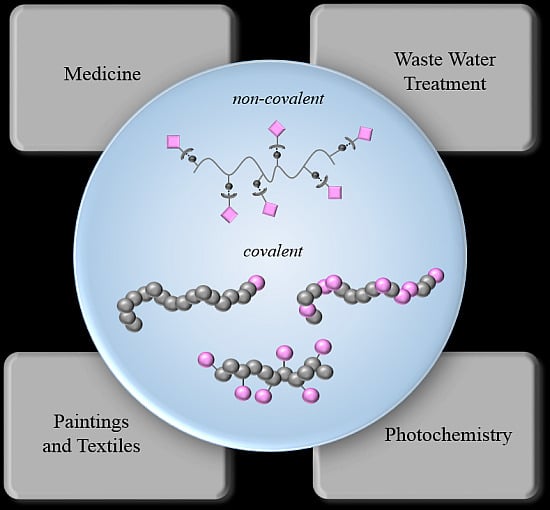
Polymers and Dyes: Developments and Applications
Abstract
:1. Introduction
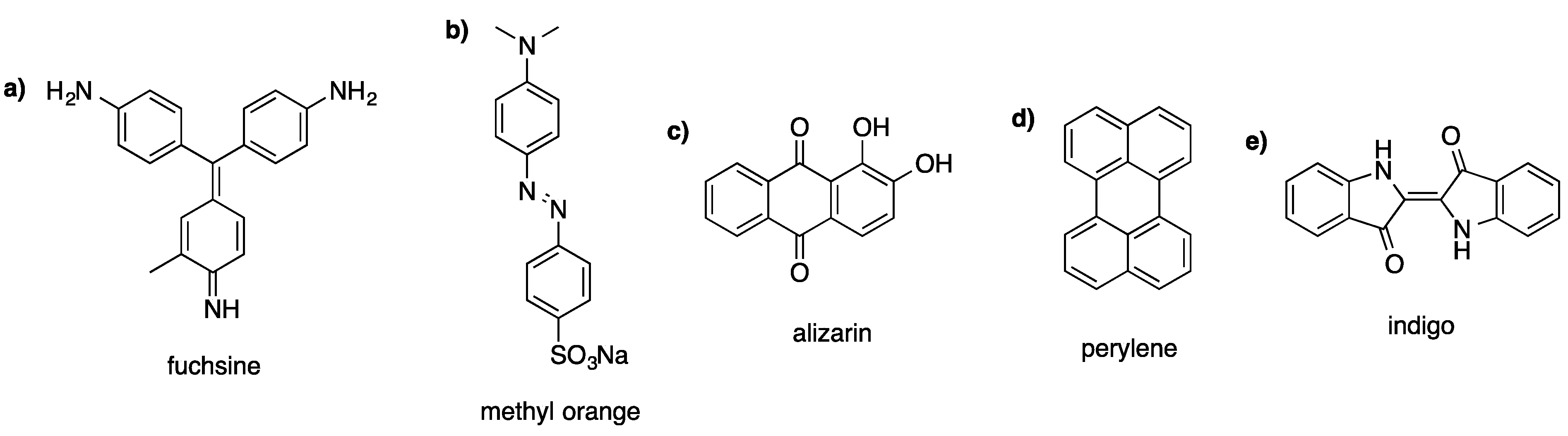
2. Preparation Techniques
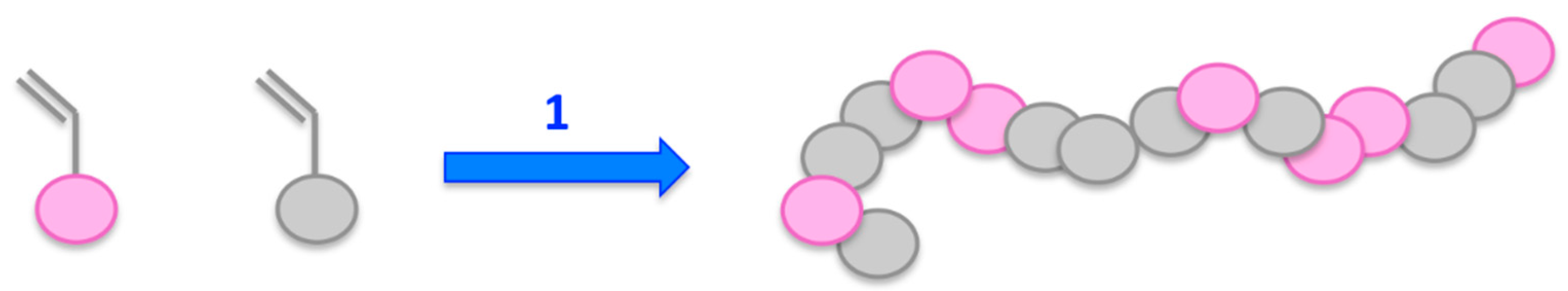
2.1. Non-Covalent Attachment
2.1.1. Sugar-Based Polymers

2.1.2. Synthetic Polymers
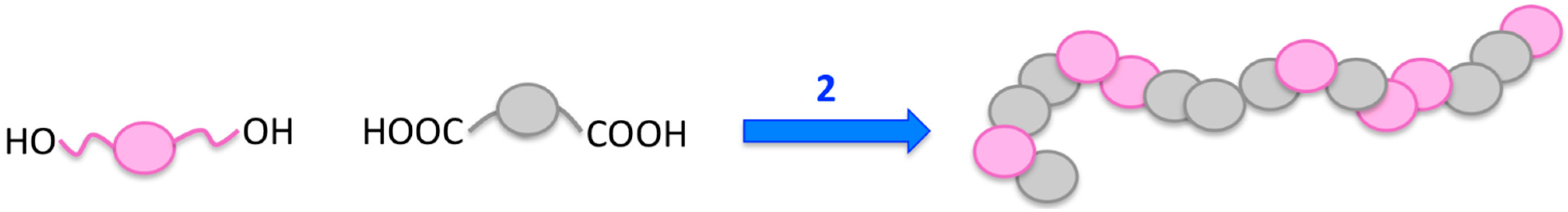
2.2. Covalent Attachment
- (1)
- Polymerization of colored monomers;
- (2)
- Polycondensation or (cross)coupling reactions of adequate dyes/dye derivatives;
- (3)
- Polymer-analogous attachment of dye molecules to preformed polymers;
- (4)
- Preparation of high molecular weight derivatives of single chromophores (e.g., via grafting onto mechanism).
2.2.1. Polymerization of Colored Monomers
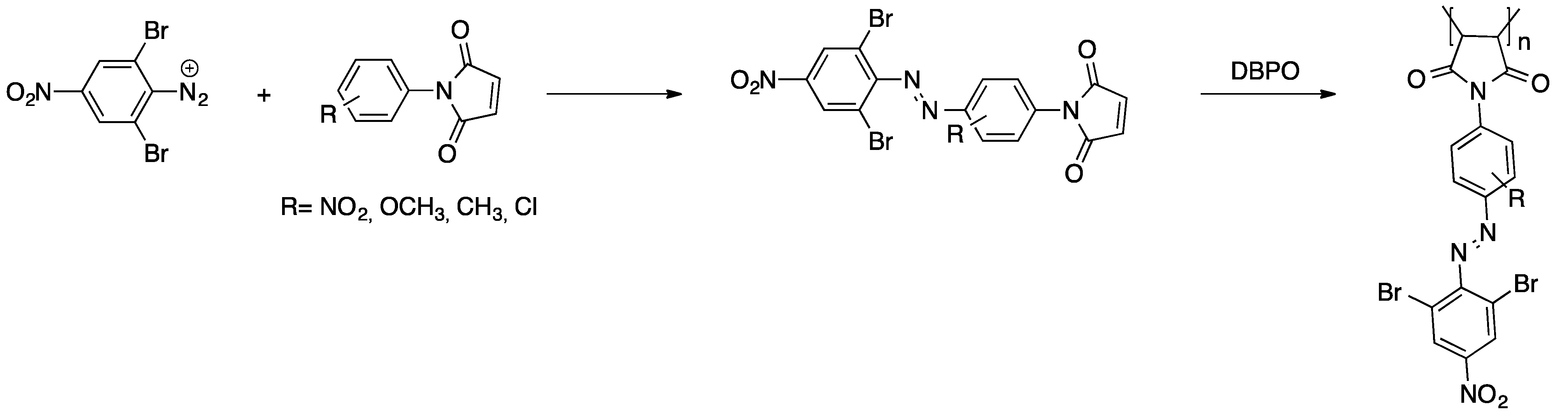
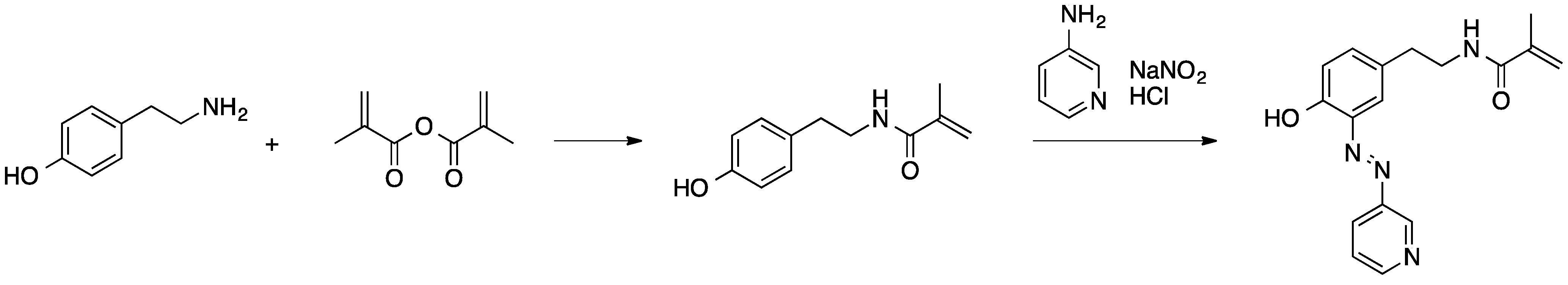

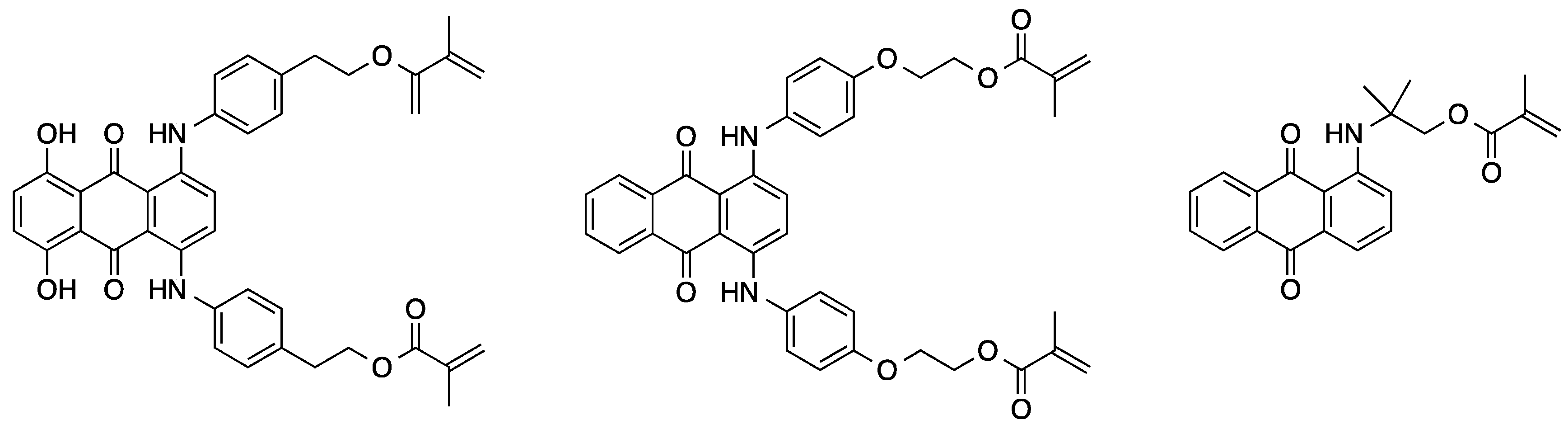
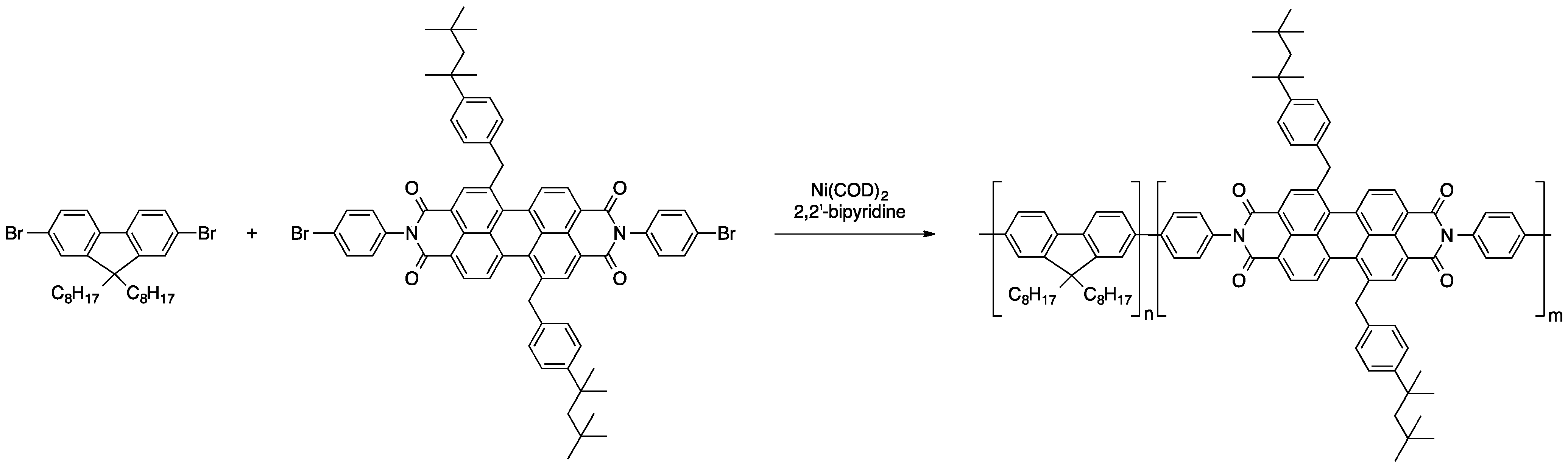
2.2.2. Polycondensation and (Cross)coupling Reactions of Adequate Dye Derivatives
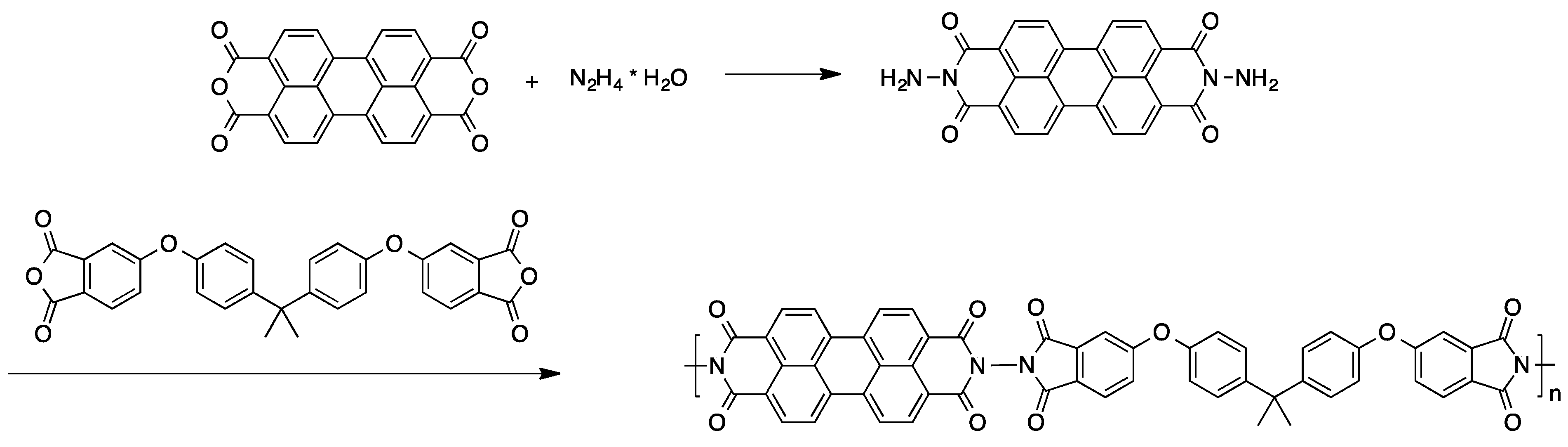
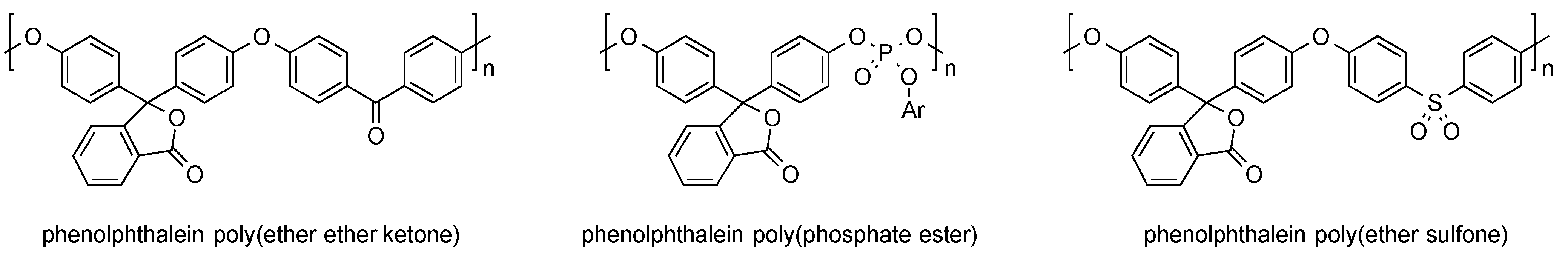
2.2.3. Polymer-Analogous Attachment of Dye Molecules to Preformed Polymers
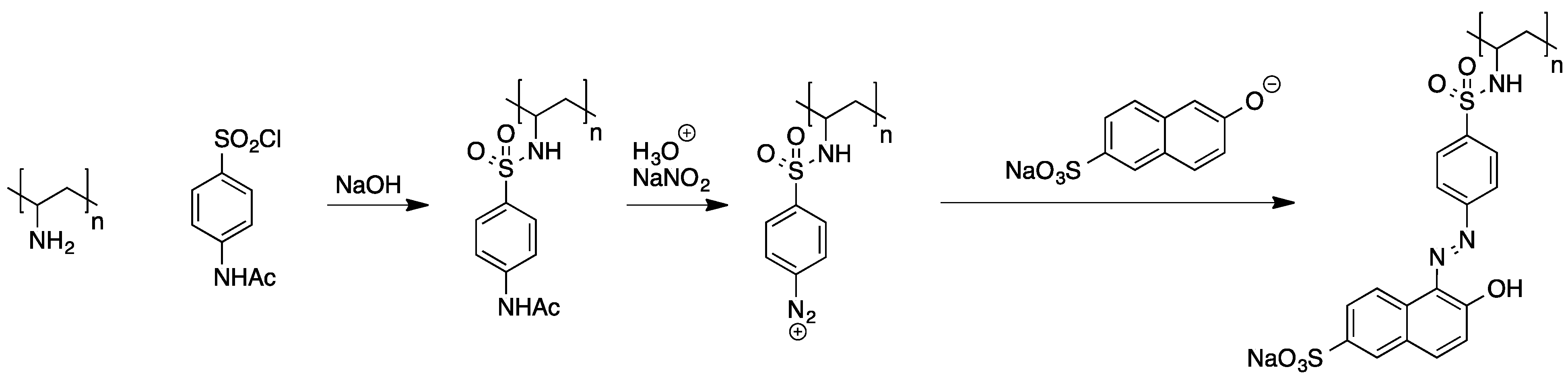
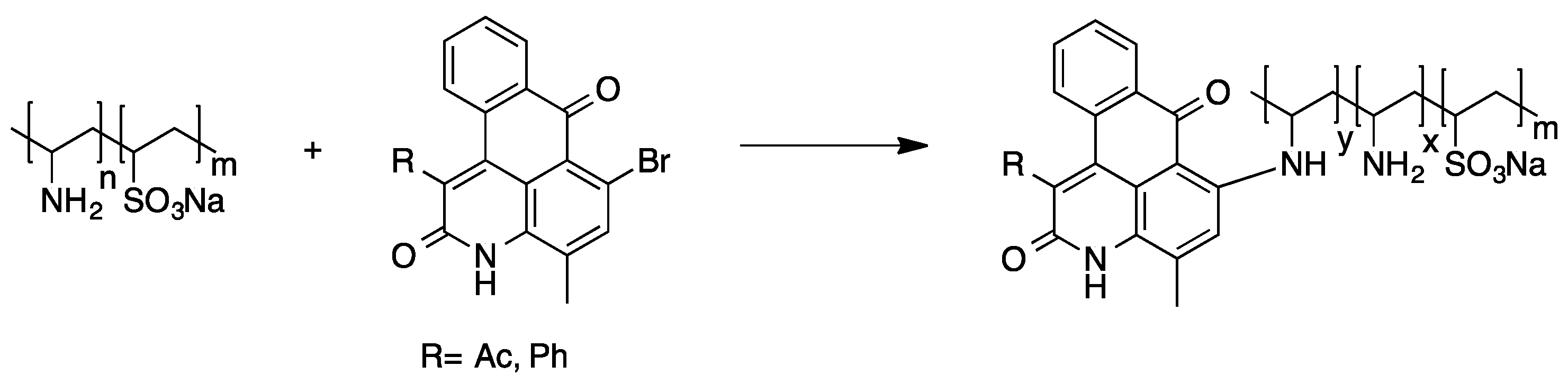
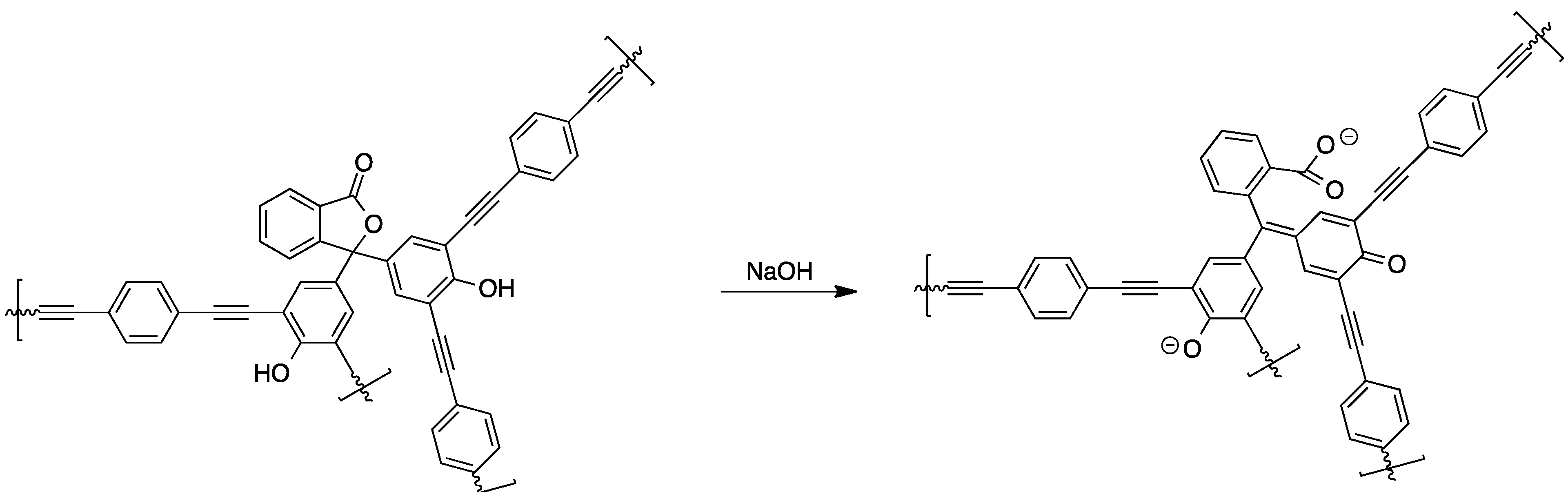
2.2.4. Preparation of High Molecular Weight Derivatives of Single Chromophores
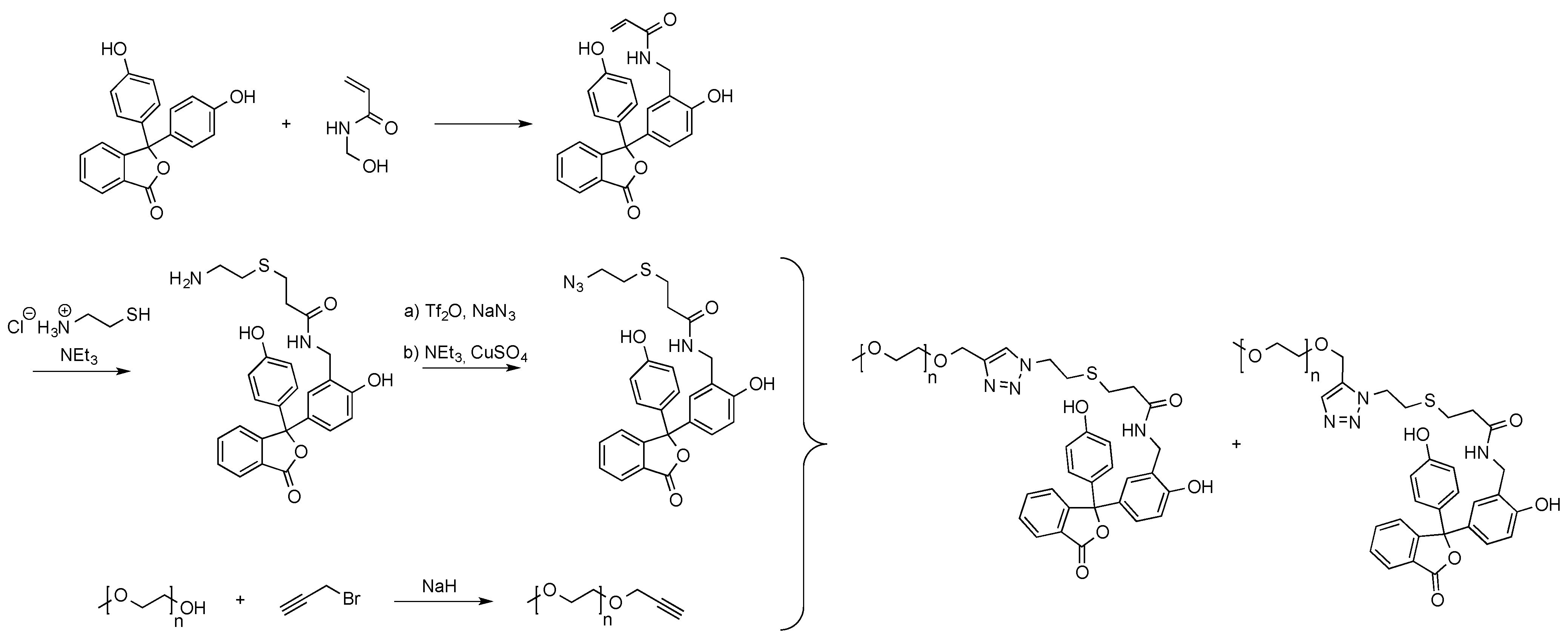
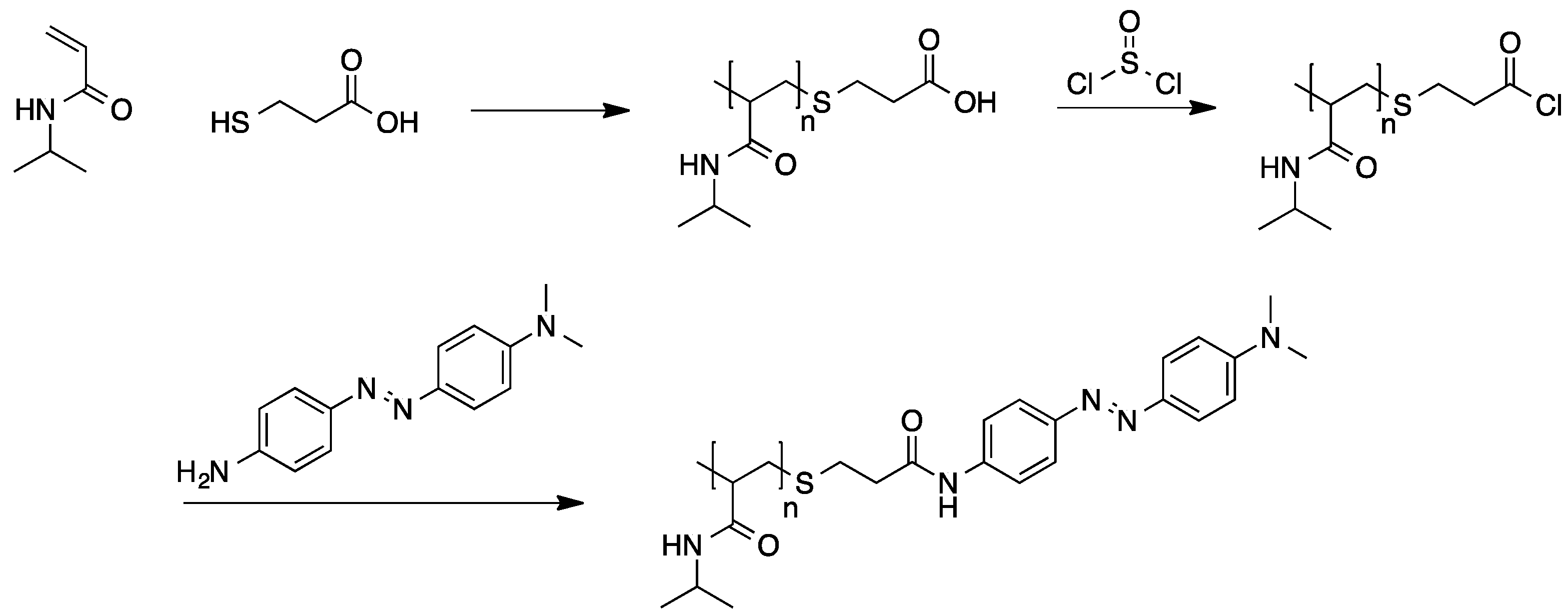
3. Applications
3.1. Waste Water Treatment
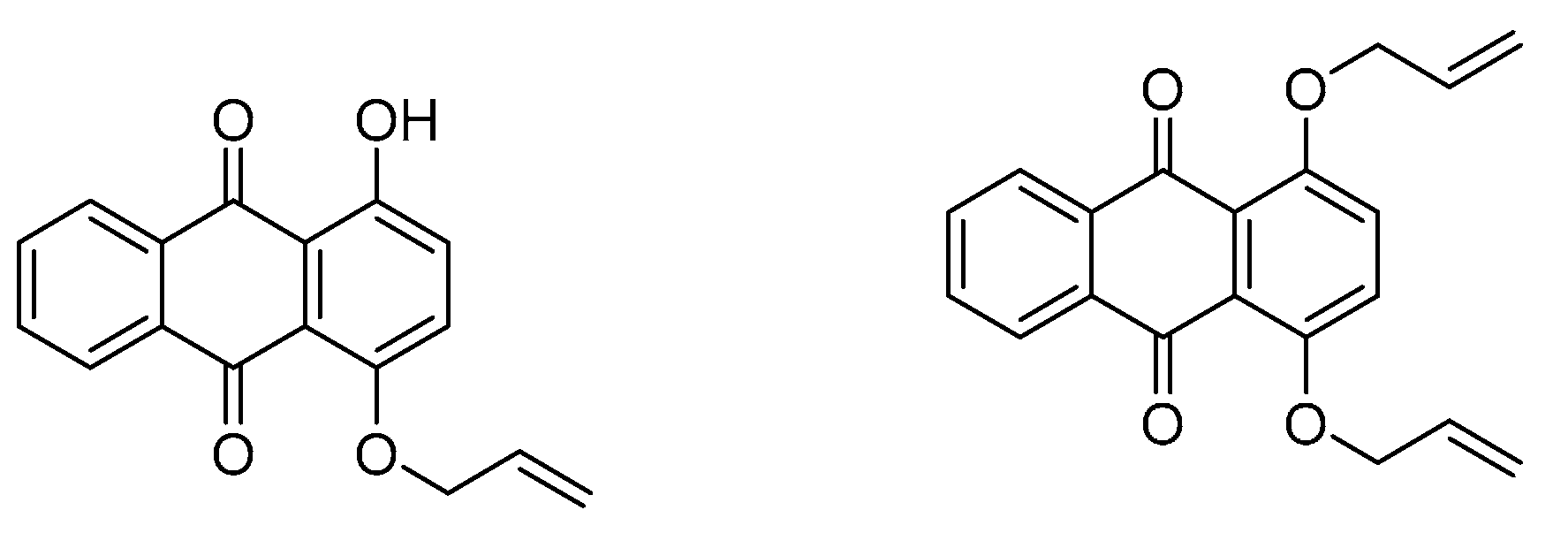
3.2. Paintings and Textiles
3.3. Medicine

3.4. Photochemistry
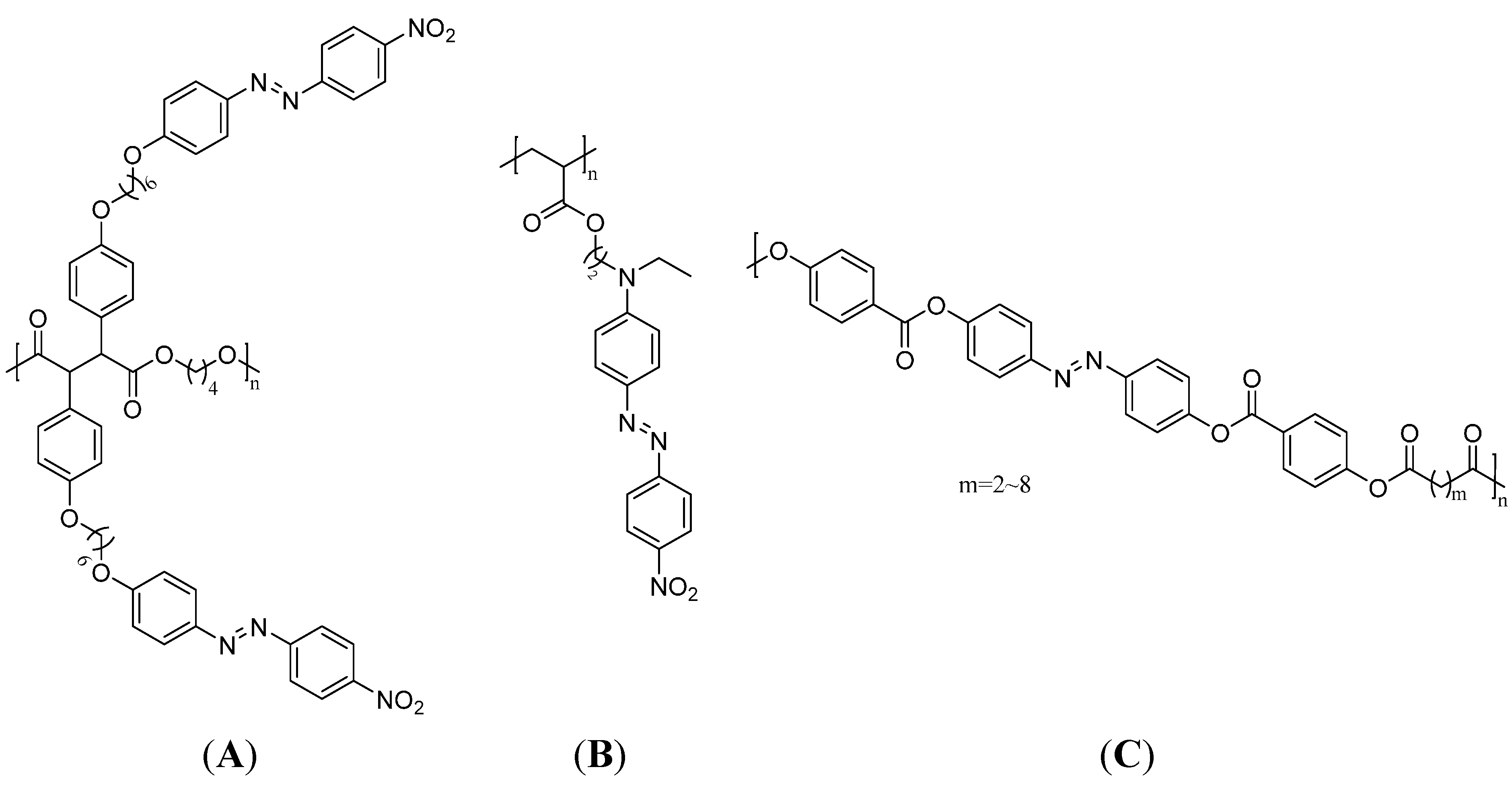
3.5. Optical Sensors

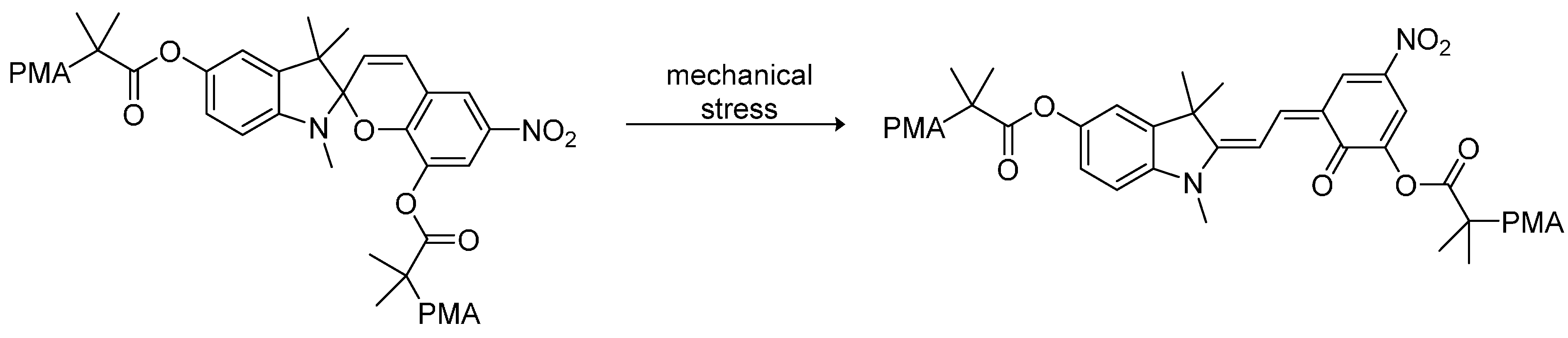
3.6. Electrochemical and Optoelectronic Applications
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Abou-Okeil, A.; El-Shafie, A.; El Zawahry, M.M. Ecofriendly laccase–hydrogen peroxide/ultrasound-assisted bleaching of linen fabrics and its influence on dyeing efficiency. Ultrason. Sonochem. 2010, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Lewis, D.; Broadbent, P. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Color. Technol. 2008, 124, 186–194. [Google Scholar] [CrossRef]
- Baptista, M.S.; Indig, G.L. Effect of BSA binding on photophysical and photochemical properties of triarylmethane dyes. J. Phys. Chem. B 1998, 102, 4678–4688. [Google Scholar] [CrossRef]
- Mula, S.; Ray, A.K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. Design and development of a new pyrromethene dye with improved photostability and lasing efficiency: Theoretical rationalization of photophysical and photochemical properties. J. Org. Chem. 2008, 73, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Nazarpoor, K.; Karimi, L. Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J. Clean. Prod. 2011, 19, 1045–1051. [Google Scholar] [CrossRef]
- Sulak, M.T.; Yatmaz, H.C. Removal of textile dyes from aqueous solutions with eco-friendly biosorbent. Desalin. Water Treat. 2012, 37, 169–177. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Robra, K.-H.; Cavaco-Paulo, A.; Gübitz, G.M. Decolorization and detoxification of textile dyes with a laccase from trametes hirsuta. Appl. Environ. Microb. 2000, 66, 3357–3362. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pavlostathis, S.G. Decolorization and toxicity of reactive anthraquinone textile dyes under methanogenic conditions. Water Res. 2004, 38, 1838–1852. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green chemistry. Environ. Impact Assess. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Duxbury, D.F. The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 1993, 93, 381–433. [Google Scholar] [CrossRef]
- Azmi, W.; Sani, R.K.; Banerjee, U.C. Biodegradation of triphenylmethane dyes. Enzyme Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 2, pp. 707–710. [Google Scholar]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 4, pp. 409–464. [Google Scholar]
- Bergmann, E.; Engel, L.; Sándor, S. Beiträge zur kenntnis der doppelten bindung, ii.: Über die räumliche konfiguration der aromatischen azokörper. Ber. Dtsch. Chem. Ges. 1930, 63, 2572–2575. [Google Scholar]
- Kumar, G.S.; Neckers, D. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P.; Ho, M.-S.; Barrett, C. Azo polymers for reversible optical storage.6. Poly[4-[2-(methacryloyloxy)ethyl]azobenzene]. Macromolecules 1995, 28, 4179–4183. [Google Scholar] [CrossRef]
- Natansohn, A.; Xie, S.; Rochon, P. Azo polymers for reversible optical storage. 2. Poly[4'-[[2-(acryloyloxy)ethyl]ethylamino]-2-chloro-4-nitroazobenzene]. Macromolecules 1992, 25, 5531–5532. [Google Scholar]
- Kohl, C.; Becker, S.; Müllen, K. Bis(rylenedicarboximide)-a,d-1,5-diaminoanthraquinones as unique infrared absorbing dyes. Chem. Commun. 2002, 2778–2779. [Google Scholar] [CrossRef]
- Herrmann, A.; Müllen, K. From industrial colorants to single photon sources and biolabels: The fascination and function of rylene dyes. Chem. Lett. 2006, 35, 978–985. [Google Scholar] [CrossRef]
- Weil, T.; Abdalla, M.A.; Jatzke, C.; Hengstler, J.; Müllen, K. Water-soluble rylene dyes as high-performance colorants for the staining of cells. Biomacromolecules 2005, 6, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Epolito, W.; Lee, Y.; Bottomley, L.; Pavlostathis, S. Characterization of the textile anthraquinone dye reactive blue 4. Dyes Pigments 2005, 67, 35–46. [Google Scholar] [CrossRef]
- Aspland, J.R. Textile Dyeing and Coloration; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Schaetzer, H.; Raisin, H.; Mausezahl, D. Anthraquinone Dyes, Dyeing, Printing, Textiles. U.S. Patent 4,396,393, 8 February 1983. [Google Scholar]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 4, pp. 1–548. [Google Scholar]
- Johnson, M.G.; Kiyokawa, H.; Tani, S.; Koyama, J.; Morris-Natschke, S.L.; Mauger, A.; Bowers-Daines, M.M.; Lange, B.C.; Lee, K.-H. Antitumor agents—CLXVII. Synthesis and structure-activity correlations of the cytotoxic anthraquinone 1,4-bis-(2,3-epoxypropylamino)-9, 10-anthracenedione, and of related compounds. Bioorgan. Med. Chem. 1997, 5, 1469–1479. [Google Scholar]
- Agarwal, S.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Oshio, H.; Kawamura, N. Quantitative analysis of the laxative components in rhubarb by high performance liquid chromatography. Jpn. J. Pharmacogn. 1985, 39, 131–138. [Google Scholar] [CrossRef]
- Brown, J.P.; Brown, R.J. Mutagenesis by 9,10-anthraquinone derivatives and related compounds in salmonella typhimurium. Mutat. Res. 1976, 40, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.N. Absorption and emission spectroscopy and photochemistry of 1,10-anthraquinone derivatives: A review. J. Photochem. Photobiol. 1990, 53, 141–167. [Google Scholar] [CrossRef]
- Rondão, R.; de Melo, J.S.; Schaberle, F.; Voss, G. Excited state characterization of a polymeric indigo. Phys. Chem. Chem. Phys. 2012, 14, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Truckmeier, S. Naturfarbstoffe: Farben mit geschichte. Chem. Unserer Zeit 2003, 37, 402–409. [Google Scholar] [CrossRef]
- W. Kratzert, R.P. Farbstoffe; Quelle & Meyer: Heidelberg, Germany, 1981. [Google Scholar]
- Friedländer, P. Über indigoide farbstoffe. Ber. Dtsch. Chem. Ges. 1908, 29, 359–374. [Google Scholar]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.M.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D. Indirubin, the active constituent of a chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, E.Y.; Sezgin, M.; Yilmaz, A.; Yilmaz, M. Synthesis of β-cyclodextrin and starch based polymers for sorption of azo dyes from aqueous solutions. Bioresour. Technol. 2008, 99, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Yilmaz, E.; Yilmaz, M.; Bartsch, R.A. Removal of azo dyes from aqueous solutions using calix[4]arene and β-cyclodextrin. Dyes Pigments 2007, 74, 54–59. [Google Scholar] [CrossRef]
- Akceylan, E.; Bahadir, M.; Yilmaz, M. Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J. Hazard. Mater. 2009, 162, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigments 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Trellenkamp, T.; Ritter, H. Poly(N-vinylpyrrolidone) bearing covalently attached cyclodextrin via click-chemistry: Synthesis, characterization, and complexation behavior with phenolphthalein. Macromolecules 2010, 43, 5538–5543. [Google Scholar] [CrossRef]
- Taguchi, K. Transient binding of phenolphthalein-β-cyclodextrin complex: An example of induced geometrical distortion. J. Am. Chem. Soc. 1986, 108, 2705–2709. [Google Scholar] [CrossRef]
- Ramos-Ortiz, G.; Maldonado, J.; Meneses-Nava, M.; Barbosa-García, O.; Olmos, M.; Cha, M. Third-harmonic generation performance of organic polymer films doped with triphenylmethane derivative dyes. Opt. Mater. 2007, 29, 636–641. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, S.H.; Antonietti, M.; Böttcher, C.; Faul, C.F. Synthesis of supramolecular polymers by ionic self-assembly of oppositely charged dyes. Chem. Eur. J. 2005, 11, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Şolpan, D.; Duran, S.; Saraydin, D.; Güven, O. Adsorption of methyl violet in aqueous solutions by poly (acrylamide-co-acrylic acid) hydrogels. Radiat. Phys. Chem. 2003, 66, 117–127. [Google Scholar] [CrossRef]
- Ngo, T.T.; Liotta, C.L.; Eckert, C.A.; Kazarian, S.G. Supercritical fluid impregnation of different azo-dyes into polymer: In situ UV/Vis spectroscopic study. J. Supercrit. Fluids 2003, 27, 215–221. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R.; Maka, T.; Romanov, S.G.; Sotomayor Torres, C.M. Dye-containing polymer beads as photonic crystals. Chem. Mater. 2000, 12, 2508–2512. [Google Scholar] [CrossRef]
- Seeboth, A.; Kriwanek, J.; Vetter, R. The first example of thermochromism of dyes embedded in transparent polymer gel networks. J. Mater. Chem. 1999, 9, 2277–2278. [Google Scholar] [CrossRef]
- Donati, F.; Pucci, A.; Ruggeri, G. Temperature and chemical environment effects on the aggregation extent of water soluble perylene dye into vinyl alcohol-containing polymers. Phys. Chem. Chem. Phys. 2009, 11, 6276–6282. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.R.; Burnworth, M.; Khariwala, D.; Hiltner, A.; Mather, P.T.; Simha, R.; Weder, C. Deformation-induced color changes in mechanochromic polyethylene blends. Macromolecules 2007, 40, 2400–2408. [Google Scholar] [CrossRef]
- Donati, F.; Pucci, A.; Cappelli, C.; Mennucci, B.; Ruggeri, G. Modulation of the optical response of polyethylene films containing luminescent perylene chromophores. J. Phys. Chem. B 2008, 112, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D. Polymeric dyes. Aldrichim. Acta 1981, 14, 23–29. [Google Scholar]
- Robello, D.R. Linear polymers for nonlinear optics. I. Polyacrylates bearing aminonitro-stilbene and-azobenzene dyes. J. Polym. Sci. Pol. Chem. 1990, 28, 1–13. [Google Scholar]
- Maradiya, H.R.; Patel, V.S. Synthesis, characterization and application of monomeric and polymeric dyes based on N-arylmaleimides. High Perform. Polym. 2000, 12, 335–348. [Google Scholar] [CrossRef]
- Maradiya, H.R.; Patel, V.S. Studies of novel monomeric and polymeric azo disperse dyes. J. Appl. Polym. Sci. 2002, 84, 1380–1389. [Google Scholar] [CrossRef]
- Retzmann, N.; Maatz, G.; Ritter, H. Host–guest-driven color change in water: Influence of cyclodextrin on the structure of a copper complex of poly((4-hydroxy-3-(pyridin-3-yldiazenyl)phenethyl) methacrylamide-co-dimethylacrylamide). Beilstein J. Org. Chem. 2014, 10, 2480–2483. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Kungwatchakun, D. Photoresponsive polymers. Mechanochemistry of polyacrylamide gels having triphenylmethane leuco derivatives. Macromol. Rapid Comm. 1984, 5, 829–832. [Google Scholar]
- Fleischmann, C.; Cheng, J.; Tabatabai, M.; Ritter, H. Extended applicability of classical phenolphthalein: Color changing polymeric materials derived from pH-sensitive acrylated phenolphthalein derivatives. Macromolecules 2012, 45, 5343–5346. [Google Scholar] [CrossRef]
- Fleischmann, C.; Ritter, H. Color indicator for supramolecular polymer chemistry: Phenolphthalein-containing thermo-and pH-sensitive N-(isopropyl) acrylamide copolymers and β-cyclodextrin complexation. Macromol. Rapid Comm. 2013, 34, 1085–1089. [Google Scholar] [CrossRef]
- Hetzer, M.; Fleischmann, C.; Schmidt, B.V.; Barner-Kowollik, C.; Ritter, H. Visual recognition of supramolecular graft polymer formation via phenolphthalein–cyclodextrin association. Polymer 2013, 54, 5141–5147. [Google Scholar] [CrossRef]
- Dollendorf, C.; Kreth, S.K.; Choi, S.W.; Ritter, H. Polymerization of novel methacrylated anthraquinone dyes. Beilstein J. Org. Chem. 2013, 9, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sastre, R.; Costela, A. Polymeric solid-state dye lasers. Adv. Mater. 1995, 7, 198–202. [Google Scholar] [CrossRef]
- Serin, J.; Schultze, X.; Adronov, A.; Fréchet, J.M. Synthesis and study of the absorption and luminescence properties of polymers containing Ru(BpyMe2)32+ chromophores and coumarin laser dyes. Macromolecules 2002, 35, 5396–5404. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Color change of N-isopropylacrylamide copolymer bearing reichardts dye as optical sensor for lower critical solution temperature and for host–guest interaction with β-cyclodextrin. J. Am. Chem. Soc. 2007, 129, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
- Shiba, M.; Hiramasu, H.; Nakano, H.; Kawano, Y.; Shigeri, Y.; Kondo, T. Synthesis of high polymers with a light absorption band in the visible region by interfacial polycondensation reaction. Polym. J. 1973, 4, 366–371. [Google Scholar] [CrossRef]
- Ghassemi, H.; Hay, A.S. Red pigmentary polyimides from N,N'-diamino-3,4,9,10-perylenetetracarboxylic acid bisimide. Macromolecules 1994, 27, 4410–4412. [Google Scholar] [CrossRef]
- Ego, C.; Marsitzky, D.; Becker, S.; Zhang, J.; Grimsdale, A.C.; Müllen, K.; MacKenzie, J.D.; Silva, C.; Friend, R.H. Attaching perylene dyes to polyfluorene: Three simple, efficient methods for facile color tuning of light-emitting polymers. J. Am. Chem. Soc. 2003, 125, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroglu, F.E.; Alexander, S.C.; Ji, D.; Prusty, D.K.; Borsch, M.; Herrmann, A. Poly(BODIPY)s: A new class of tunable polymeric dyes. Macromolecules 2009, 42, 6529–6536. [Google Scholar] [CrossRef]
- Morgan, P. Linear condensation polymers from phenolphthalein and related compounds. J. Polym. Sci. 1964, 2, 437–459. [Google Scholar] [CrossRef]
- Kishore, K.; Kannan, P. Synthesis, spectral, thermal, and flammability studies of phenolphthalein polyphosphate esters. J. Polym. Sci. Pol. Chem. 1990, 28, 3481–3486. [Google Scholar] [CrossRef]
- Wang, M.; An, Q.-F.; Wu, L.-G.; Mo, J.-X.; Gao, C.-J. Preparation of pH-responsive phenolphthalein poly(ether sulfone) membrane by redox-graft pore-filling polymerization technique. J. Membrane Sci. 2007, 287, 257–263. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.-G.; Zheng, X.-C.; Mo, J.-X.; Gao, C.-J. Surface modification of phenolphthalein poly(ether sulfone) ultrafiltration membranes by blending with acrylonitrile-based copolymer containing ionic groups for imparting surface electrical properties. J. Colloid Interface Sci. 2006, 300, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kiskan, B.; Antonietti, M.; Weber, J. Teaching new tricks to an old indicator: pH-Switchable, photoactive microporous polymer networks from phenolphthalein with tunable CO2 adsorption power. Macromolecules 2012, 45, 1356–1361. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Osburn, P.L.; Li, C. Soluble polymer-supported catalysts containing azo dyes. Org. Lett. 2002, 4, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.J.; Otteson, K.M.; Wang, P.C.; Wingard, R.E., Jr. Soluble functional polymers. 2. Utilization of water-insoluble chromophores in water-soluble polymeric dyes. Macromolecules 1978, 11, 320–324. [Google Scholar]
- Schaap, A.P.; Thayer, A.L.; Blossey, E.C.; Neckers, D.C. Polymer-based sensitizers for photooxidations. II. J. Am. Chem. Soc. 1975, 97, 3741–3745. [Google Scholar] [CrossRef]
- Milliken Chemical. Available online: http://millikenchemical.com/versatint-water-soluble-colorant/ (accessed on 21 February 2015).
- Fleischmann, C.; Wöhlk, H.; Ritter, H. End group functionalization of poly(ethylene glycol) with phenolphthalein: Towards star-shaped polymers based on supramolecular interactions. Beilstein J. Org. Chem. 2014, 10, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Maatz, G.; Maciollek, A.; Ritter, H. Cyclodextrin-induced host–guest effects of classically prepared poly(NIPAM) bearing azo-dye end groups. Beilstein J. Org. Chem. 2012, 8, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Alinsafi, A.; Khemis, M.; Pons, M.; Leclerc, J.; Yaacoubi, A.; Benhammou, A.; Nejmeddine, A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. 2005, 44, 461–470. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-S.; Chen, M.-L. Decolourization of dye-containing solutions by electrocoagulation. J. Appl. Electrochem. 1994, 24, 785–790. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Studies on adsorption of dyes on β-cyclodextrin polymer. Bioresource Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Vebrel, J.; Knorr, M.; Sauvin, G.; Conte, E. Starch-Modified Filters Used for the Removal of Dyes from Waste Water; Macromolecular Symposia; Wiley Online Library: Weinheim, Gemany, 2003; pp. 165–172. [Google Scholar]
- Chiou, M.; Li, H. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Progr. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Torres, J.D.; Faria, E.A.; Dias, S.C.L. Comparative adsorption studies of indigo carmine dye on chitin and chitosan. J. Colloid Interface Sci. 2004, 277, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, N.; Khojasteh, Z.; Sharghi, H.; Shamsipur, M. Lead ion-selective membrane electrodes based on some recently synthesized 9,10-anthraquinone derivatives. Anal. Chim. Acta 1998, 360, 203–208. [Google Scholar] [CrossRef]
- Reza Pouretedal, H.; Forghaniha, A.; Sharghi, H.; Shamsipur, M. Lead-selective membrane potentiometric sensor based on a recently synthesized bis(anthraquinone) sulfide derivative. Anal. Lett. 1998, 31, 2591–2605. [Google Scholar] [CrossRef]
- Rahmani, A.; Barzegar, M.; Shamsipur, M.; Sharghi, H.; Mousavi, M. New potentiometric membrane sensors responsive to Pb(ii) based on some recently synthesized 9,10-anthraquinone derivatives. Anal. Lett. 2000, 33, 2611–2629. [Google Scholar] [CrossRef]
- Voss, G.; Drechsler, M.; Eller, S.; Gradzielski, M.; Gunzelmann, D.; Mondal, S.; van Smaalen, S.; Voertler, C.S. Polymeric colorants: Statistical copolymers of indigo building blocks with defined structures. Helv. Chim. Acta 2009, 92, 2675–2697. [Google Scholar] [CrossRef]
- Peters, A.T. 1,5-Dihydroxy-4,8-diamino-and 1,8-dihydroxy-4,5-diaminoanthraquinone-β-ethers and thioethers. Blue dyes for synthetic polymer fibres. J. Chem. Technol. Biot. 1990, 48, 135–143. [Google Scholar]
- Dixit, B.C.; Patel, H.M.; Desai, D.J.; Dixit, R.B. Studies on dyeing performance of novel acid azo dyes and mordent acid azo dyes based on 2,4-dihydroxybenzophenone. E-J. Chem. 2009, 6, 315–322. [Google Scholar] [CrossRef]
- Asquith, R.S.; Blair, H.S.; Crangle, A.A.; Riordan, E. Self-coloured polymers based on anthraquinone residues. J. Soc. Dyers Colour 1977, 93, 114–125. [Google Scholar] [CrossRef]
- Schmidt, A.; Bach, E.; Schollmeyer, E. The dyeing of natural fibres with reactive disperse dyes in supercritical carbon dioxide. Dyes Pigments 2003, 56, 27–35. [Google Scholar] [CrossRef]
- Lewis, D.M.; Vo, L.T. Dyeing cotton with reactive dyes under neutral conditions. Color. Technol. 2007, 123, 306–311. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Burkinshaw, S.; Mignanelli, M.; Froehling, P.; Bide, M. The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dyes Pigments 2000, 47, 259–267. [Google Scholar] [CrossRef]
- Ebbesen, M.F.; Bienk, K.; Deleuran, B.W.; Howard, K.A. Extended blood circulation and joint accumulation of a p(HPMA-co-AzMA)-based nanoconjugate in a murine model of rheumatoid arthritis. Mol. Cell. Ther. 2014, 2, 29. [Google Scholar] [CrossRef]
- Ebbesen, M.F.; Schaffert, D.H.; Crowley, M.L.; Oupický, D.; Howard, K.A. Synthesis of click-reactive hpma copolymers using raft polymerization for drug delivery applications. J. Polym. Sci. Pol. Chem. 2013, 51, 5091–5099. [Google Scholar] [CrossRef]
- Xie, S.; Natansohn, A.; Rochon, P. Recent developments in aromatic azo polymers research. Chem. Mater. 1993, 5, 403–411. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P.; Gosselin, J.; Xie, S. Azo polymers for reversible optical storage. 1. Poly[4'-[[2-(acryloyloxy)ethyl]ethylamino]-4-nitroazobenzene]. Macromolecules 1992, 25, 2268–2273. [Google Scholar]
- Xu, G.; Yang, Q.G.; Si, J.; Liu, X.; Ye, P.; Li, Z.; Shen, Y. Application of all-optical poling in reversible optical storage in azopolymer films. Opt. Commun. 1999, 159, 88–92. [Google Scholar] [CrossRef]
- Weigert, F. Über einen neuen effekt der strahlung. Z. Phys. 1921, 5, 410–427. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Xia, X.; Ge, J.; Xu, Q.; Lu, J. Flash memory effects based on styrene/maleimiade copolymers with pendant azobenzene chromophores. Eur. Polym. J. 2011, 47, 1160–1167. [Google Scholar] [CrossRef]
- Pham, V.P.; Galstyan, T.; Granger, A.; Lessard, R.A. Novel azo dye-doped poly (methyl methacrylate) films as optical data storage media. Jpn. J. Appl. Phys. 1997, 36, 429. [Google Scholar] [CrossRef]
- Crenshaw, B.R.; Weder, C. Deformation-induced color changes in melt-processed photoluminescent polymer blends. Chem. Mater. 2003, 15, 4717–4724. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G. Mechanochromic polymer blends. J. Mater. Chem. 2011, 21, 8282–8291. [Google Scholar] [CrossRef]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-linked addition polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, G.; Wong, B.M.; McElhanon, J.R. Stress sensing in polycaprolactone films via an embedded photochromic compound. ACS App. Mat. Interfaces 2010, 2, 1594–1600. [Google Scholar] [CrossRef]
- Le Gall, T.; Reiman, K.H.; Grossel, M.C.; Owen, J.R. Poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene): A new organic polymer as positive electrode material for rechargeable lithium batteries. J. Power Sources 2003, 119, 316–320. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Anthraquinone based polymer as high performance cathode material for rechargeable lithium batteries. Chem. Commun. 2009, 448–450. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, S.; Han, S.; Yang, Z.; Ye, L.; Yang, T. Organic light-emitting diodes with azo films as electrodes. Synth. Met. 2000, 114, 251–254. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Müller, D.C.; Nothofer, H.-G.; Scherf, U.; Neher, D.; Bräuchle, C.; Meerholz, K. Improving the performance of doped π-conjugated polymers for use in organic light-emitting diodes. Nature 2000, 405, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Benning, S.; Kitzerow, H.-S.; Bock, H.; Achard, M.-F. Fluorescent columnar liquid crystalline 3,4,9,10-tetra-(N-alkoxycarbonyl)-perylenes. Liq. Cryst. 2000, 27, 901–906. [Google Scholar] [CrossRef]
- Ranke, P.; Bleyl, I.; Simmerer, J.; Haarer, D.; Bacher, A.; Schmidt, H. Electroluminescence and electron transport in a perylene dye. Appl. Phys. Lett. 1997, 71, 1332–1334. [Google Scholar] [CrossRef]
- Li, L.; Guan, M.; Cao, G.; Li, Y.; Zeng, Y. Highly efficient and stable organic light-emitting diodes employing MoO3-doped perylene-3,4,9,10-tetracarboxylic dianhydride as hole injection layer. Appl. Phys. A 2010, 99, 251–254. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and Dyes: Developments and Applications. Polymers 2015, 7, 717-746. https://doi.org/10.3390/polym7040717
Fleischmann C, Lievenbrück M, Ritter H. Polymers and Dyes: Developments and Applications. Polymers. 2015; 7(4):717-746. https://doi.org/10.3390/polym7040717
Chicago/Turabian StyleFleischmann, Carolin, Melanie Lievenbrück, and Helmut Ritter. 2015. "Polymers and Dyes: Developments and Applications" Polymers 7, no. 4: 717-746. https://doi.org/10.3390/polym7040717
APA StyleFleischmann, C., Lievenbrück, M., & Ritter, H. (2015). Polymers and Dyes: Developments and Applications. Polymers, 7(4), 717-746. https://doi.org/10.3390/polym7040717