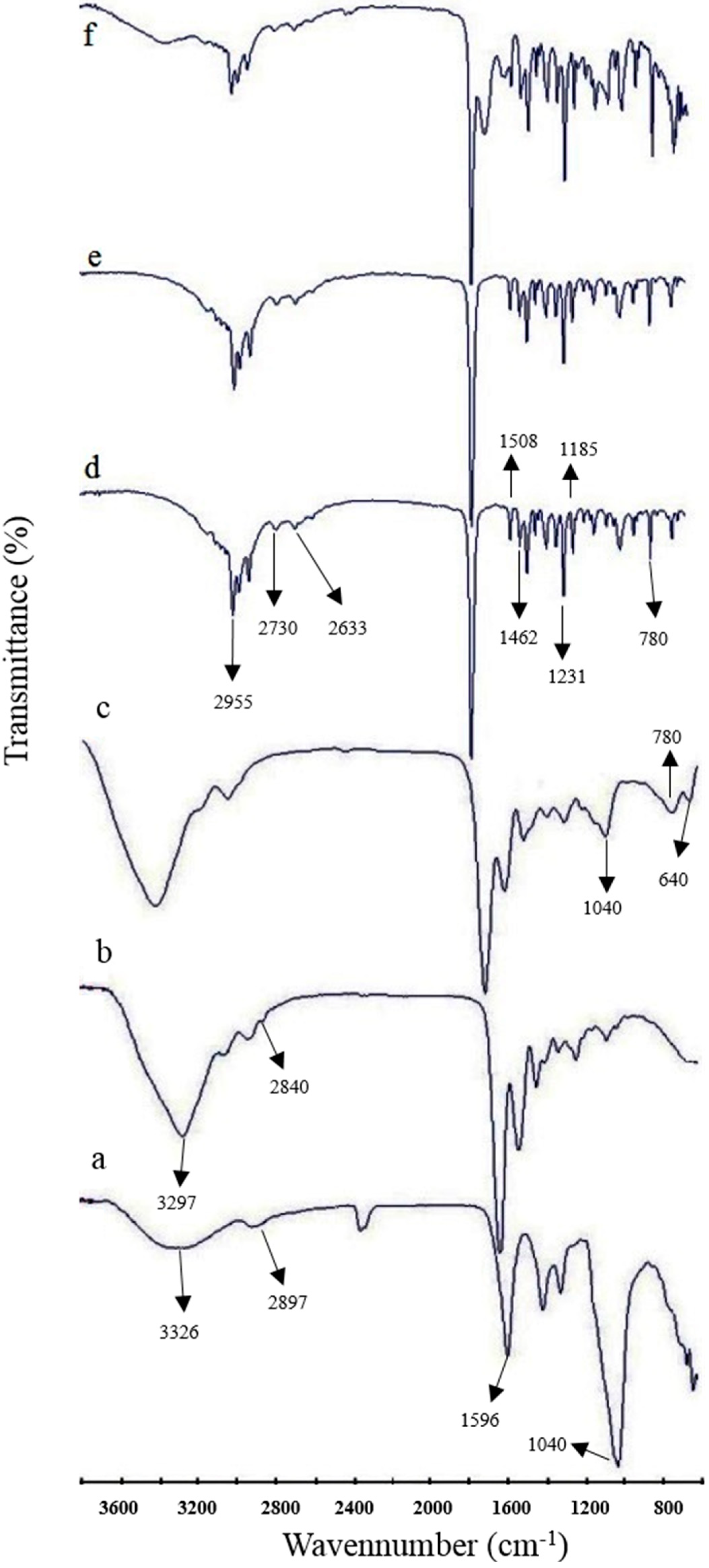
3.3. Fourier Transform Infrared Spectroscopy Studies
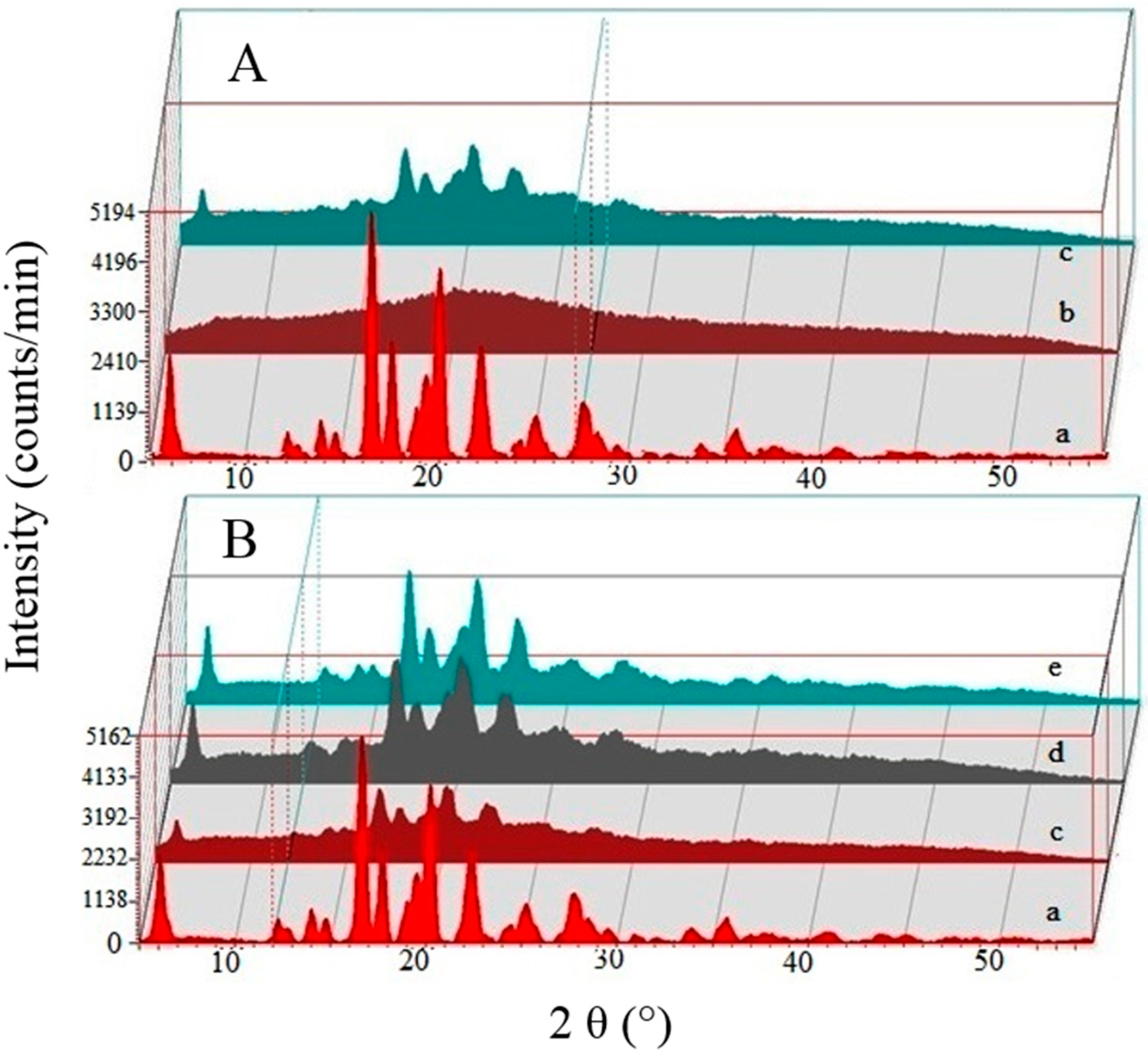
3.4. X-Ray Diffractometry
3.5. Differential Scanning Calorimetry
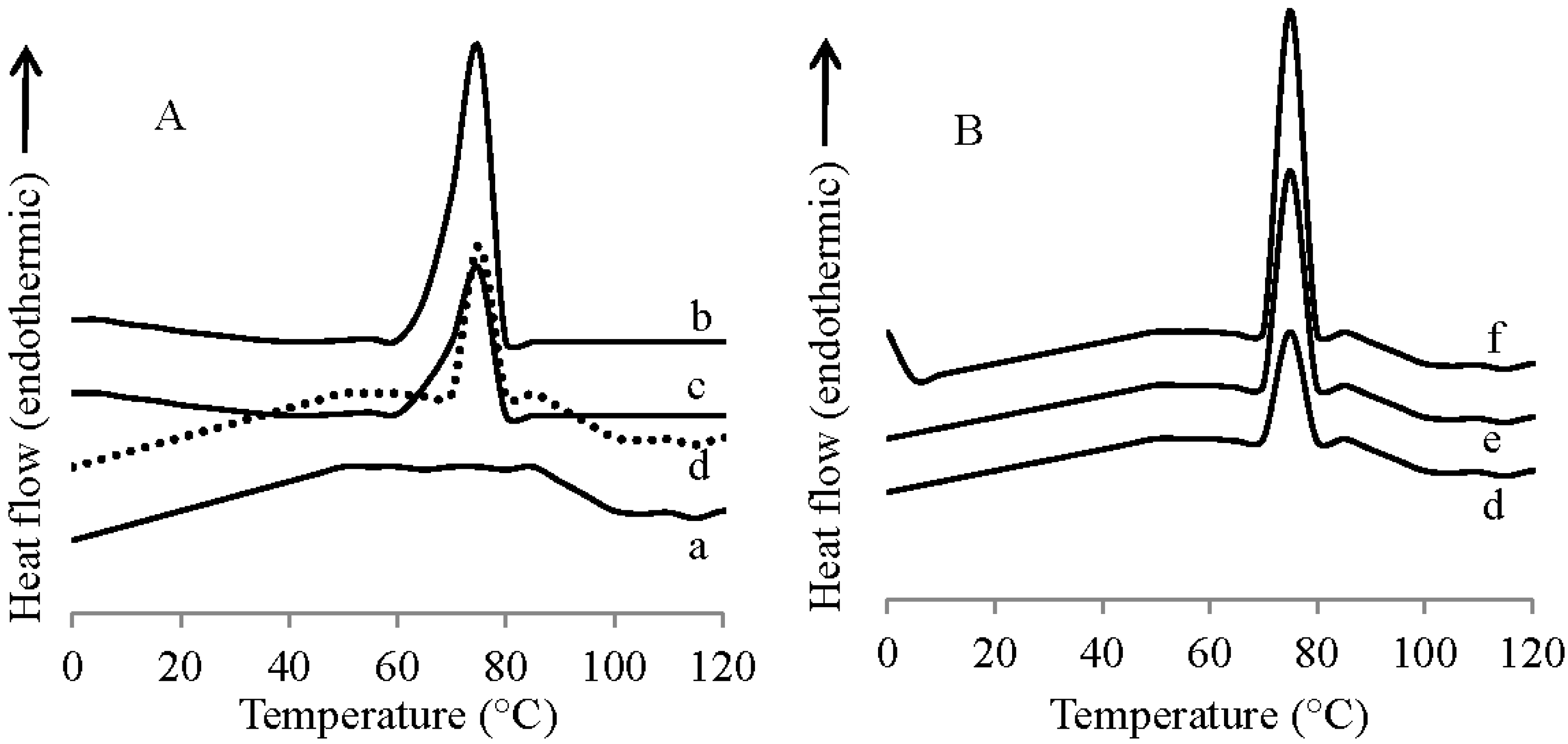
3.6. Thermogravimetric Analysis
3.7. Optical Microscopy and FESEM

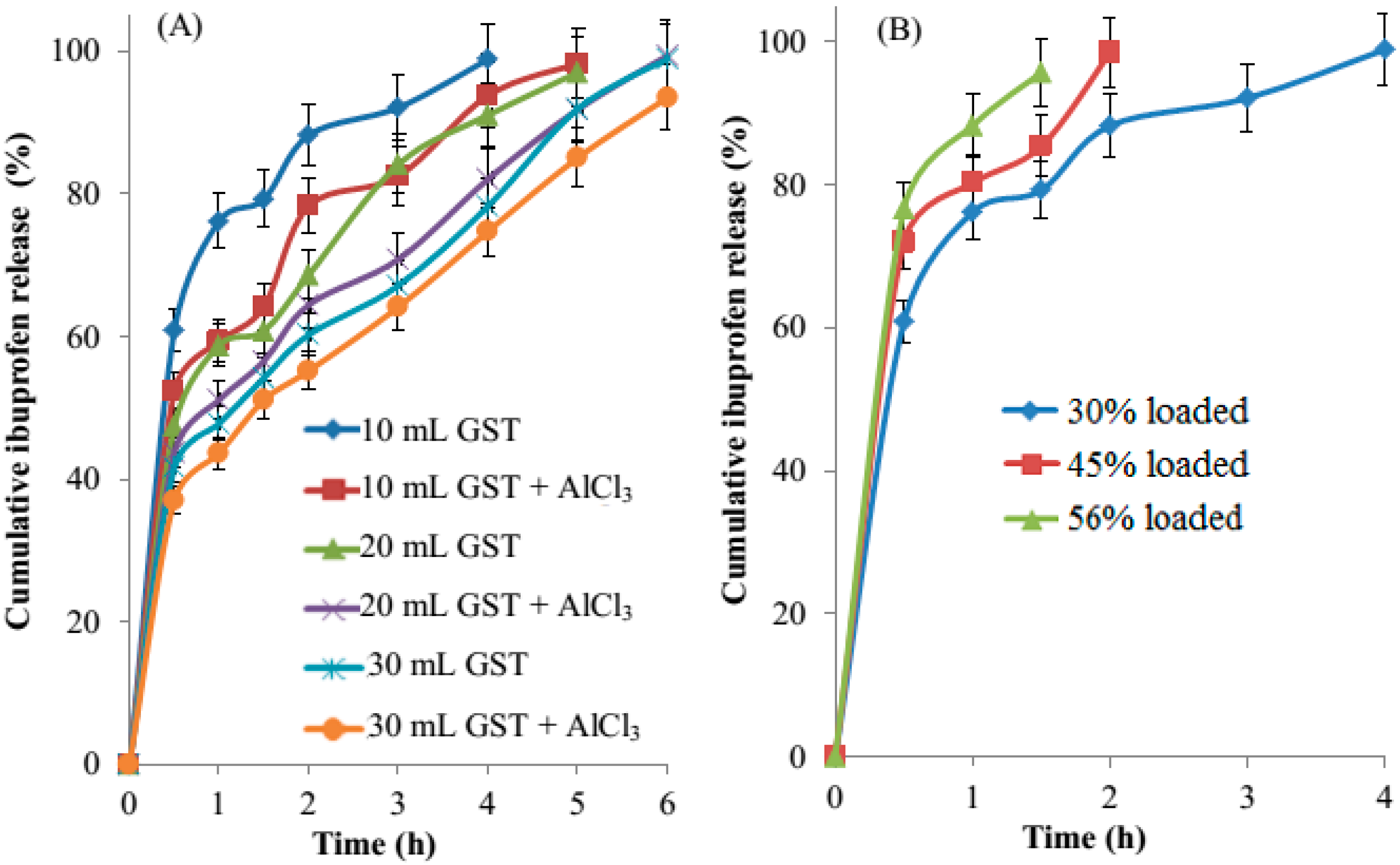
3.8. In Vitro Drug Release
3.9. Release Kinetics
3.10. Residual Glutaraldehyde
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pushpamalar, V.; Langford, S.; Ahmad, M.; Lim, Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.; Ahmad, M.; Hashim, K.; Lim, Y. Absorption characterization of Ca2+, Na+, and K+ on irradiation cross-linked carboxymethyl sago pulp hydrogel. J. Appl. Polym. Sci. 2013, 128, 1828–1833. [Google Scholar]
- Pushpamalar, V. Preparation and Characterisation of Carboxymethyl Cellulose and Carboxymethyl Cellulose Hydrogels from Sago Waste; Monash University: Clayton, Australia, 2010. [Google Scholar]
- Thenapakiam, S.; Kumar, D.G.; Pushpamalar, J.; Saravanan, M. Aluminium and radiation cross-linked carboxymethyl sago pulp beads for colon targeted delivery. Carbohydr. Polym. 2013, 94, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Deasy, P.B. Microencapsulation and Related Drug Processes; Marcel Dekker Inc.: New York, NY, USA, 1984. [Google Scholar]
- Polk, A.; Amsden, B.; de Yao, K.; Peng, T.; Goosen, M. Controlled release of albumin from chitosan—Alginate microcapsules. J. Pharm. Sci. 1994, 83, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Alexander, H.; Inchikel, A.; Stevenson, W.T. Microcapsules through polymer complexation: Part 3: Encapsulation and culture of human burkitt lymphoma cells in vitro. Biomaterials 1995, 16, 325–335. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Saravanan, M.; Rao, K.P. Pectin–gelatin and alginate–gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethy1ene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Pronounced activity of enzymes through the incorporation into the core of polyion complex micelles made from charged block copolymers. J. Control. Release 2001, 72, 85–91. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Perry, S.L.; Li, Y.; Priftis, D.; Leon, L.; Tirrell, M. The effect of salt on the complex coacervation of vinyl polyelectrolytes. Polymers 2014, 6, 1756–1772. [Google Scholar] [CrossRef]
- Chollakup, R.; Beck, J.B.; Dirnberger, K.; Tirrell, M.; Eisenbach, C.D. Polyelectrolyte molecular weight and salt effects on the phase behavior and coacervation of aqueous solutions of poly(acrylic acid) sodium salt and poly(allylamine) hydrochloride. Macromolecules 2013, 46, 2376–2390. [Google Scholar] [CrossRef]
- Wong, R.C.; Kang, S.; Heezen, J.L.; Voorhees, J.J.; Ellis, C.N. Oral ibuprofen and tetracycline for the treatment of acne vulgaris. J. Am. Acad. Dermatol. 1984, 11, 1076–1081. [Google Scholar] [CrossRef]
- Curhan, S.G.; Eavey, R.; Shargorodsky, J.; Curhan, G.C. Analgesic use and the risk of hearing loss in men. Am. J. Med. 2010, 123, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: Population based nested case-control analysis. BMJ 2005, 330, 1366. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Pouretedal, H.R.; Vosoughi, V. Preparation and characterization of Ibuprofen nanoparticles by using solvent/antisolvent precipitation. Open Conf. Proc. J. 2011, 2, 88–94. [Google Scholar] [CrossRef]
- Saravanan, M.; Bhaskar, K.; Rao, G.S.; Dhanaraju, M. Ibuprofen-loaded ethylcellulose/polystyrene microspheres: An approach to get prolonged drug release with reduced burst effect and low ethylcellulose content. J. Microencapsul. 2003, 20, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Boppana, R.; Kulkarni, R.V.; Mutalik, S.S.; Setty, C.M.; Sa, B. Interpenetrating network hydrogel beads of carboxymethylcellulose and egg albumin for controlled release of lipid lowering drug. J. Microencapsul. 2010, 27, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Bhaskar, K.; Maharajan, G.; Pillai, K.S. Development of gelatin microspheres loaded with diclofenac sodium for intra-articular administration. J. Drug Target. 2011, 19, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Charpentier-Valenza, D.; Merle, L.; Mocanu, G.; Picton, L.; Muller, G. Rheological properties of hydrophobically modified carboxymethylcelluloses. Carbohydr. Polym. 2005, 60, 87–94. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Hu, H.; Zhai, M.; Peng, J.; Nho, Y.; Li, J.; Wei, G. Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl. Instrum. Methods. Phys. Res. Section B 2007, 265, 385–389. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Garrigues, S.; Gallignani, M.; de la Guardia, M. FIA-FT-IR determination of ibuprofen in pharmaceuticals. Talanta 1993, 40, 89–93. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Davé, R. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Garnero, C.; Aloisio, C.; Longhi, M. Ibuprofen-maltodextrin interaction: Study of enantiomeric recognition and complex characterization. Pharmacol. Pharm. 2013, 4, 18. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Batycky, R.P.; Hanes, J.; Langer, R.; Edwards, D.A. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J. Pharm. Sci. 1997, 86, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S.; Peppas, N.A. Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers. Polymer 1999, 40, 3383–3398. [Google Scholar] [CrossRef]
- Hedayati, R.; Jahanshahi, M.; Attar, H. Fabrication and characterization of albumin-acacia nanoparticles based on complex coacervation as potent nanocarrier. J. Chem. Technol. Biotechnol. 2012, 87, 1401–1408. [Google Scholar] [CrossRef]
- Devi, N.; Hazarika, D.; Deka, C.; Kakati, D. Study of complex coacervation of gelatin A and sodium alginate for microencapsulation of olive oil. J. Macromol. Sci. Part A 2012, 49, 936–945. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Sorenson, D.; Verduzco, G.; Ames, Z.R.; Mansour, M. Evaluation of disinfectants forcitrus packinghouses. Citrograph 2011, 49, 50–55. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniyandy, S.; Sathasivam, T.; Veeramachineni, A.K.; Janarthanan, P. Dual Cross-Linked Carboxymethyl Sago Pulp-Gelatine Complex Coacervates for Sustained Drug Delivery. Polymers 2015, 7, 1088-1105. https://doi.org/10.3390/polym7061088
Muniyandy S, Sathasivam T, Veeramachineni AK, Janarthanan P. Dual Cross-Linked Carboxymethyl Sago Pulp-Gelatine Complex Coacervates for Sustained Drug Delivery. Polymers. 2015; 7(6):1088-1105. https://doi.org/10.3390/polym7061088
Chicago/Turabian StyleMuniyandy, Saravanan, Thenapakiam Sathasivam, Anand Kumar Veeramachineni, and Pushpamalar Janarthanan. 2015. "Dual Cross-Linked Carboxymethyl Sago Pulp-Gelatine Complex Coacervates for Sustained Drug Delivery" Polymers 7, no. 6: 1088-1105. https://doi.org/10.3390/polym7061088
APA StyleMuniyandy, S., Sathasivam, T., Veeramachineni, A. K., & Janarthanan, P. (2015). Dual Cross-Linked Carboxymethyl Sago Pulp-Gelatine Complex Coacervates for Sustained Drug Delivery. Polymers, 7(6), 1088-1105. https://doi.org/10.3390/polym7061088