Modeling the Pyrolysis and Combustion Behaviors of Non-Charring and Intumescent-Protected Polymers Using “FiresCone”
Abstract
:1. Introduction
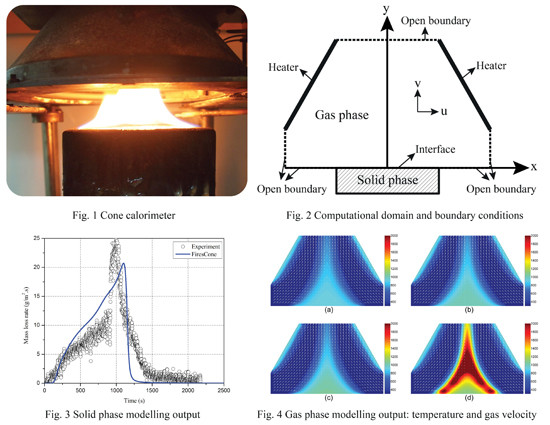
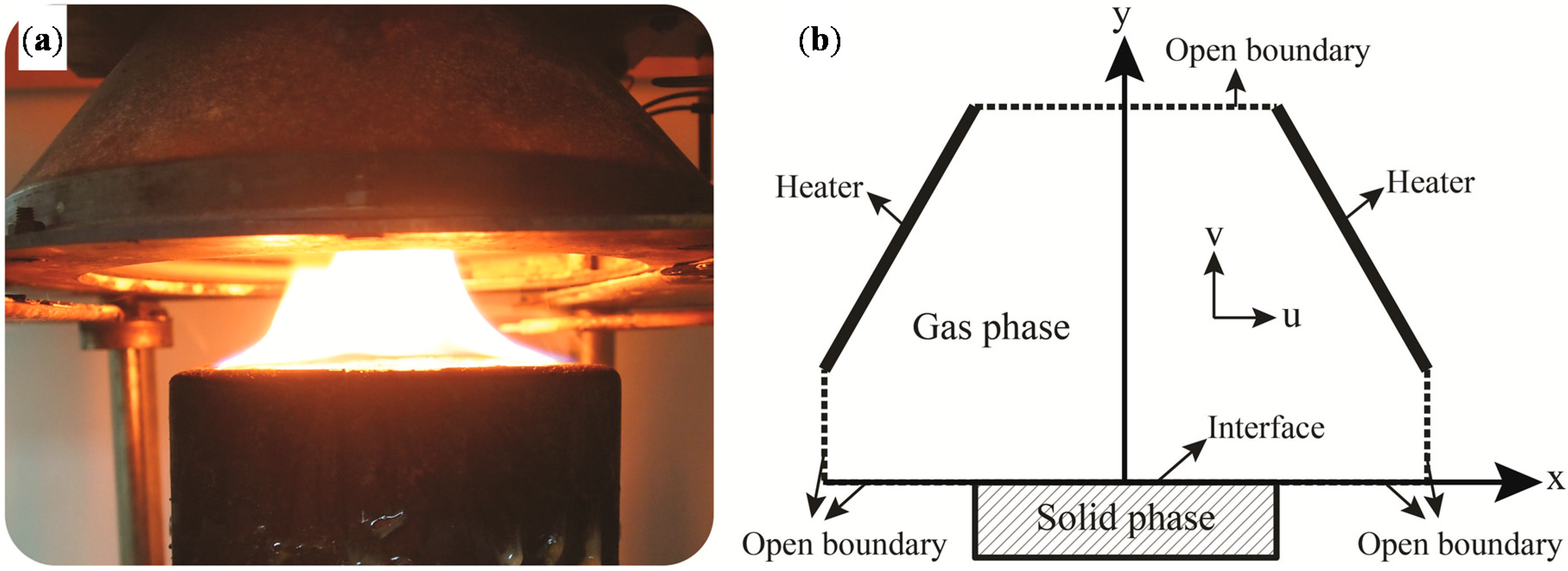
2. Experimental Methodology
| Item | Description | Thickness (mm) | Heat flux (kW/m2) | Formula |
|---|---|---|---|---|
| Material | PMMA | 10, 20 | 25, 50, 75 | –C5H8O2– |
| PC | 10, 20 | 25, 50, 75 | –C16H14O3– | |
| Computational domain | Gas phase | Width 80 mm | ||
| Height 100 mm | ||||
| Solid phase | Depend on sample thickness | |||
3. Mathematical Model
3.1. Major Assumptions
3.2. The Governing Equations
3.2.1. The Solid Phase
3.2.2. The Gas Phase
3.3. The Numerical Approach
4. Results and Discussion
4.1. Non-Charring Polymer
4.1.1. Thermal Properties of PMMA
| Property | Unit | PMMA | bPMMA | Char (nominal) | Gas |
|---|---|---|---|---|---|
| Pyrolysis reaction rate | 1/s | [15] | [15] | - | - |
| Melting rate | 1/s | [2] | - | - | - |
| Density | kg/m3 | 1,202.9 [27] | 1,000.0 | 1,202.9 | 1.205 |
| Heat of pyrolysis | kJ/kg | 1,350 | 1,350 | - | - |
| Gas permeability | m2 | 5.80 × 10−16 | 5.80 × 10−16 | 5.80 × 10−16 | - |
| Water permeability | m2 | 3.79 × 10−19 | 3.79 × 10−19 | 3.79 × 10−19 | - |
| Diffusion coefficient of water | m2/s | 5.11 × 10−8 | 5.11 × 10−8 | 5.11 × 10−8 | - |
| Diffusion coefficient of gas | m2/s | 1.85 × 10−9 | 1.85 × 10−9 | 1.85 × 10−9 | - |
| Specific heat capacity | J/kg·K | 1,500 [37] | 2,200 [37] | 2,200 | 1,000 [38] |
| Surface emissivity | - | 0.96 [39] | 0.96 [39] | 0.96 | - |
| Thermal conductivity | W/m·K | 0.16 [37] | 0.21 [37] | 0.21 | 0.03 [40] |
| Absorption coefficient | 1/m | 960 [39] | 960 [39] | 0.1 | - |
| Dynamic viscosity | Pa·s | - | - | - | 2.0 × 10−5 [41] |
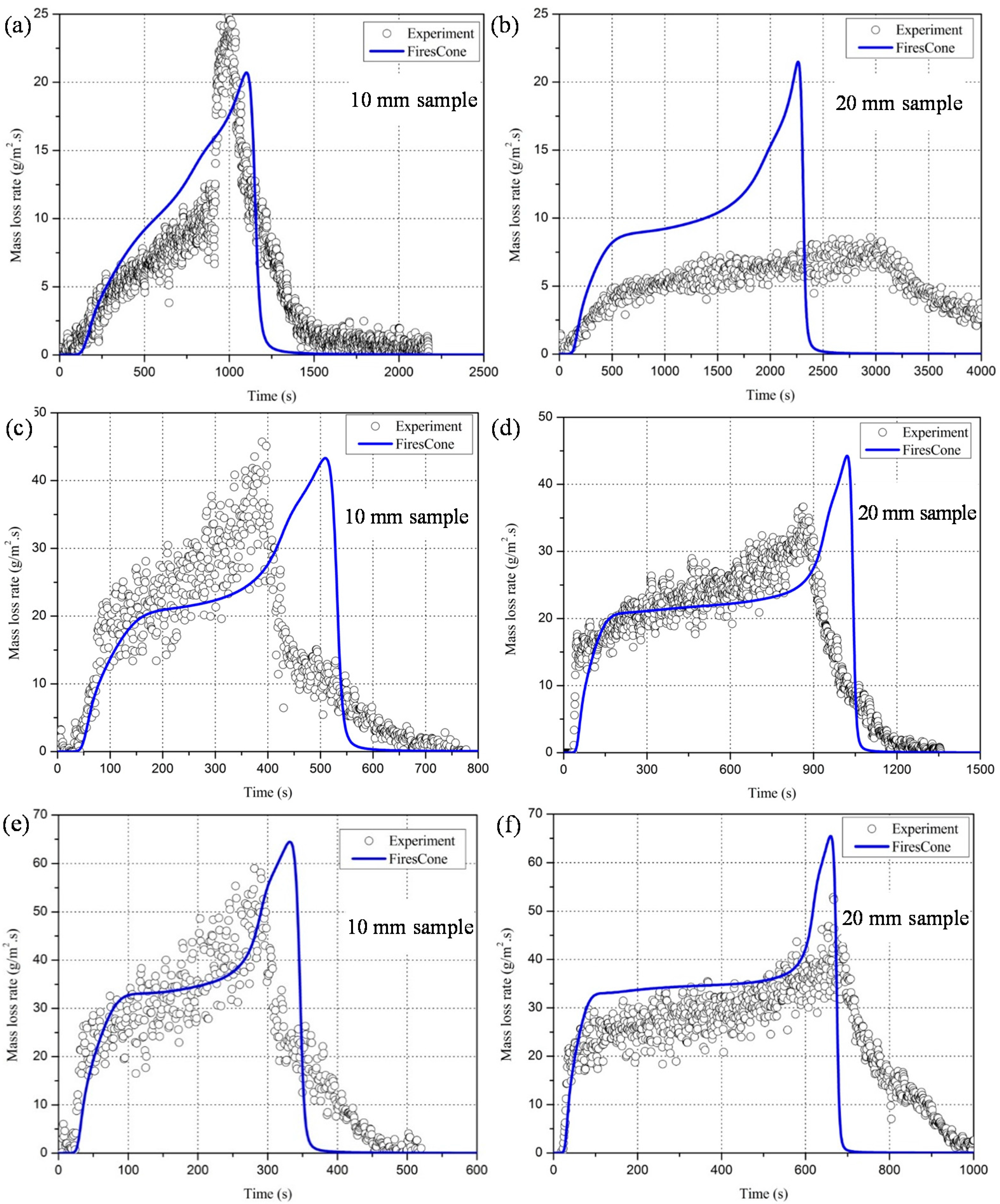
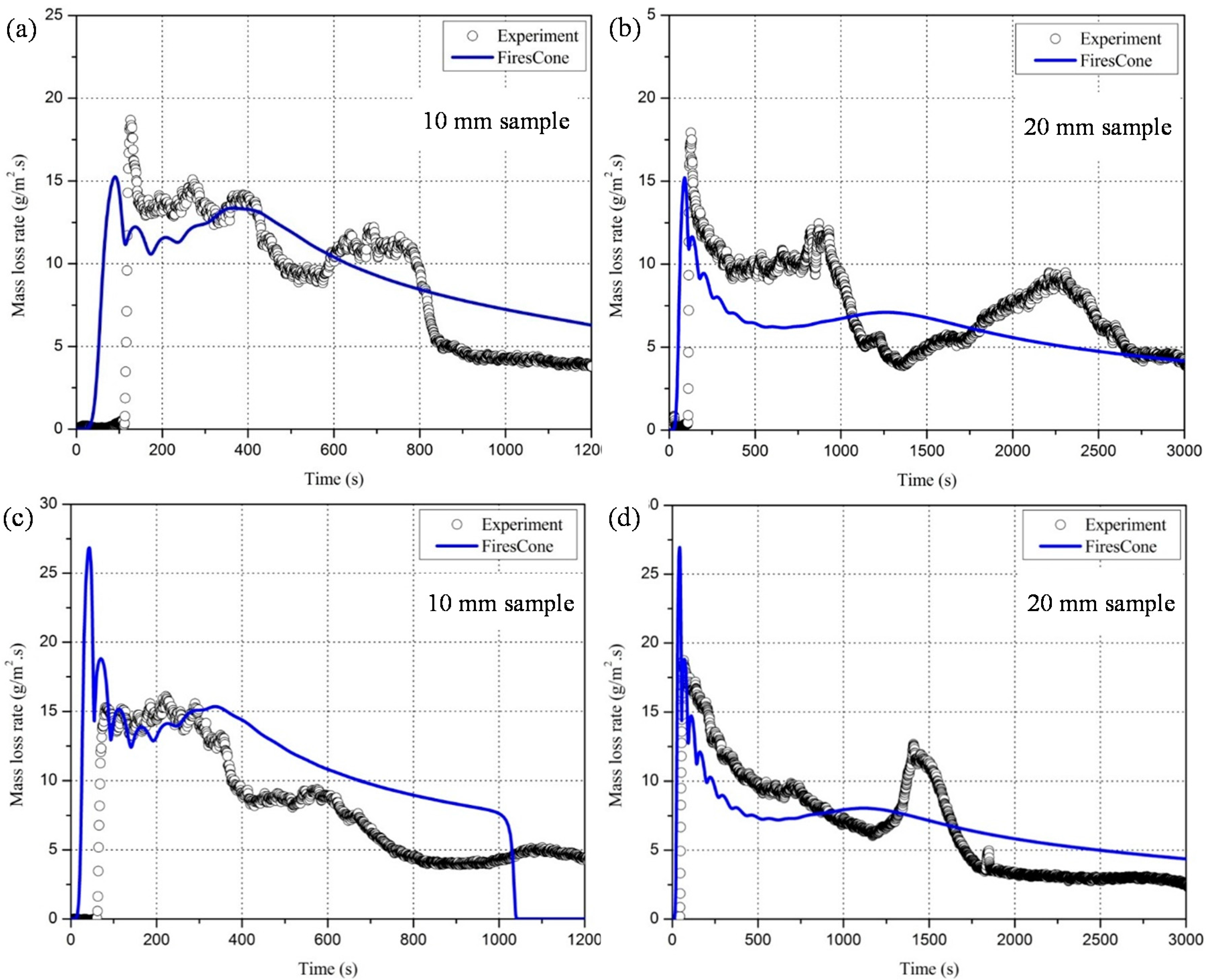
4.1.2. Mass Loss Rate
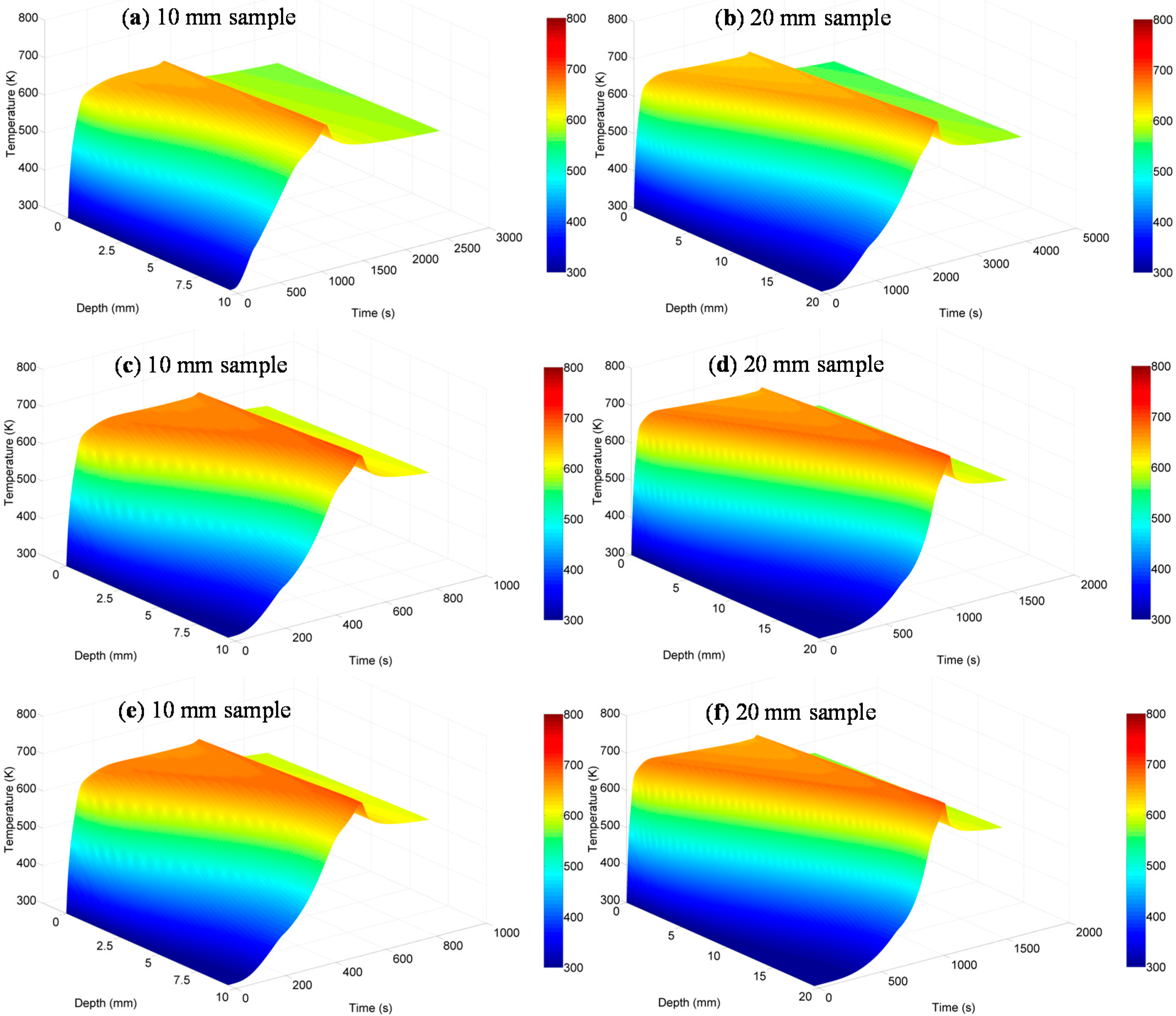
4.1.3. Temperatures Inside the Solid Phase
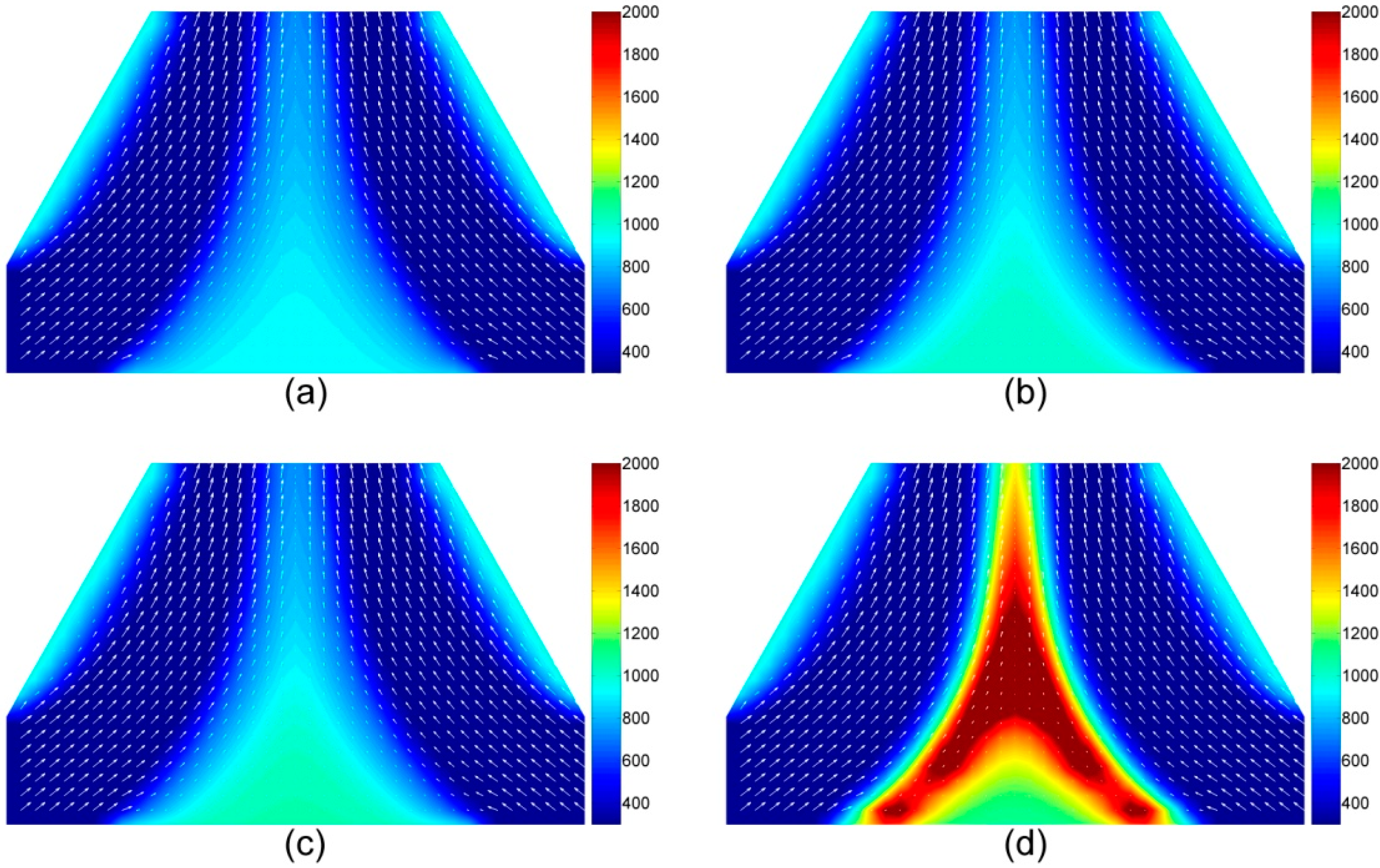
4.1.4. The Temperature and Velocity of Volatiles in the Gas Phase
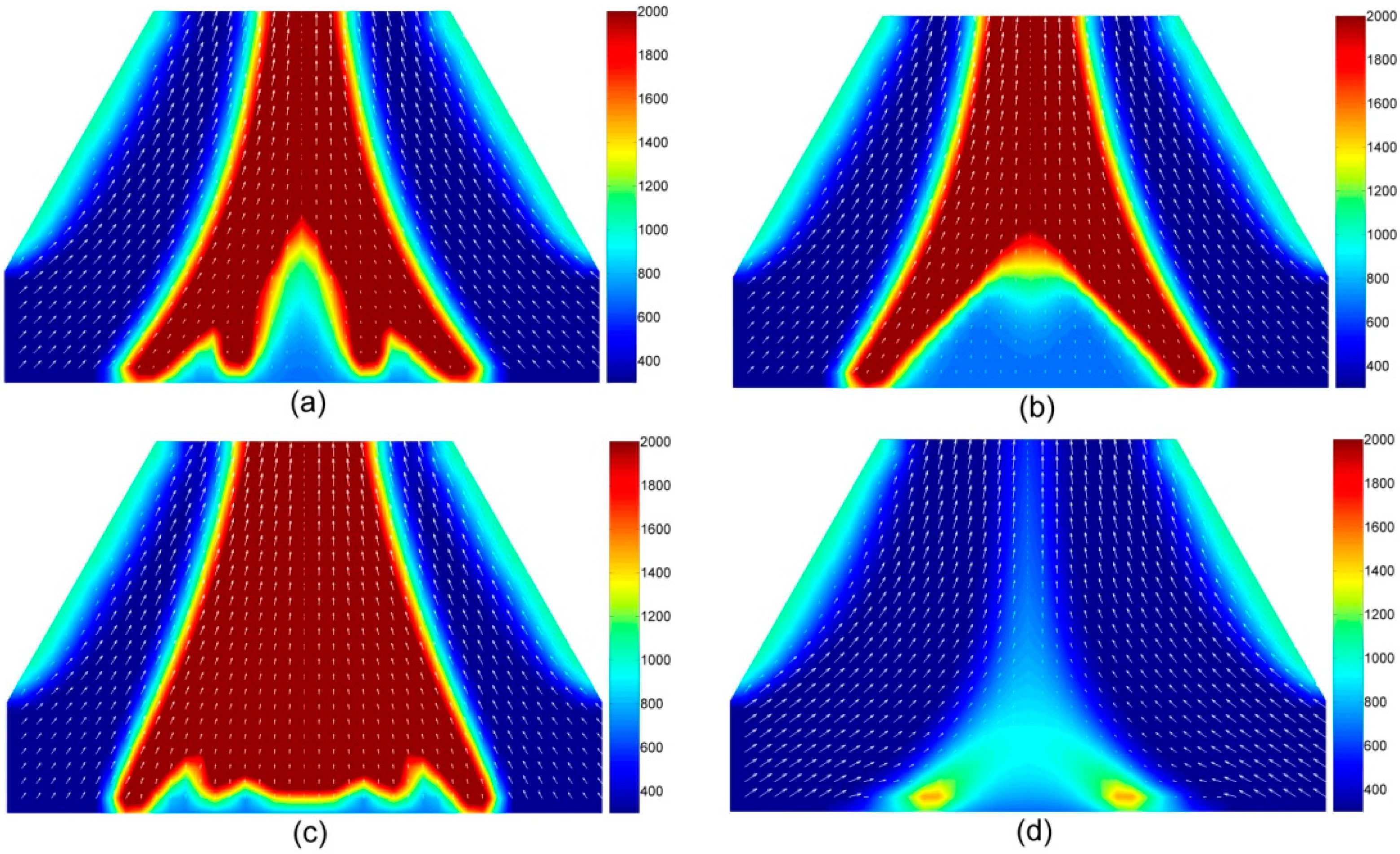
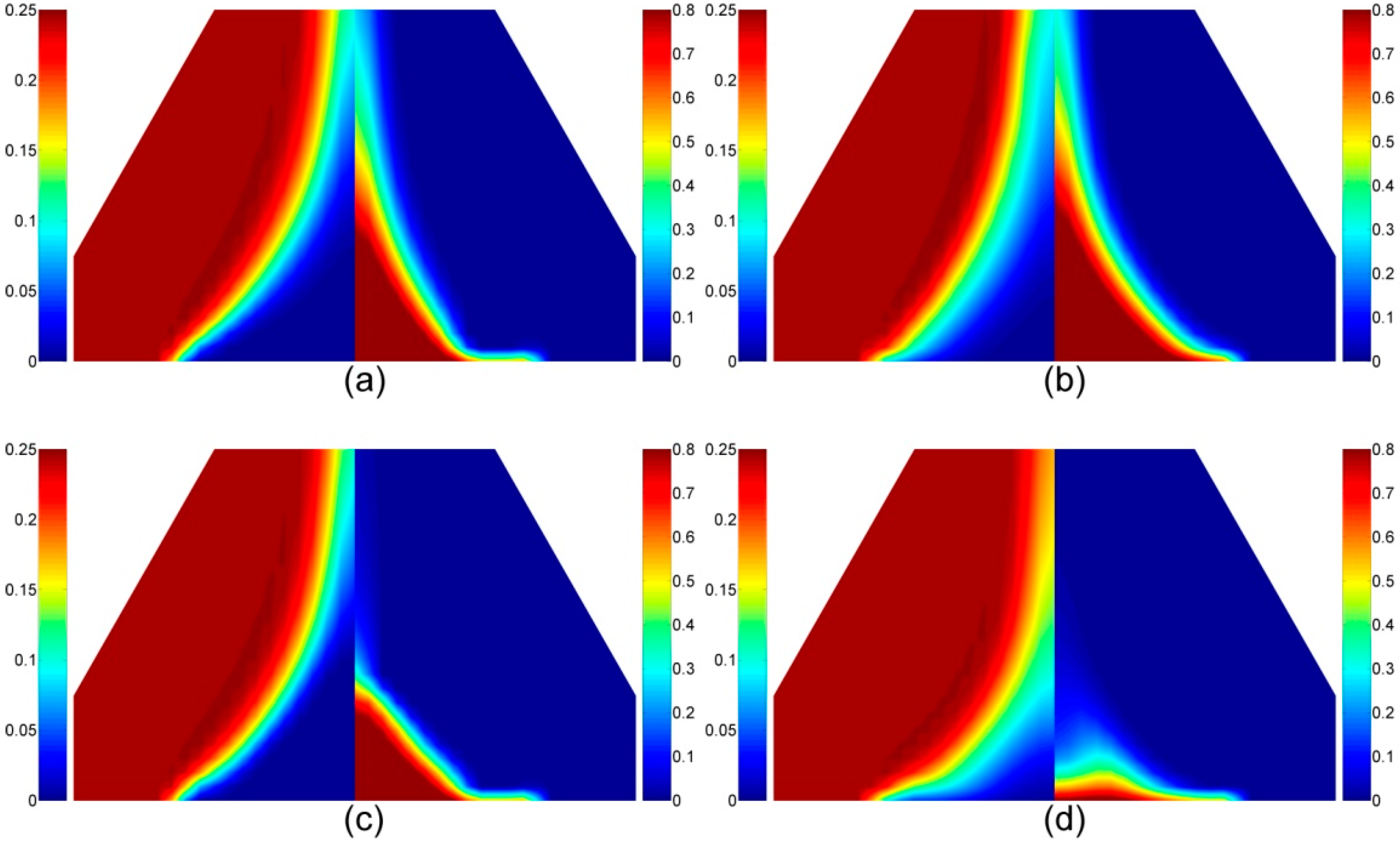
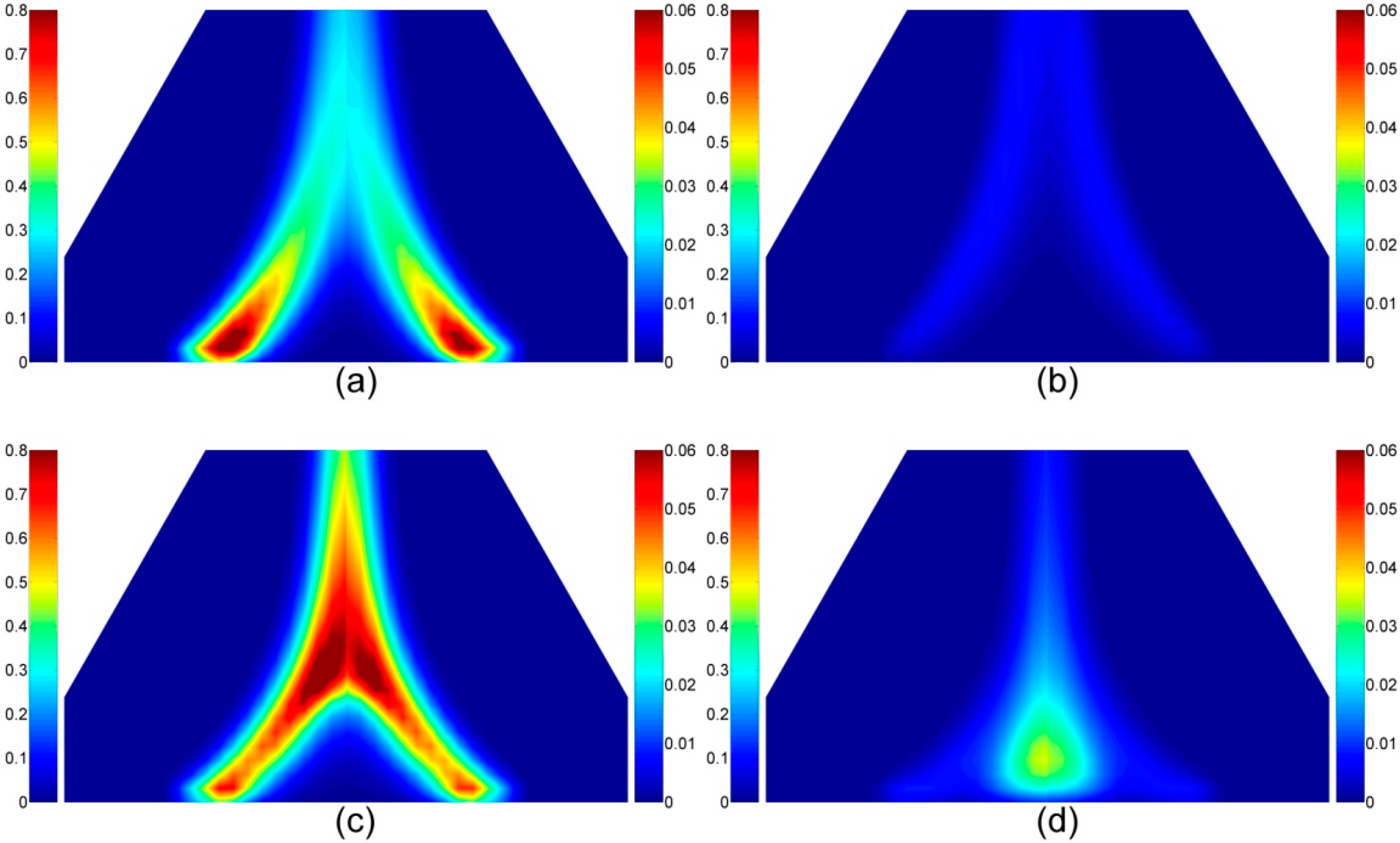
4.1.5. Volatile Components in the Gas Phase
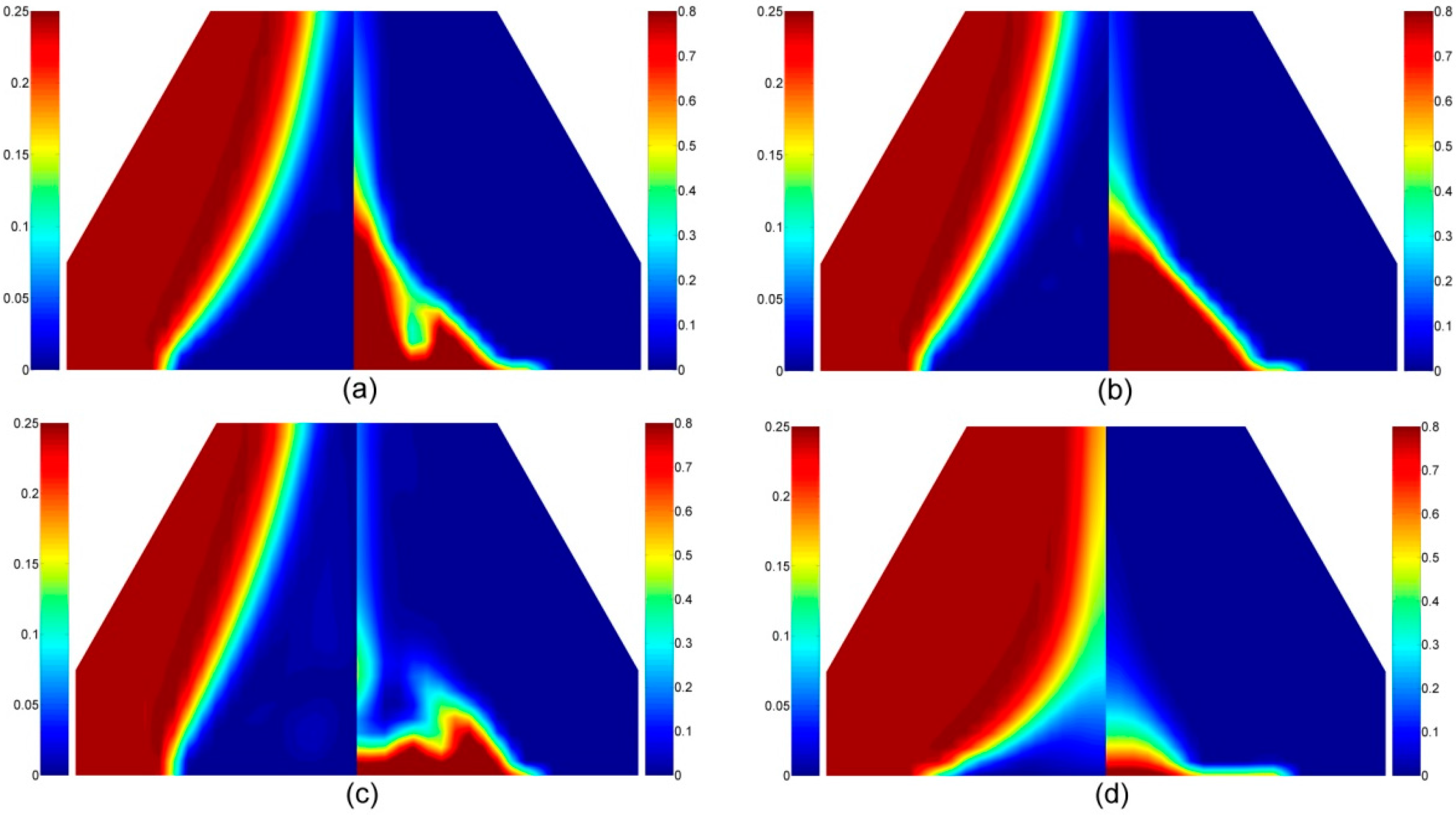
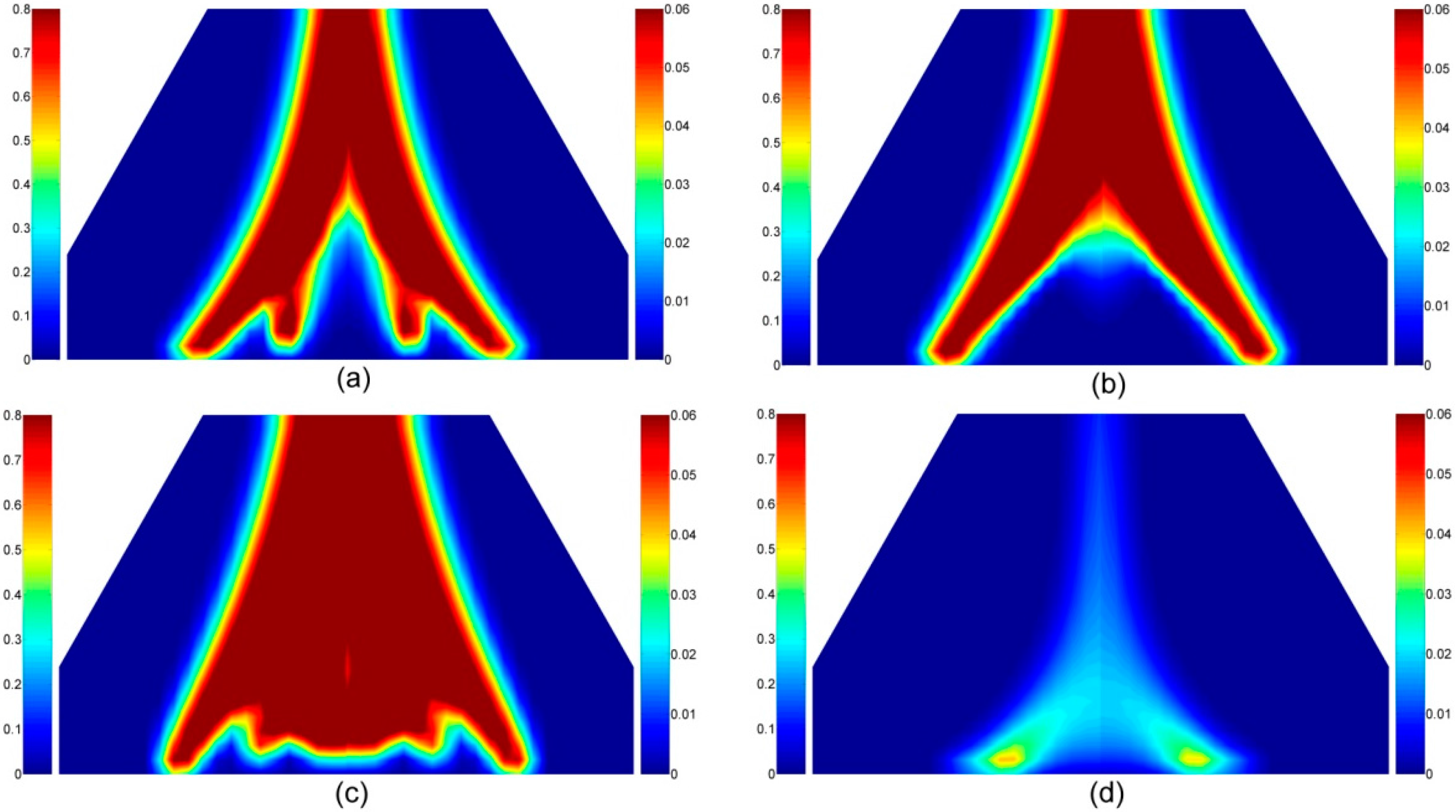
4.2. Intumescent-Protected Polymer
4.2.1. Thermal Properties of PC
| Property | Unit | PC | Char/Ash |
|---|---|---|---|
| Pyrolysis reaction rate | 1/s | [4] | [43] |
| Density | kg/m3 | 1,226.7 (Measured) | 100 (char); 55 (ash) |
| Yield | kg/kg | 0.25 (char yield) | 0.25 (Ash yield) |
| Heat of pyrolysis | kJ/kg | 400 | 580 |
| Gas permeability | m2 | 5.80 × 10−17 | 5.80 × 10−16 |
| Water permeability | m2 | 3.79 × 10−20 | 3.79 × 10−19 |
| Diffusion coefficient of water | m2/s | 5.11 × 10−9 | 5.11 × 10−8 |
| Diffusion coefficient of gas | m2/s | 1.85 × 10−10; | 1.85 × 10−9 |
| Specific heat capacity | J/kg·K | 1,220 [44] | 1,350 [45] |
| Surface emissivity | - | 0.96 | 0.90 |
| Thermal conductivity | W/m·K | 0.30 | 0.15 |
4.2.2. The Mass Loss Rate of the PC Samples
4.2.3. The Temperatures Inside the Solid Phase

4.2.4. The Temperature and Velocity of the Volatiles in the Gas Phase
4.2.5. The Volatiles Species in the Gas Phase
5. Main Conclusions
Author Contributions
Conflicts of Interest
Nomenclature
| A | pre-exponential, or frequency factor (s−1) | λ | thermal conductivity (W·m−1·K−1) |
| Cp | specific heat capacity (J·kg−1·K−1) | μ | dynamic viscosity (Pa·s) |
| D | diffusivity coefficient (m2·s−1) | υ | viscosity, stoichiometric coefficient |
| E | activation energy (J·mol−1) | ρ | density (kg·m−3) |
| h | specific enthalpy of materials (J) | ϕ | porosity (-) |
| k | reaction rate constant (s−1) | ||
| MLR | mass loss rate (g·s−1·m−2) | Subscripts and Superscripts | |
| n | order of reaction (-) | ext | external |
| N | number of species | f | flame |
| P | pressure (Pa) | g | gas phase, or gas species |
| heat flux (kW·m−2) | l | liquid water | |
| Q | heat of reaction (J·kg−1) | pyr | pyrolysis process |
| R | Universal gas constant (J·mol−1·K−1) | rad | radiation process |
| t | time (s) | reac | reaction process |
| T | absolute temperature (K) | s | solid phase, or solid species |
| u, v | velocity (m·s−1) | ||
| x, y | Cartesian coordinates (m) | Abbreviations | |
| Y | mass fraction (-) | CFD | computational fluid dynamics |
| EHC | effective heat of combustion | ||
| Greek letters | Fuel | general designation of gases volatiles | |
| γ | permeability (m2) | HRR | heat release rate |
| κ | absorption coefficient (m−1) | MLR | mass loss rate |
| Δ | change in variable value (-) | PC | polycarbonate |
| Δh | heat of reaction (kJ/kg) | PMMA | polymethyl methacrylate |
| Θ | production or reaction rate (kg·m−3·s−1) | ||
References
- Stec, A.A.; Hull, T.R.; Purser, D.A.; Purser, J.A. Fire toxicity assessment: comparison of asphyxiant yields from laboratory and large scale flaming fires. In Proceedings of the 11th International Conference on Fire Safety Science, Christchurch, New Zealand, 10–14 February 2014; pp. 404–408.
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for non-charring polymers. Combust. Flame 2013, 160, 1287–1297. [Google Scholar] [CrossRef]
- Griffin, G.J. The modeling of heat transfer across intumescent polymer coatings. J. Fire Sci. 2010, 28, 249–277. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. A review of fire processes modeling of combustible materials under external heat flux. Fuel 2013, 106, 30–50. [Google Scholar] [CrossRef]
- Karim, M.R.; Naser, J. Progress in numerical modelling of packed bed biomass combustion. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 December 2014; pp. 1–4.
- Novozhilov, V.; Moghtaderi, B.; Fletcher, D.F.; Kent, J.H. Computational fluid dynamics modelling of wood combustion. Fire Saf. J. 1996, 27, 69–84. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Galgano, A.; di Blasi, C. Modeling the propagation of drying and decomposition fronts in wood. Combust. Flame 2004, 139, 16–27. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling the effects of high radiative heat fluxes on intumescent material decomposition. J. Anal. Appl. Pyrol. 2004, 71, 721–737. [Google Scholar] [CrossRef]
- Di Blasi, C. Transition between regimes in the degradation of thermoplastic polymers. Polym. Degrad. Stab. 1999, 64, 359–367. [Google Scholar] [CrossRef]
- Kim, E.; Dembsey, N. Parameter estimation for comprehensive pyrolysis modeling: guidance and critical observations. Fire Technol. 2015, 51, 443–477. [Google Scholar] [CrossRef]
- Lautenberger, C. Gpyro3D: A three dimensional generalized pyrolysis model. In Proceedings of the 11th International Conference on Fire Safety Science, Christchurch, New Zealand, 10–14 February 2014; pp. 193–207.
- Lautenberger, C.; Fernandez-Pello, C. Generalized pyrolysis model for combustible solids. Fire Saf. J. 2009, 44, 819–839. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Leventon, I.T.; Lyon, R.E. Two-dimensional model of burning for pyrolyzable solids. Fire Mater. 2014, 38, 391–408. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Lyon, R.E.; Linteris, G.T. Prediction of the burning rates of non-charring polymers. Combust. Flame 2009, 156, 1068–1083. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Walters, R.N.; Lyon, R.E. Prediction of the burning rates of charring polymers. Combust. Flame 2010, 157, 2024–2034. [Google Scholar] [CrossRef]
- Riccio, A.; Damiano, M.; Zarrelli, M.; Scaramuzzino, F. Three-dimensional modeling of composites fire behavior. J. Reinf. Plast. Compos. 2014, 33, 619–629. [Google Scholar] [CrossRef]
- Marquis, D.M.; Pavageau, M.; Guillaume, E.; Chivas-Joly, C. Modelling decomposition and fire behavioubehavior of small samples of a glass-fibre-reinforced polyester/balsa-cored sandwich material. Fire Mater. 2013, 37, 413–439. [Google Scholar] [CrossRef]
- Yuen, R.K.K.; Yeoh, G.H.; Davis, G.D.V.; Leonardi, E. Modelling the pyrolysis of wet wood—II. Three-dimensional cone calorimeter simulation. Int. J. Heat Mass Transfer 2007, 50, 4387–4399. [Google Scholar] [CrossRef]
- Anderson, C.E.; Wauters, D.K. A thermodynamic heat transfer model for intumescent systems. Int. J. Eng. Sci. 1984, 22, 881–889. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. A model to predict carbon monoxide of woods under external heat flux—Part I: Theory. Proced. Eng. 2013, 62, 413–421. [Google Scholar] [CrossRef]
- Feih, S.; Mathys, Z.; Gibson, A.G.; Mouritz, A.P. Modelling the compression strength of polymer laminates in fire. Compos. Appl. Sci. Manuf. 2007, 38, 2354–2365. [Google Scholar] [CrossRef]
- Henderson, J.B.; Wiecek, T.E. A mathematical model to predict the thermal response of decomposing, expanding polymer composites. J. Compos. Mater. 1987, 21, 373–393. [Google Scholar] [CrossRef]
- Staggs, J.E.J. A simple model of polymer pyrolysis including transport of volatiles. Fire Saf. J. 2000, 34, 69–80. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L.; Novozhilov, V. A model to predict fire behaviors of combustible materials under external heat flux. In Proceedings of the 14th International Conference and Exhibition on Fire and Materials, San Francisco, CA, USA, 2–4 February 2015; pp. 256–270.
- Stoliarov, S.I.; Lyon, R.E. Thermo-kinetic model of burning for pyrolyzing materials. In Proceeding of the 9th International Symposium on Fire Safety Science, University of Karlsruhe, German, 21–26 September 2008; pp. 38–50.
- Shi, L.; Chew, M.Y.L. Fire behaviors of polymers under autoignition conditions in a cone calorimeter. Fire Saf. J. 2013, 61, 243–253. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. Influence of moisture on autoignition of woods in cone calorimeter. J. Fire Sci. 2012, 30, 158–169. [Google Scholar] [CrossRef]
- Font, R.; Marcilla, A.; Verdu, E.; Devesa, J. Kinetics of the pyrolysis of almond shells and almond shells impregnated with cobalt dichloride in a fluidized bed reactor and in a pyroprobe 100. Ind. Eng. Chem. Res. 1990, 29, 1846–1855. [Google Scholar] [CrossRef]
- Ragland, K.W.; Aerts, D.J. Properties of wood for combustion analysis. Bioresour. Technol. 1991, 37, 161–168. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Santoro, A.; Hernandez, E.G. Pyrolytic behavior and products of some wood varieties. Combust. Flame 2001, 124, 165–177. [Google Scholar] [CrossRef]
- Thunman, H.; Niklasson, F.; Johnston, F.; Leckner, B. Composition of volatile gases and thermochemical properties of wood for modeling of fixed or fluidized beds. Energy Fuels 2001, 15, 1488–1497. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Sparano, S.; Mantia, B.L. Drying characteristics of wood cylinders for conditions pertinent to fixed-bed countercurrent gasification. Biomass Bioenerg. 2003, 25, 45–58. [Google Scholar] [CrossRef]
- Bhuiyan, A.A.; Naser, J. Computational modelling of co-firing of biomass with coal under oxy-fuel condition in a small scale furnace. Fuel 2015, 143, 455–466. [Google Scholar] [CrossRef]
- Bhuiyan, A.A.; Naser, J. Numerical modelling of oxy fuel combustion, the effect of radiative and convective heat transfer and burnout. Fuel 2015, 139, 268–284. [Google Scholar] [CrossRef]
- MatWeb, Your Source for Materials Information. Database of material properties. Available online: www.matweb.com (accessed on 20 April 2015).
- Chew, M.Y.L.; Shi, L. Behaviors of materials at elevated temperatures. In Fire Protection—For Building Professionals; NUS Internal Electronic Module Coursework: Singapore, 2012. [Google Scholar]
- Schmal, D.; Duyzer, J.H.; van Heuven, J.W. A model for the spontaneous heating of coal. Fuel 1985, 64, 963–972. [Google Scholar] [CrossRef]
- Jiang, F.H.; de Ris, J.L.; Khan, M.M. Absorption of thermal energy in PMMA by in-depth radiation. Fire Saf. J. 2009, 44, 106–112. [Google Scholar] [CrossRef]
- Gronli, M.G.; Melaaen, M.C. Mathematical model for wood pyrolysis—Comparison of experimental measurements with model predictions. Energy Fuels 2000, 14, 791–800. [Google Scholar] [CrossRef]
- Sinha, P.K.; Wang, C.Y. Pore-network modeling of liquid water transport in gas diffusion layer of a polymer electrolyte fuel cell. Electrochimica Acta 2007, 52, 7936–7945. [Google Scholar] [CrossRef]
- Tewarson, A. Generation of heat and chemical compounds in fires. In SFPE Handbook of Fire Protection Engineering; National Fire Protection Association: Quincy, MA, USA, 2002. [Google Scholar]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Lyon, R.E.; Janssens, M.L. Polymer flammability; Report No.: DOT/FAA/AR-05/14; Federal Aviation Administration: Springfield, VA, USA, May 2005.
- Alves, S.S.; Figueiredo, J.L. A model for pyrolysis of wet wood. Chem. Eng. Sci. 1989, 44, 2861–2869. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. Experimental study of carbon monoxide for woods under spontaneous ignition condition. Fuel 2012, 102, 709–715. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Chew, M.Y.L.; Novozhilov, V.; Joseph, P. Modeling the Pyrolysis and Combustion Behaviors of Non-Charring and Intumescent-Protected Polymers Using “FiresCone”. Polymers 2015, 7, 1979-1997. https://doi.org/10.3390/polym7101495
Shi L, Chew MYL, Novozhilov V, Joseph P. Modeling the Pyrolysis and Combustion Behaviors of Non-Charring and Intumescent-Protected Polymers Using “FiresCone”. Polymers. 2015; 7(10):1979-1997. https://doi.org/10.3390/polym7101495
Chicago/Turabian StyleShi, Long, Michael Yit Lin Chew, Vasily Novozhilov, and Paul Joseph. 2015. "Modeling the Pyrolysis and Combustion Behaviors of Non-Charring and Intumescent-Protected Polymers Using “FiresCone”" Polymers 7, no. 10: 1979-1997. https://doi.org/10.3390/polym7101495
APA StyleShi, L., Chew, M. Y. L., Novozhilov, V., & Joseph, P. (2015). Modeling the Pyrolysis and Combustion Behaviors of Non-Charring and Intumescent-Protected Polymers Using “FiresCone”. Polymers, 7(10), 1979-1997. https://doi.org/10.3390/polym7101495