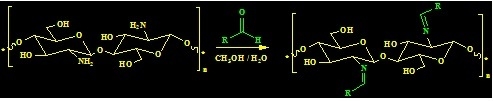
Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Chitosan
2.3. General Procedures for Chitosan Schiff-Base Synthesis
2.4. General Procedures for Reduction of Imine by NaBH4
2.5. Antimicrobial Activity
2.6. Antiproliferative Activity Screening
3. Results and Discussion
3.1. Synthesis of Chitosan Schiff’s Base (CSB) and Chitosan Amine Derivatives
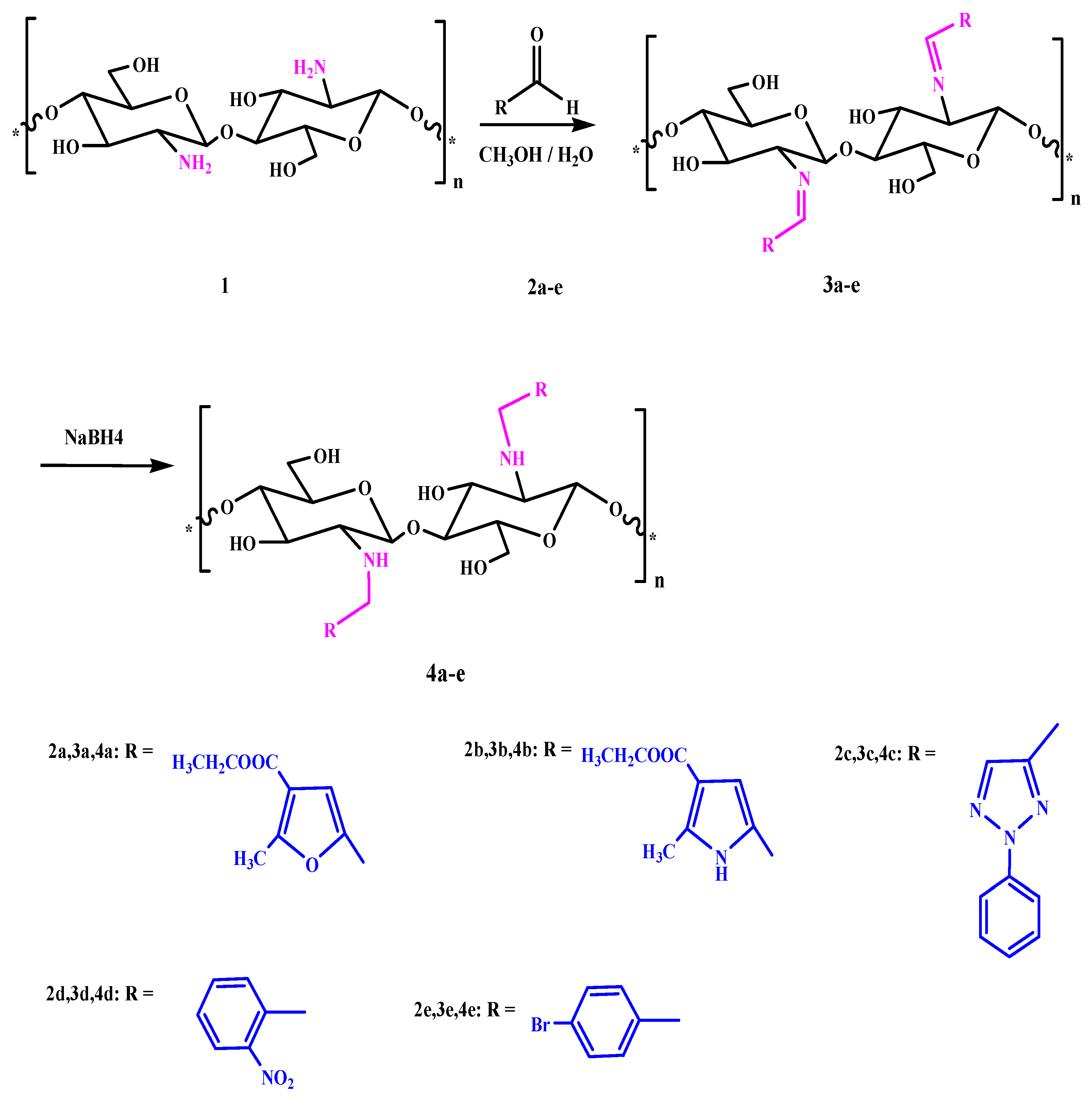
3.2. Characterization of Chitosan-Imine and Chitosan-Amine Derivatives
| Compound Numner | γ KBr Max cm−1 | |||
|---|---|---|---|---|
| C=N | COOEt | NH | OH | |
| 3a | 1642 | 1687 | - | 3440 |
| 3b | 1599 | 1655 | 2976 | 3322 |
| 3c | 1639 | - | - | 3455 |
| 3d | 1638 | - | - | 3480 |
| 3e | 1637 | - | - | 3466 |
| 4a | - | 1647 | 3254 | 3322 |
| 4b | - | 1659 | 3334 | 3387 |
| 4c | - | - | 3260 | 3321 |
| 4d | - | - | 3331 | 3340 |
| 4e | - | - | 3264 | 3398 |
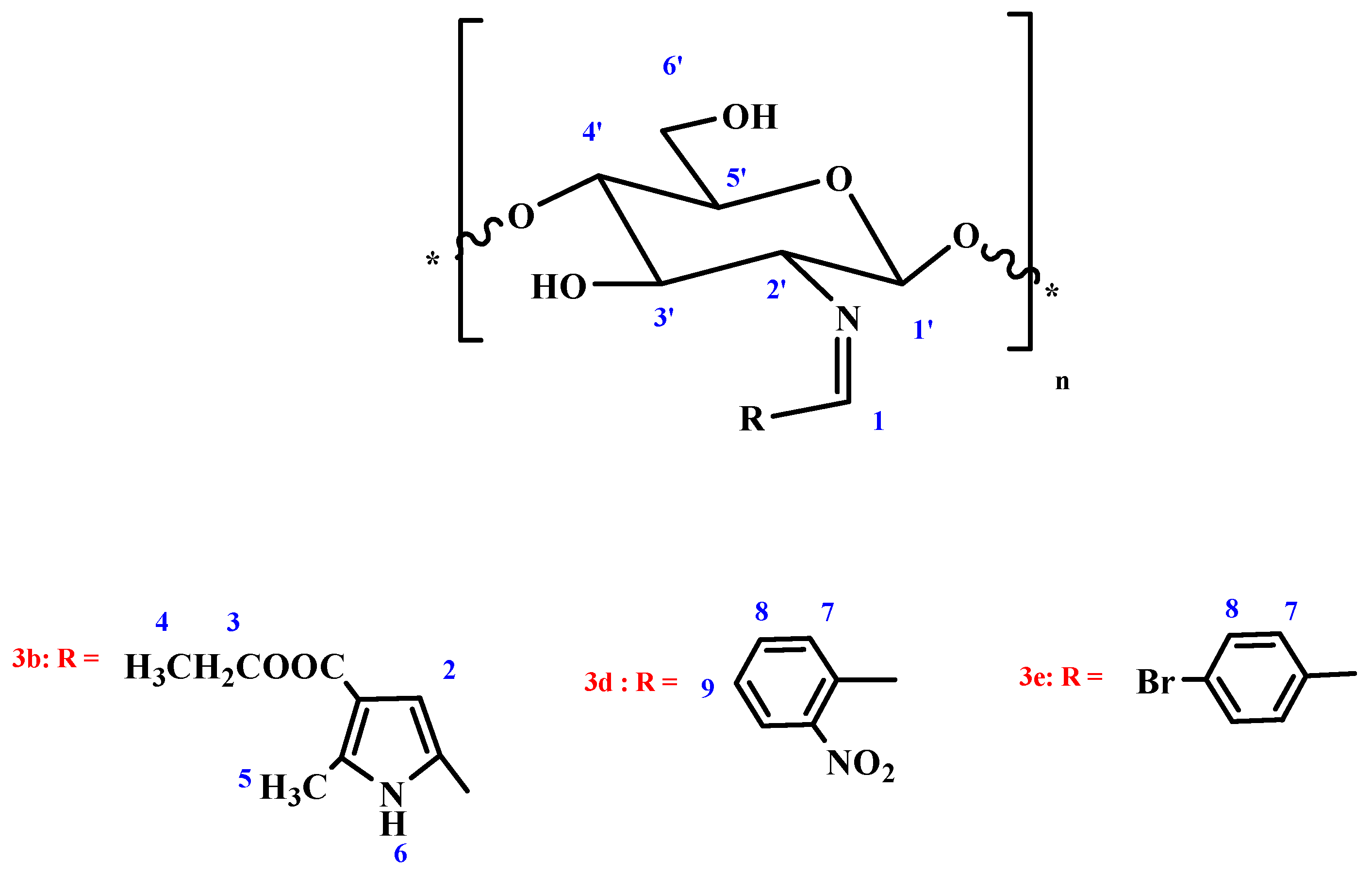
| Compound Numner | δ (ppm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | ||||||||
| 3b | 6.51 (s) | 6.55 (s) | 1.93 (s) | 3.17 (s) | 3.25 (d) | 10.33 (bs) | - | - | - | |||||||
| 3d | 6.77 (s) | - | - | - | - | - | 6.98 (d) | 7.83 (d) | 7.83 (d) | |||||||
| 3e | 7.42 (s) | - | - | - | - | - | 7.53 (d) | 7.84 (d) | - | |||||||
| Compound Numner | δ (ppm) | |||||||||||||||
| H-1′ | H-2′ | H-3′ | H-4′ | H-5′ | H-6a′, H-6b′ | 3′-OH | 6′-OH | |||||||||
| 3b | 2.38 (m) | 2.41 (m) | 2.41 (m) | 2.61 (d) | 2.61 (d) | 2.75 (m) | 4.53 (bs) | 4.53 (bs) | ||||||||
| 3d | 3.56 (m) | 3.68 (m) | 3.83 (d) | 3.83 (d) | 3.90 (m) | 4.07 (m) | 5.44 (bs) | 5.44 (bs) | ||||||||
| 3e | 3.27 (m) | 3.38 (m) | 3.54 (d) | 3.61 (m) | 3.61 (m) | 3.73 (m) | 5.73 (bs) | 5.73 (bs) | ||||||||
| Sample | Tested Microorganisms | |||
|---|---|---|---|---|
| Gram Positive Bacteria | Gram Negative Bacteria | |||
| S. aureus | B. subtilis | P. aeruginosa | E. coli | |
| 3a | 22.2 ± 0.58 | 24.6 ± 0.25 | NA | 21.4 ± 0.63 |
| 3b | 18.2 ± 0.63 | 20.4 ± 0.58 | NA | 18.3 ± 0.72 |
| 3c | 16.7 ± 0.36 | 19.2 ± 0.27 | 13.3 ± 0.36 | 13.6 ± 0.36 |
| 3d | 11.3 ± 0.63 | 14.2 ± 0.58 | NA | 11.1 ± 0.63 |
| 3e | 21.4 ± 0.63 | 22.3 ± 0.72 | NA | 21.2 ± 0.63 |
| Ampicillin | 23.8 ± 0.2 | 32.4 ± 0.3 | NA | NA |
| Gentamicin | NA | NA | 17.3 ± 0.1 | 19.9 ± 0.3 |
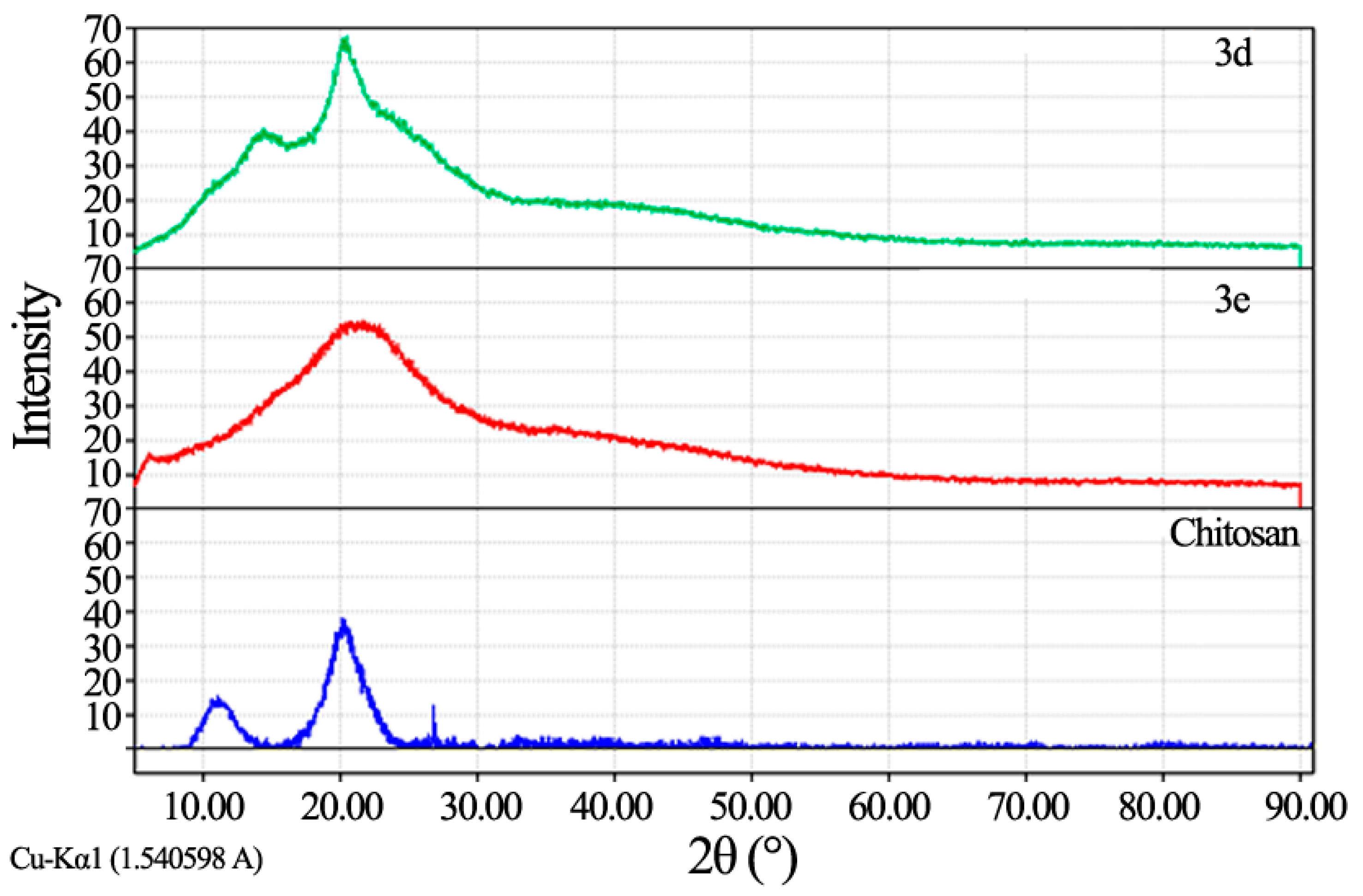
3.3. Antimicrobial Activity
| Sample | Tested Microorganisms | |
|---|---|---|
| A. fumigates | S. rumracemosum | |
| 3a | 21.3 ± 0.63 | NA |
| 3b | 16.3 ± 0.72 | NA |
| 3c | 16.8 ± 0.39 | 13.4 ± 0.58 |
| 3d | 13.2 ± 0.72 | NA |
| 3e | 19.6 ± 0.58 | NA |
| Amphotericin B | 23.7 ± 0.1 | 19.7 ± 0.2 |
| Tested Microorganism | Samples | Standard | ||||
|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 3d | 3e | ||
| Fungi | MIC (µg/mL) | AmphotericinB | ||||
| Aspergillus Fumigates | 1.95 | 31.25 | 62.5 | 62.5 | 3.9 | 0.98 |
| Gram positive bacteria | - | Ampicillin | ||||
| Staphilococcus aureus | 1.95 | 7.81 | 125 | 125 | 1.95 | 0.98 |
| Bacillis subtilis | 0.98 | 3.9 | 62.5 | 125 | 1.95 | 0.49 |
| Gram negative bacteria | - | Gentamicin | ||||
| Escherichia coli | 1.95 | 7.81 | 125 | 125 | 1.95 | 3.9 |
3.4. Antiproliferative Activity Screening
| Sample conc. (μg/mL) | Viability % | |||||
|---|---|---|---|---|---|---|
| 3c | Doxorubicin (std.) | |||||
| HepG-2 | MCF-7 | HCT-116 | HepG-2 | MCF-7 | HCT-116 | |
| 0 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 1 | 0.58716 | 0.652078 | 0.83326 | 0.5451 | 0.4256 | 0.3902 |
| 2.5 | 0.19456 | 0342510 | 0.54419 | 0.3903 | 0.2989 | 0.2900 |
| 5 | 0.25484 | 0.330447 | 0.29740 | 0.2500 | 0.2000 | 0.1990 |
| 10 | 0.20469 | 0.320388 | 0.25028 | 0.1959 | 0.1443 | 0.1510 |
| IC50 | 1.21 | 1.54 | 2.89 | 1.2 | 0.44 | 0.47 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, M.; Ma, Y.; Wang, C.; Liu, H.; Li, Q.; Fei, M. Synthesis, anti-oxidant activity, and biodegradability of a novel recombinant polysaccharide derived from chitosan and lactose. Carbohydr. Polym. 2015, 118, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Noguchi, K.; Yanagi, M.; Shinoyama, H.; Kagawa, Y.; Hirata, H.; Yabuki, M.; Fujii, T. Primary structre of chitosanase produced by Bacillus circulans MH-K1. J. Gen. Appl. Microbiol. 1992, 38, 135–144. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F.H.; Yu, R.F.; Lee, Y.C. Using chitosan as coagulant in recovery of organic matters from the mash and lautar wastewater of brewery. J. Polym. Environ. 2005, 13, 383–388. [Google Scholar] [CrossRef]
- Bugg, T.D.; Walsh, C.T. Intracellular steps of bacterial cell wall peptidoglycan biosynthesis: Enzymology, antibiotics, and antibiotic-resistance. Nat. Prod. Rep. 1992, 9, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Tasaih, M.L. Effect of temperature on the intrinsic viscosity and conformation of chitosans in dilute HCl solution. Int. J. Biol. Macromol. 1998, 23, 135–141. [Google Scholar] [CrossRef]
- Kpping-Hggrd, M.; Tubulekas, I.; Guan, H.; Edwards, K.; Nilsson, M.; Varum, K.; Artursson, P. Chitosan as a nonviral gene delivery system. Structure–property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001, 8, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Yuan, S.; Wei, T. New contact lens based on chitosan/gelatin composites. J. Bioact. Compat. Polym. 2004, 19, 467–479. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Esam, A.E.; Mohamed, M.N.; Abdul, H.Y. Rheological and morphological studies of chitosan/agar/poly(vinyl alcohol) blends. J. Appl. Sci. Res. 2010, 6, 460–468. [Google Scholar]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Org. 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharides mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activity of chito-oligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003, 51, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Saranya, N.; Moorthi, A.; Saravanan, S.; Devi, M.P.; Selvamurugan, N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromol. 2011, 48, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Köping-Höggård, M.; Mel’nikova, Y.S.; Vårum, K.M.; Lindman, B.; Artursson, P. Relationship between the physical shape and the efficiency of oligomeric chitosan as a gene delivery system in vitro and in vivo. J. Gene Med. 2003, 5, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Mao, H.Q.; Huang, S.K.; Leong, K.W. Oral gene delivery with chitosan—DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999, 5, 387–391. [Google Scholar] [PubMed]
- Brode, G.L.; Goddard, E.D.; Harris, W.C.; Salensky, G.A. Cationic polysaccharides for cosmetics and therapeutics. In Cosmetics and Pharmaceutical Applications of Polymers; Gebelein, C.G., Cheng, T.C., Yang, Y.C., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 117–128. [Google Scholar]
- Akiyama, K.; Fujita, T.; Kuroshima, K.; Sanake, T.; Yokota, A.; Takata, R. Purification and gene cloning of chitosanase from Bacillus ehimensis EAG1. J. Biosci. Bioeng. 1999, 87, 383–385. [Google Scholar] [CrossRef]
- Masson, J.Y.; Bocher, I.; Neugebauer, W.A.; Ramotar, D.; Brzezinski, R. A new chitosanase gene from a Nocardioides sp. Is a third member of glycosyl hydrolase family 46. Microbiology 1995, 141, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Beloshenko, V.A.; Askadskii, A.A.; Varyukhin, V.N. Promising ways of structural modification of polymers and polymer composites with the use of high pressure. Russ. Chem. Rev. 1998, 67, 951–973. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Swenson, J.M.; Tenover, F.C.; Ferraro, M.J.; Hindler, J.A.; Murray, P.R. Development of interpretive criteria and quality control limits for broth micro dilution and disk diffusion antimicrobial susceptibility testing of Streptococcus pneumonia. J. Clin. Microbiol. 1994, 32, 2448–2459. [Google Scholar] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of antibacterial effect of silver ions on Escherichia coli and Straphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Dina, R.; Kristine, V.; Albert, H.; Hans-Georg, S. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar]
- El-Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Eweis, M.; Elkholy, S.S.; Elsabee, M.Z. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int. J. Biol. Macromol. 2006, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelwahab, H.E.; Hassan, S.Y.; Yacout, G.A.; Mostafa, M.A.; El Sadek, M.M. Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives. Polymers 2015, 7, 2690-2700. https://doi.org/10.3390/polym7121532
Abdelwahab HE, Hassan SY, Yacout GA, Mostafa MA, El Sadek MM. Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives. Polymers. 2015; 7(12):2690-2700. https://doi.org/10.3390/polym7121532
Chicago/Turabian StyleAbdelwahab, Huda E., Seham Y. Hassan, Galila A. Yacout, Mohamed A. Mostafa, and Mohamed M. El Sadek. 2015. "Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives" Polymers 7, no. 12: 2690-2700. https://doi.org/10.3390/polym7121532
APA StyleAbdelwahab, H. E., Hassan, S. Y., Yacout, G. A., Mostafa, M. A., & El Sadek, M. M. (2015). Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives. Polymers, 7(12), 2690-2700. https://doi.org/10.3390/polym7121532