The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Hydrogels
2.3. pH Determination
2.4. Particles Size Analysis
2.5. Drug Content
2.6. Determination of Gelation Time
2.7. Morphology of the Hydrogels
2.8. Viscosity Measurement
2.9. Mechanical Properties
2.10. Swelling Index Study
2.11. Determination of the in Vitro Mucoadhesive Properties
2.12. Stability Data
In Vitro Release of CLO
2.13. Cell Culture
2.14. Preparation of CS Solutions for Cytotoxicity Studies
2.15. MTT Assay
2.16. Flow Cytometry Assessment of Annexin V Binding
2.17. Fluorescent Microscopy Assay
2.18. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Hydrogels
| Component (g) | Formulation | |||
|---|---|---|---|---|
| Unmodified chitosan | Ion crosslinked chitosan | |||
| F1 | F2 | F3 | F4 | |
| CLO * | 2.0 | 2.0 | 2.0 | 2.0 |
| CS | 3.0 | 4.0 | 3.0 | 4.0 |
| Glycerolum 86% | 5.0 | 5.0 | 5.0 | 5.0 |
| Cremophor EL | 6.0 | 6.0 | 6.0 | 6.0 |
| β-GP 45% (w/w) | - | - | 4.2 ** | 5.59 ** |
| d-Glucono-1,5-lactone and sodium benzoate | 1.0 | 1.0 | 1.0 | 1.0 |
| 1.8% Acetic acid ad | 100.0 | - | 100.0 | - |
| 2.4% Acetic acid ad | - | 100.0 | - | 100.0 |
| Parameter | Formulation | |||
|---|---|---|---|---|
| Unmodified chitosan | Ion crosslinked chitosan | |||
| F1 | F2 | F3 | F4 | |
| pH | 4.06 ± 0.02 | 4.08 ± 0.01 | 4.54 ± 0.04 | 4.56 ± 0.03 |
| Drug particles size (μm) (mean ± S.D.) | 5.9–49.5 (26.8 ± 22.1) | 5.8–47.2 (27.5 ± 20.4) | 4.1–39.9 (22.9 ± 17.0) | 3.8–41.5 (24.1 ± 16.4) |
| Gelation time at 37 °C (min) | nt. | nt. | 197 | 228 |
| Drug content (%) | 95.6 ± 4.9 | 98.2 ± 3.5 | 97.3 ± 4.1 | 97.4 ± 3.3 |
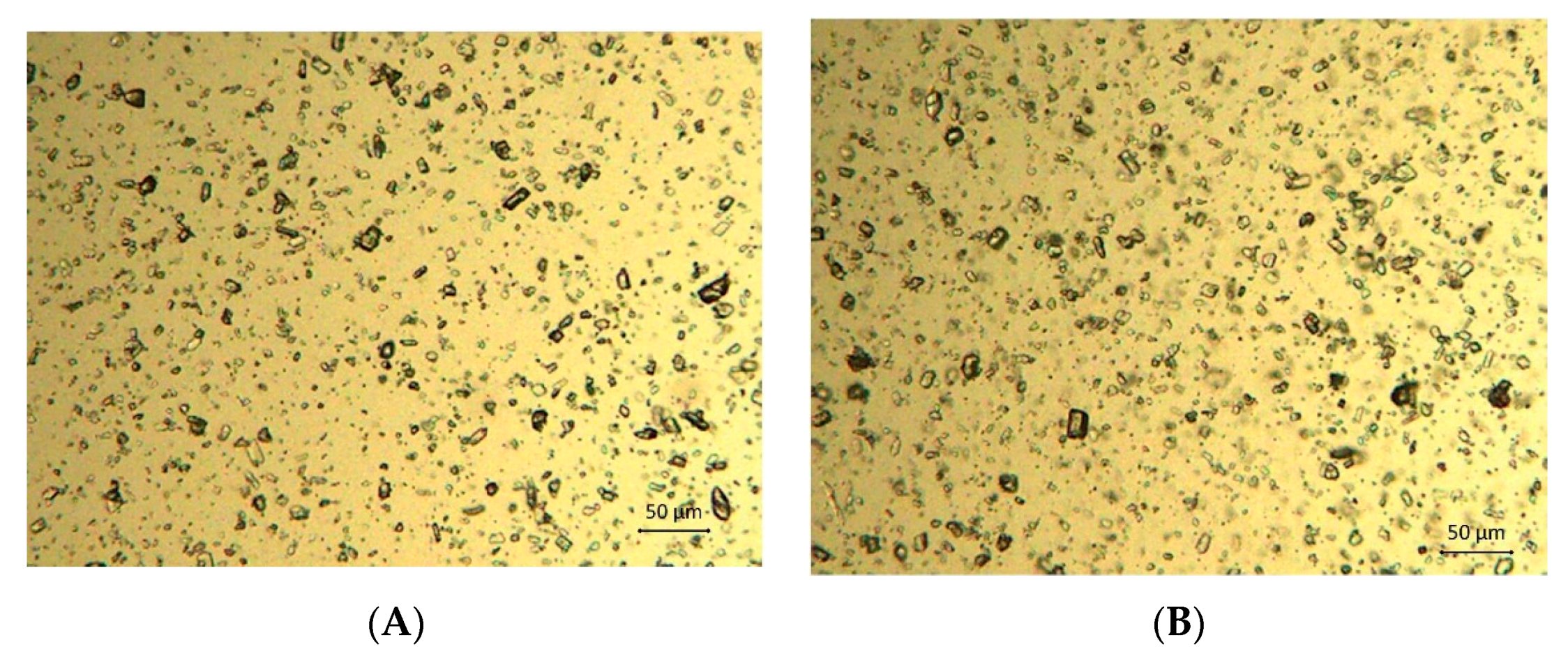

3.2. Viscosity and Mechanical Measurements
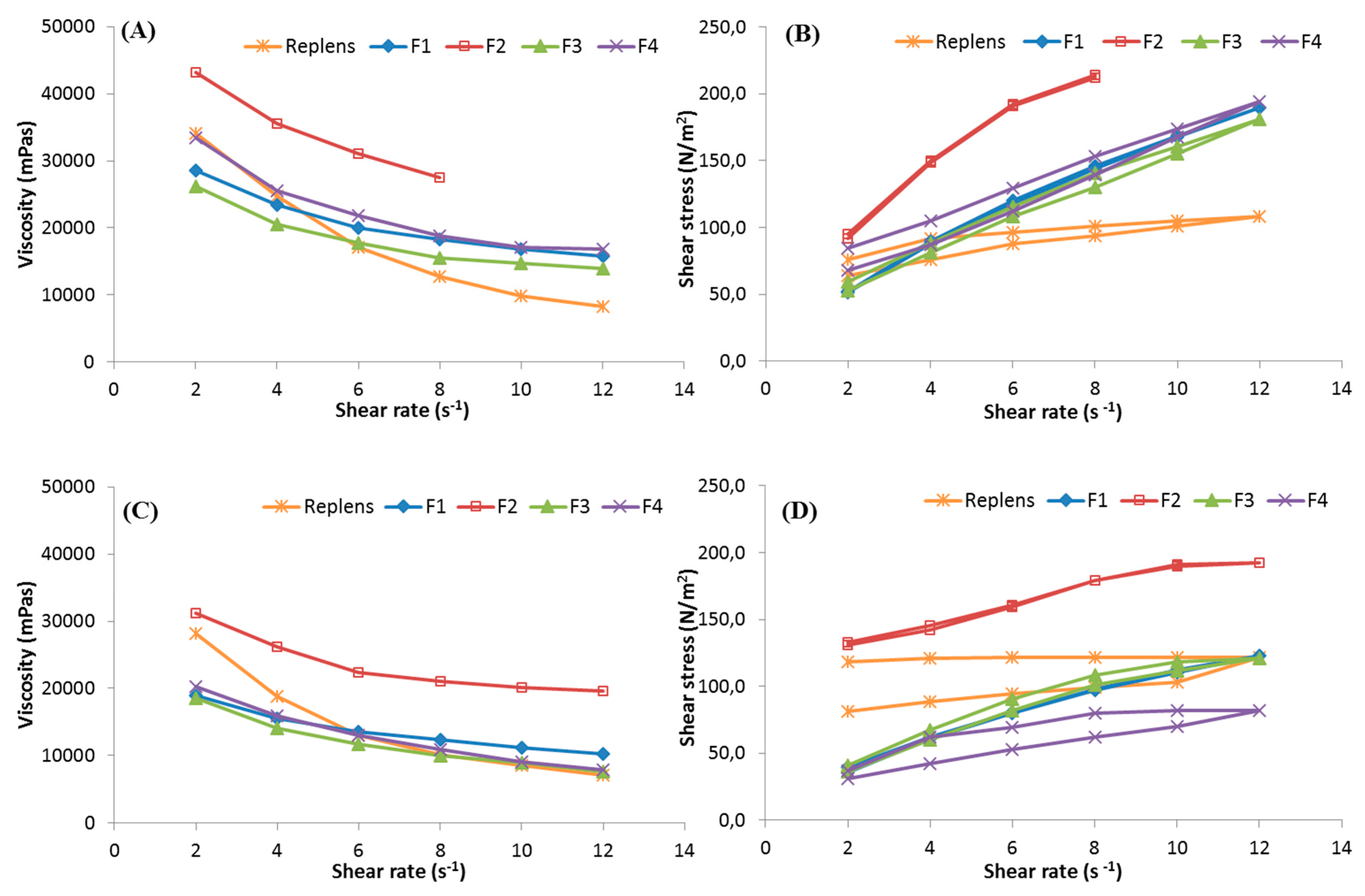
| Formulation | Viscosity (mPas) * | Viscosity (mPas) ** | Hardness (mN) | Cohesiveness (µJ) | Compressibility (mN) |
|---|---|---|---|---|---|
| Replens™ | 17,068 ± 92 | 13,047 ± 87 | 494.5 ± 10.1 | 38.6 ± 0.9 | 1,064 ± 28 |
| F1 | 19,987 ± 101 | 13,493 ± 100 | 588.6 ± 17.4 | 40.7 ± 0.1 | 1,195 ± 13 |
| F2 | 31,048 ± 85 | 23,876 ± 91 | 978.2 ± 14.9 | 67.7 ± 0.9 | 1,875 ± 31 |
| F3 | 17,809 ± 78 | 11,651 ± 81 | 562.6 ± 13.5 | 39.1 ± 0.9 | 1,096 ± 19 |
| F4 | 21,828 ± 112 | 13,013 ± 105 | 654.0 ± 12.6 | 42.2 ± 2.8 | 1,173 ± 52 |
3.3. Swelling Index and Mucoadhesion Studies
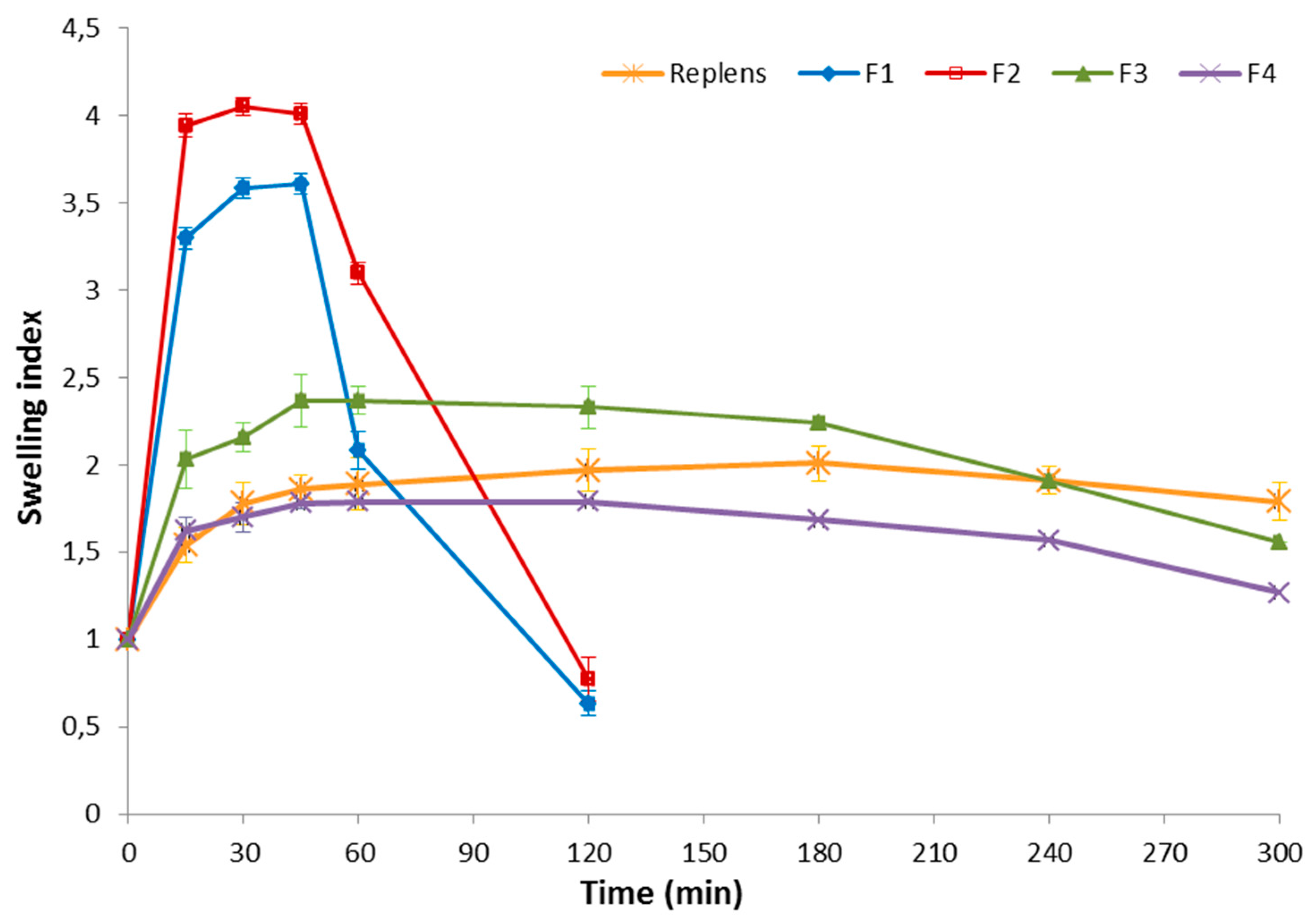
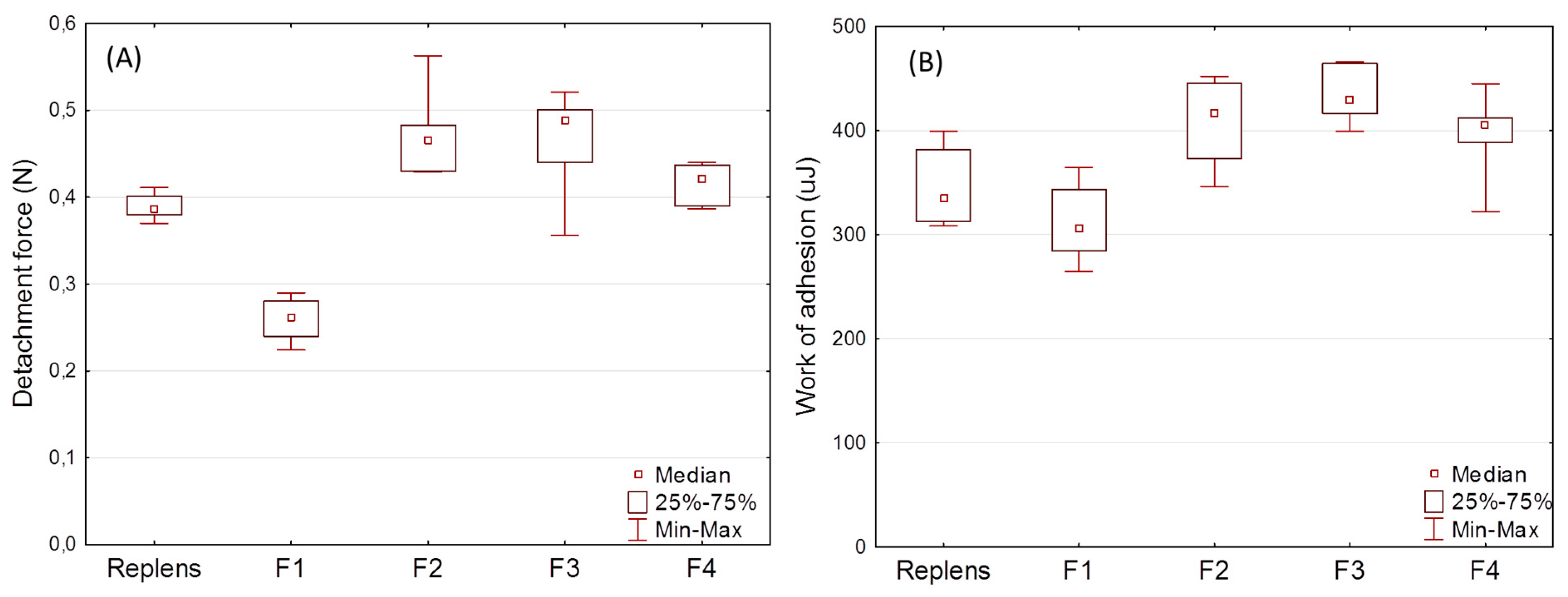
3.4. Stability Studies
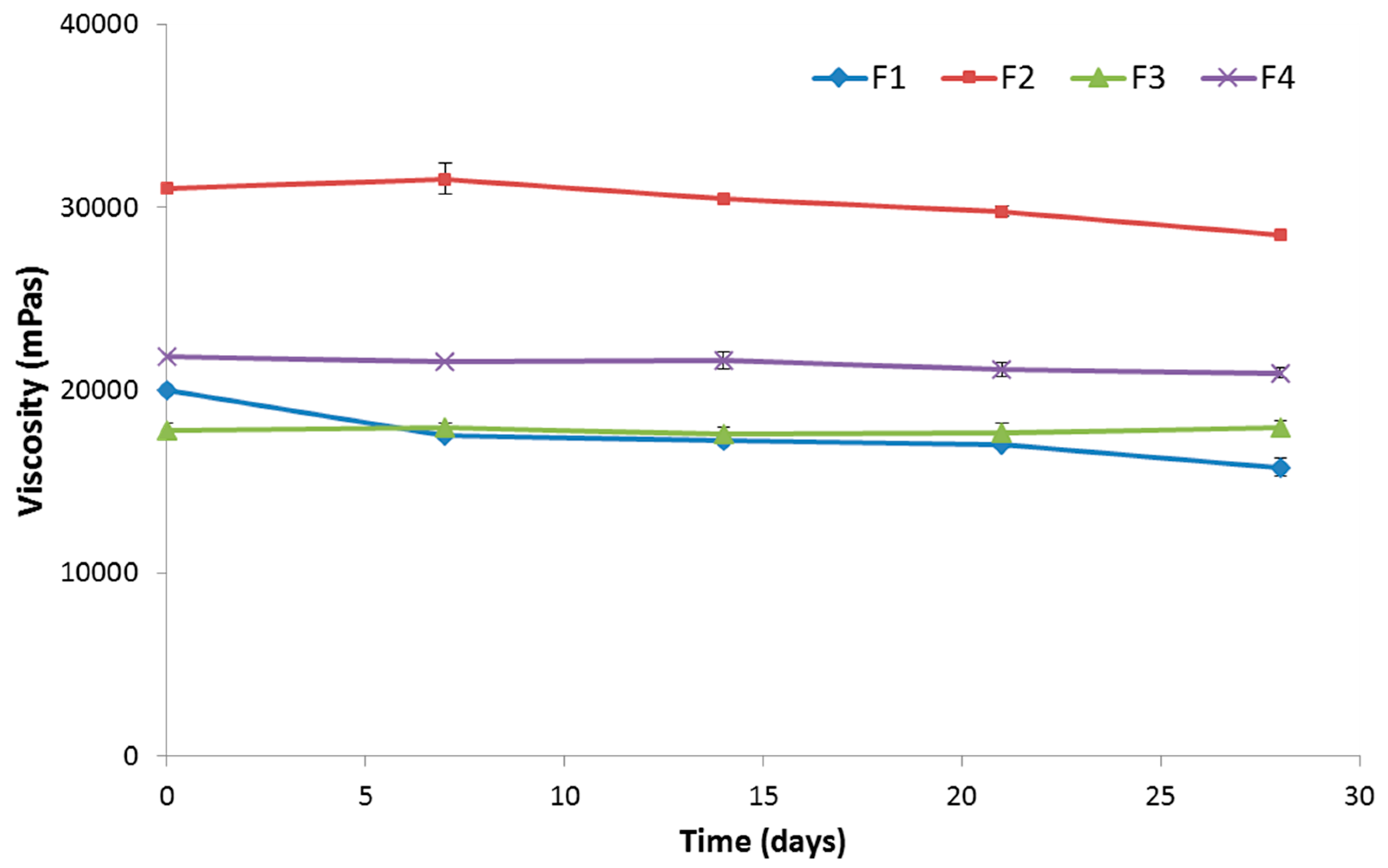
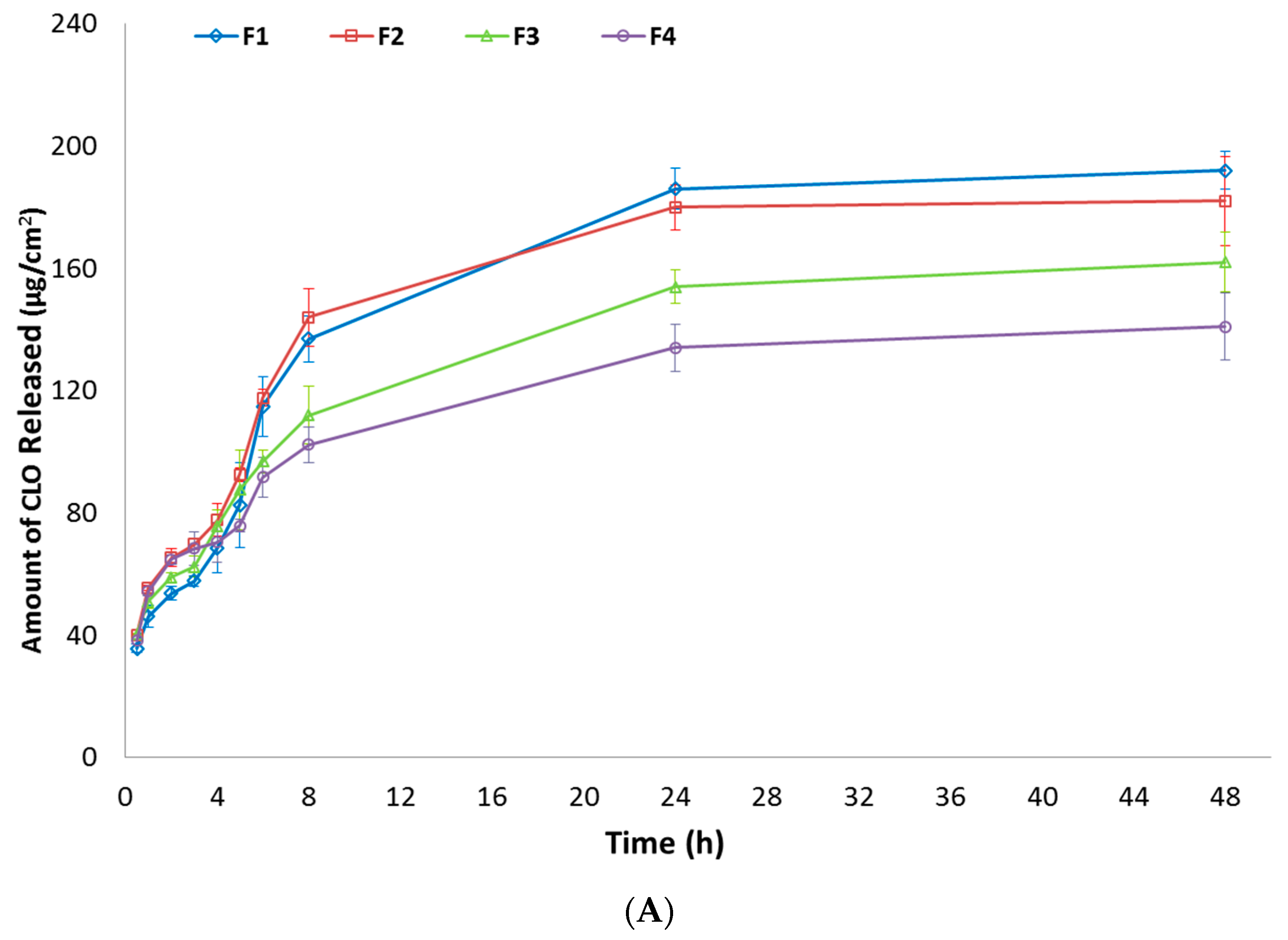

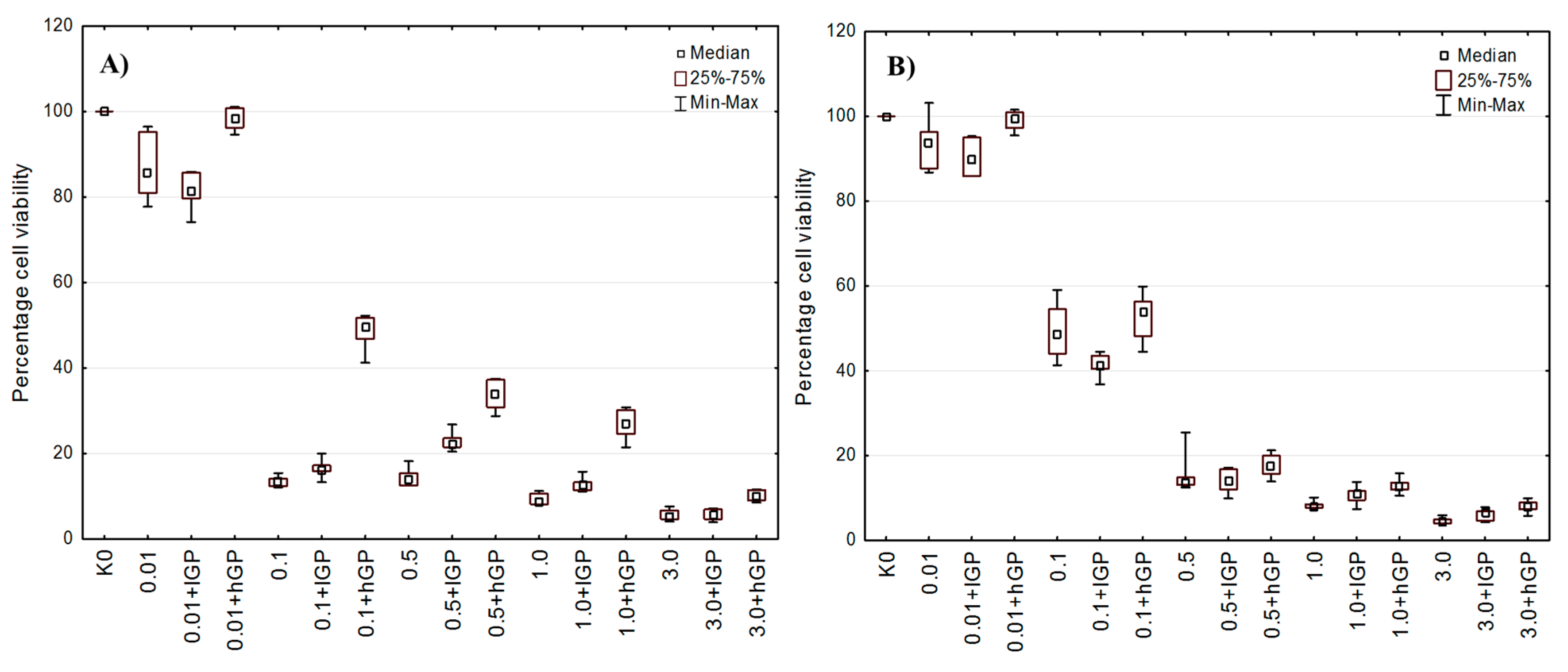
3.5. In Vitro Cytotoxicity Evaluation
| Abbreviation | CS (mg·mL−1) + β-GP (mg·mL−1) | ||||
|---|---|---|---|---|---|
| CS/lGP | 0.01 + 0.006 | 0.1 + 0.063 | 0.5 + 0.315 | 1.0 + 0.630 | 3.0 + 1.890 |
| CS/hGP | 0.01 + 0.04 | 0.1 + 0.40 | 0.5 + 2.00 | 1.0 + 4.00 | 3.0 + 12.00 |
| Abbreviation | Description | |
|---|---|---|
| K0 | Non treated cells | |
| K1 | Cells treated with: | Acetic buffer pH 4.0 |
| K2 | Acetic buffer pH 4.5 | |
| K3 | Acetic buffer pH 7.0 | |
| K4 | Aqueous solution of low β-GP * | |
| K5 | Aqueous solution of high β-GP * | |
| 0.01–3.0 | Concentrations of unmodified CS (mg·mL−1) | |
| (0.01–3.0) + lGP | CS crosslinked with low β-GP (CS:β-GP 1.0:0.63) | |
| (0.01–3.0) + hGP | CS crosslinked with high β-GP (CS:β-GP 1.0:4.0) | |
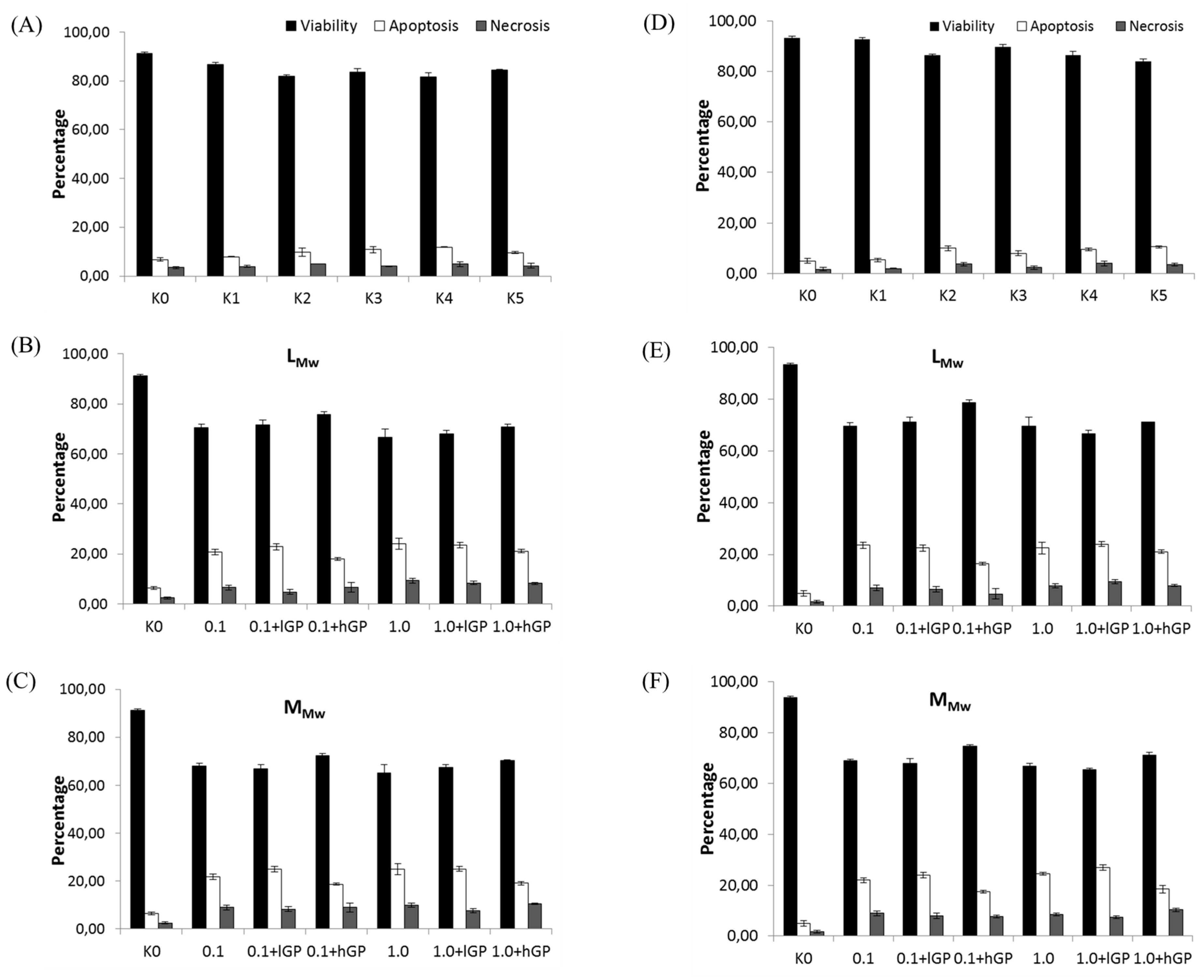
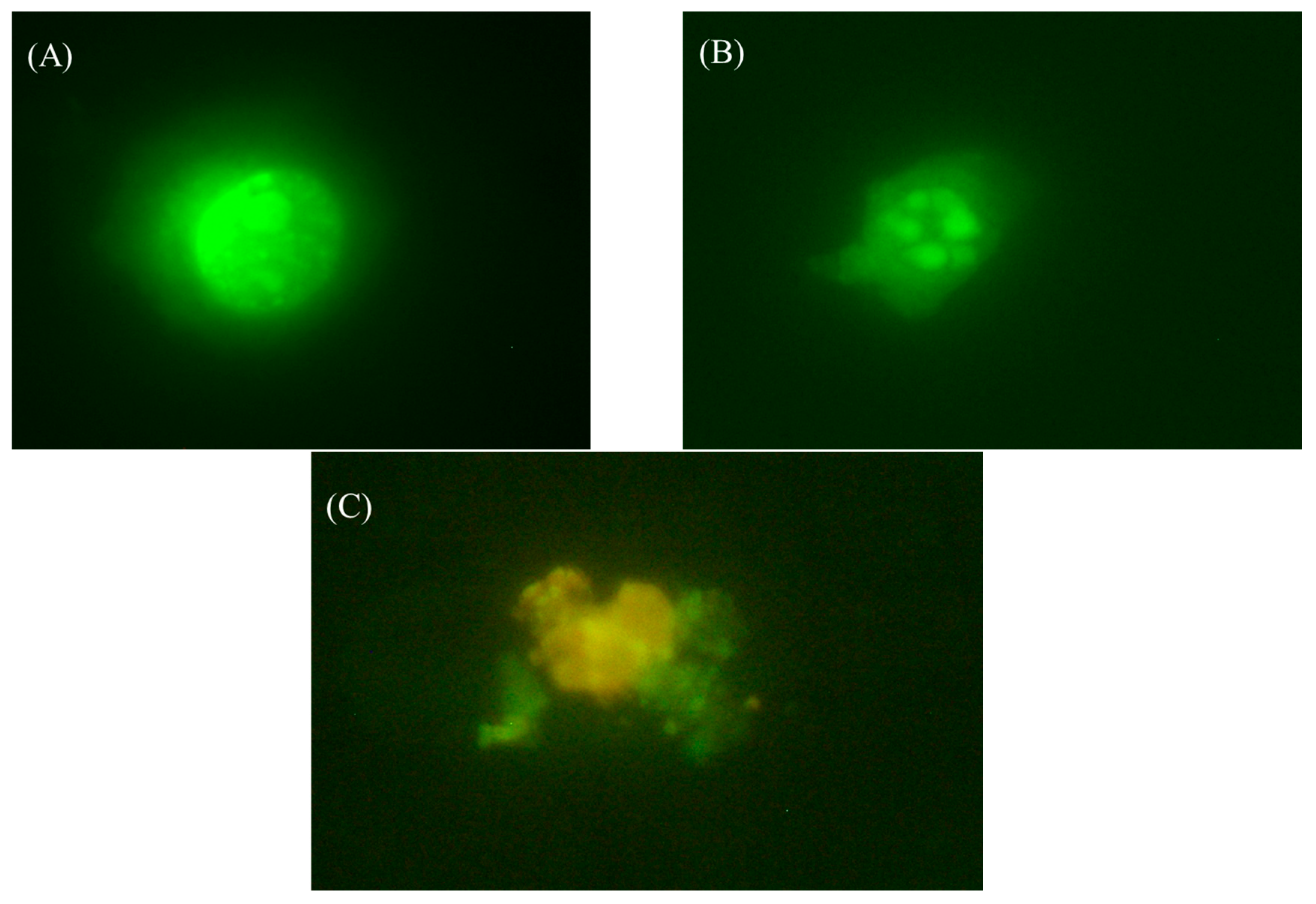
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chien, Y.W. Drug delivery: Vaginal Route. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, pp. 1339–1361. [Google Scholar]
- Sandri, G.; Rossi, S.; Ferrari, S.; Bonferoni, M.C.; Caramella, C. Strategies to Improve Systemic and Local Availability of Drugs Administered via Vaginal Route. In Enhancement in Drug Delivery, 1st ed.; Touitou, B., Barry, B.W., Eds.; Taylor and Francis CRC Press: Boca Raton, FI, USA, 2007; pp. 441–470. [Google Scholar]
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; ElMeshad, A.N.; Fares, A.R. Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: Formulation and in vitro evaluation. AAPS PharmSciTech 2014, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.H.G.; Kazi, M. Lyophilized chitosan/xanthan polyelectrolyte complex based mucoadhesive inserts for nasal delivery of promethazine hydrochloride. Iran. J. Pharm. Res. 2014, 13, 769–784. [Google Scholar] [PubMed]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatin. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.C.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef]
- Shaji, J.; Jain, V.; Lodha, S. Chitosan: A Novel Pharmaceutical Excipient. Int. J. Pharm. Appl. Sci. 2010, 1, 11–28. [Google Scholar]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. Influence of unmodified and beta-glycerophosphate crosslinked chitosan on anti-Candida activity of clotrimazole in semi-solid delivery systems. Int. J. Mol. Sci. 2014, 15, 17765–17777. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, A.; Ribeiro, M.P.; Palmeira-de-Oliveira, R.; Gaspar, C.; Costa-de-Oliveira, S.; Correia, I.J.; Pina, V.; Martinez-de-Oliveira, J.; Queiroz, J.A.; Rodrigues, A.G. Anti-Candida activity of a chitosan hydrogel: Mechanism of action and cytotoxicity profile. Gynecol. Obstet. Investig. 2010, 70, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Almomen, A.; Cho, S.; Yang, C.H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Senyiğit, Z.A.; Karavana, S.Y.; Eraç, B.; Gürsel, O.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M. Intravaginal drug delivery systems. Design, challenges, and solutions. Am. J. Drug Deliv. 2003, 1, 241–254. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Aliaghaie, M.; Mirzadeh, H.; Dashtimoghadam, E.; Taranejoo, S. Investigation of gelation mechanism of an injectable hydrogel based on chitosan by rheological measurements for a drug delivery application. Soft Matter 2012, 8, 3128–3137. [Google Scholar] [CrossRef]
- Ding, K.; Yang, Z.; Zhang, Y.L.; Xu, J.Z. Injectable thermosensitive chitosan/β-glycerophosphate/collagen hydrogel maintains the plasticity of skeletal muscle satellite cells and supports their in vivo viability. Cell Biol. Int. 2013, 37, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A.; Carver, P.L. Use of azoles for systemic antifungal therapy. Adv. Pharmacol. 1997, 39, 143–189. [Google Scholar] [PubMed]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination degree of deacetylation of chitosan: Comparison of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- EMA: Analytical Sieving General Chapter. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500044305.pdf (accessed on 19 October 2015).
- Depani, B.P.; Naik, A.A.; Nair, H.A. Preparation and evaluation of chitosan based thermoreversible gels for intraperitoneal delivery of 5-fluorouracil (5-FU). Acta Pharm. 2013, 63, 479–491. [Google Scholar] [CrossRef]
- Garg, T.; Chanana, A.; Joshi, R. Preparation of chitosan scaffolds for tissue engineering using freeze drying technology. IOSR J. Pharm. 2012, 2, 72–73. [Google Scholar] [CrossRef]
- Wróblewska, M.; Winnicka, K. The effect of cationic polyamidoamine dendrimers on physicochemical characteristics of hydrogels with erythromycin. Int. J. Mol. Sci. 2015, 16, 20277–20289. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Degraff, W.; Gazdar, A.; Minna, J.; Mitchell, J. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of β-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeia, 29th ed.; the United States Pharmacopeial Convention: Rockville, MD, USA, 2011; Volume 2, pp. 1998–1999.
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Inamdar, M.S. Aqueous behaviour of chitosan. Int. J. Polym. Sci. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Garg, S.; Anderson, R.A.; Chany, C.J., II; Waller, D.P.; Diao, X.H.; Vermani, K.; Zaneveld, L.J. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM). Contraception 2001, 64, 67–75. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Dynamic rheological properties of concentrated chitosan solutions. Appl. Rheol. 2004, 14, 140–147. [Google Scholar]
- Zhang, X.Z.; Wu, D.Q.; Chu, C.C. Effect of the crosslinking level on the properties of temperature-sensitive poly(N-isopropylacrylamide) hydrogels. J. Polym. Sci. Polym. Phys. 2003, 41, 582–593. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Wan, L.S.C.; Heng, P.W.S.; Won, L.F. Relationship between swelling and drug release in a hydrophilic matrix. Drug Dev. Ind. Pharm. 1993, 19, 1201–1210. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Płaczek, M.; Sznitowska, M. The mucoadhesion phenomena and importance in drug application. Polim. Med. 2009, 39, 49–64. [Google Scholar] [PubMed]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Erbeck, D.; Uckun, F.M. A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol. Pathol. 2005, 33, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Gonçalves, I.C.; Nardecchia, S.; Amaral, I.F.; Barbosa, M.A.; Martins, M.C. Modulation of stability and mucoadhesive properties of chitosan microspheres for therapeutic gastric application. Int. J. Pharm. 2013, 454, 116–124. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Smart, J.D. An investigation into the role of water movement and mucus gel dehydratation in mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- The European Pharmacopeia, 6th ed.; Council of Europe: Strasburg, France, 2007; Volume 2, pp. 1490–1491.
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.E. Some observations on the effect of bioprocessing on biopolymer stability. J. Drug Target 2010, 18, 732–740. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin crosslinked porous chitosan collagen gelatin scaffolds using chitosan-CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sabudin, S.; Derman1, M.A.; Zainol, I.; Noorsal, K. In vitro cytotoxicity and cell seeding studies of a chitosan-silver composite for potential wound management applications. J. Eng. Sci. 2012, 8, 29–37. [Google Scholar]
- Meng, J.; Zhang, T.; Agrahari, V.; Ezoulin, M.J.; Youan, B.B. Comparative biophysical properties of tenofovir-loaded, thiolated and nonthiolated chitosan nanoparticles intended for HIV prevention. Nanomedicine 2014, 9, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Dionísio, M.; Remuñán López, C.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Pavinatto, A.; Caseli, L.; dos Santos, D.S., Jr.; Nobre, T.M.; Zaniquelli, M.E.; de Oliveira, O.N., Jr. Interaction of chitosan with cell membrane models at the air-water interface. Biomacromolecules 2007, 8, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Torres, D.; Alonso, M.J. Chitosan nanocapsules as carriers for oral peptide delivery: Effect of chitosan molecular weight and type of salt on the in vitro behaviour and in vivo effectiveness. J. Nanosci. Nanotechnol. 2006, 6, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J. Biomed. Mater. Res. A 2008, 86, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Reutelingsperger, C.P.M.; McGahon, A.J.; Rader, J.A.; van Schie, R.C. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556. [Google Scholar] [CrossRef]
- Winnicka, K.; Bielawski, K.; Bielawska, A. Synthesis and cytotoxic activity of G3 PAMAM-NH2 dendrimer-modified digoxin and proscillaridin A conjugates in breast cancer cells. Pharmacol. Rep. 2010, 62, 414–423. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Martin, D.; Lenardo, C. Morphological, biochemical and flow cytometric assays of apoptosis. Curr. Protoc. Mol. Biol. 2001, 14, 1–16. [Google Scholar] [PubMed]
- Oh, H.; Livingston, R.; Smith, K.; Abrishamian-Garcia, L. Comparative study of the time dependency of cell death assays. MIT Undergrad. Res. J. 2004, 11, 53–62. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymaǹska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K. The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers 2015, 7, 2223-2244. https://doi.org/10.3390/polym7111510
Szymaǹska E, Sosnowska K, Miltyk W, Rusak M, Basa A, Winnicka K. The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers. 2015; 7(11):2223-2244. https://doi.org/10.3390/polym7111510
Chicago/Turabian StyleSzymaǹska, Emilia, Katarzyna Sosnowska, Wojciech Miltyk, Małgorzata Rusak, Anna Basa, and Katarzyna Winnicka. 2015. "The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application" Polymers 7, no. 11: 2223-2244. https://doi.org/10.3390/polym7111510
APA StyleSzymaǹska, E., Sosnowska, K., Miltyk, W., Rusak, M., Basa, A., & Winnicka, K. (2015). The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers, 7(11), 2223-2244. https://doi.org/10.3390/polym7111510









