Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface
Abstract
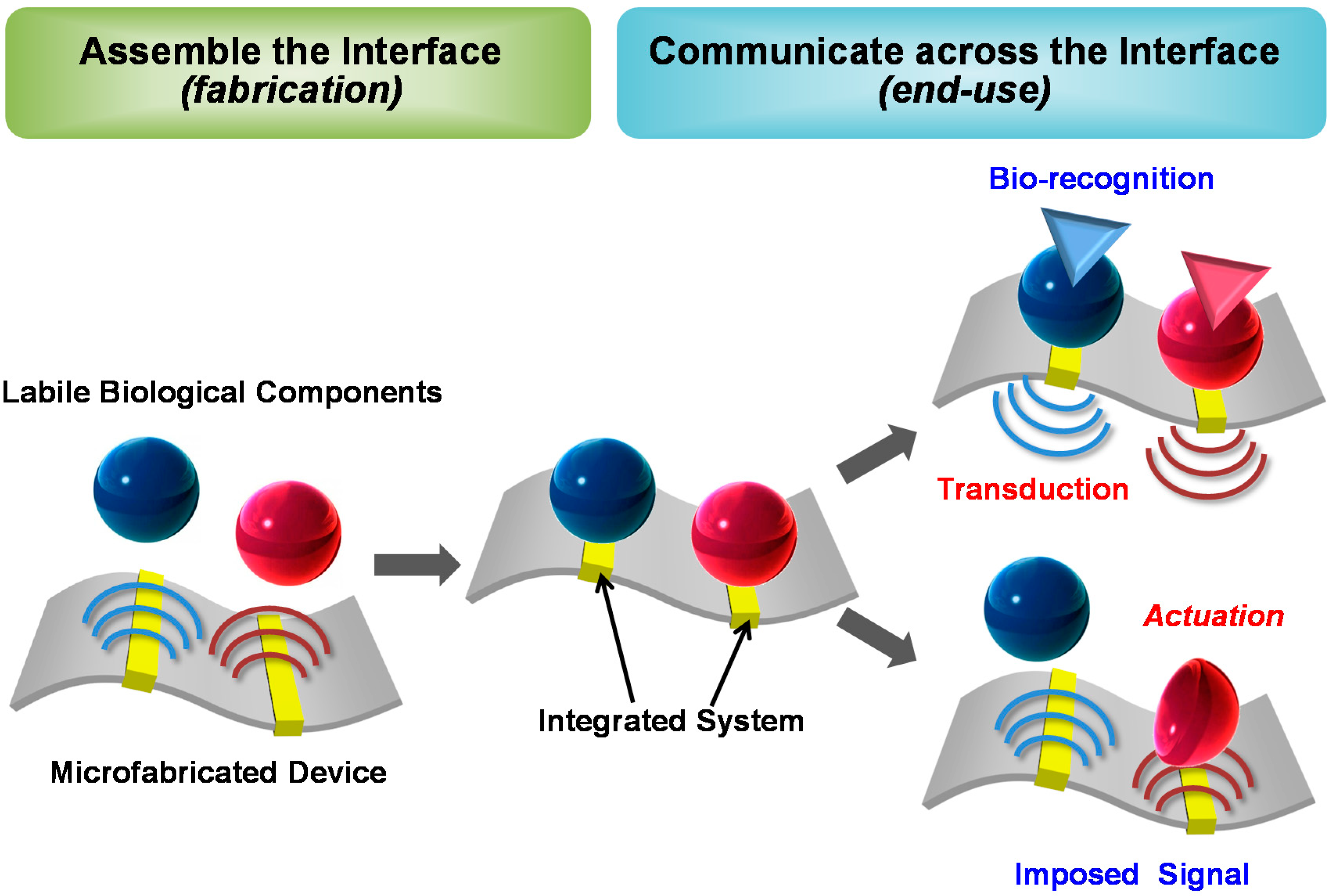
:1. Introduction: Integrating Biology into Electronics
1.1. The Opportunity. Why Interface Biology and Electronics?
| Area | Possible Applications |
|---|---|
| Analysis | Biosensors—multiplexed analysis in hand-held devices |
| Lab-on-a-Chip—high-throughput screening | |
| Smart fabrics—remote monitoring of first-responders | |
| Energy | Biofuel cells—efficient conversion of chemical and solar energy |
| Nanostructured batteries—compact storage of energy | |
| Medicine | Devices to personalize medicine—theranostics |
| Prosthetics—effective repair or restoration of function |
1.2. Vision
2. Why Chitosan?
| Property | Details |
|---|---|
| Stimuli-responsive (pH-responsive) | Chitosan undergoes a soluble to insoluble transition with a change in pH |
| Self-assembly (self-association) | Chitosan’s interchain associations can yield a 3-dimensional hydrogel network |
| Polycationic | Positive charges on chitosan’s amines can undergo electrostatic interactions with (poly)anions |
| Nucleophilic | Unshared electrons on chitosan’s amines enable chemical modification under facile conditions |
| Metal-binding | Chitosan chelates metals through interactions with its amino and hydroxyl groups |
| Oxidizable | Chitosan (like many polysaccharides) can be partially oxidized to generate reactive moieties (e.g., aldehydes) |

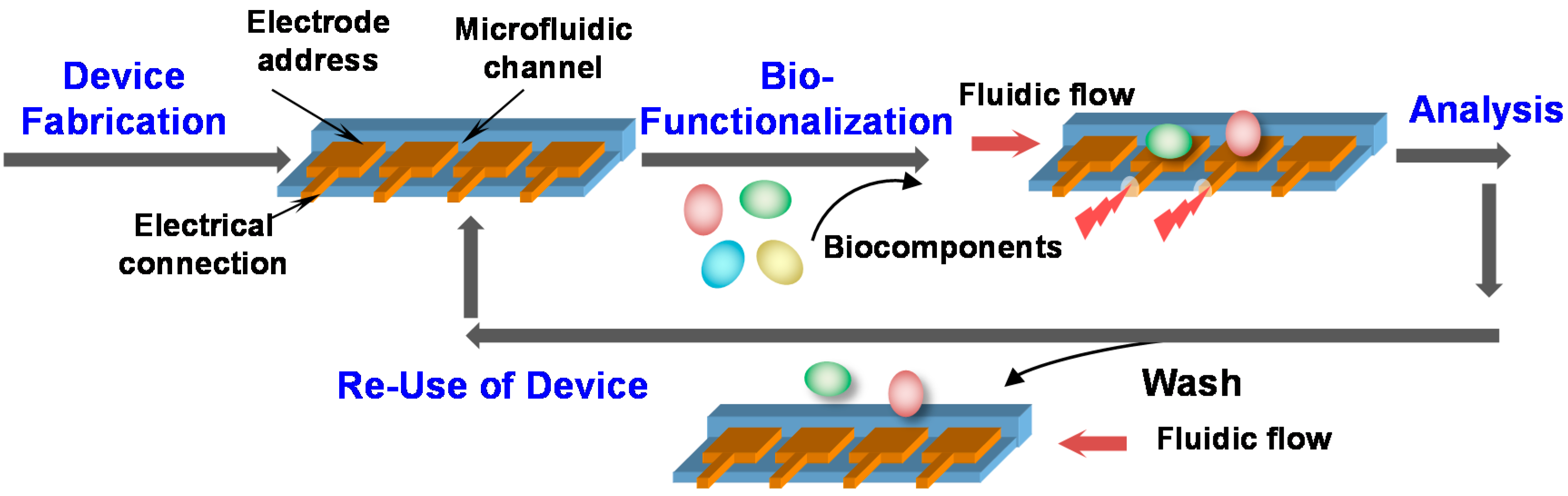
3. Fabrication
3.1. The Fabrication Challenge
| Aspect | Biological Fabrication | Microfabrication |
|---|---|---|
| Fabrication paradigm | Bottom-up & hierarchical | Top-down & monolithic |
| Common materials | Soft (e.g., proteins & polysaccharides) | Hard (e.g., silicon & metals) |
| Approach to control chemistry | Enlist molecular recognition | Exclude contaminants |
| Approach to control defects | Correct & heal | Strive for defect-free fabrication |
| Final structure | Dynamic & adaptable | Static & permanent |
3.2. Generic Fabrication: Constraints
- Post-fabrication biofunctionalization. Biological components (e.g., proteins and cells) are inherently unstable compared to traditional electronic devices, and traditional microfabrication methods are “bio-incompatible”. Thus, we believe the best strategy is to complete microfabrication of the electronics before adding biological functionality. In fact, we envision the biofabrication steps could be done on-site by end-users just prior to use;
- Erasable biofunctional films. Biofunctional films that can be washed away after use will permit re-use of the electronic device (e.g., a microfluidic device) and thus relax cost constraints imposed by single use systems;
- Fabrication from water. Water is the medium of biology and thus fabrication methods must embrace and guide interactions in water to generate hydrogel-based interfaces between the biology and the electronics;
- Fabrication must be simple, rapid and programmable. We use electrical signals for spatiotemporal programmability and molecular recognition for chemoselectivity.
3.3. Biofabrication to Build the Interface
3.4. Chitosan’s Cathodic Electrodeposition
3.4.1. Mechanism
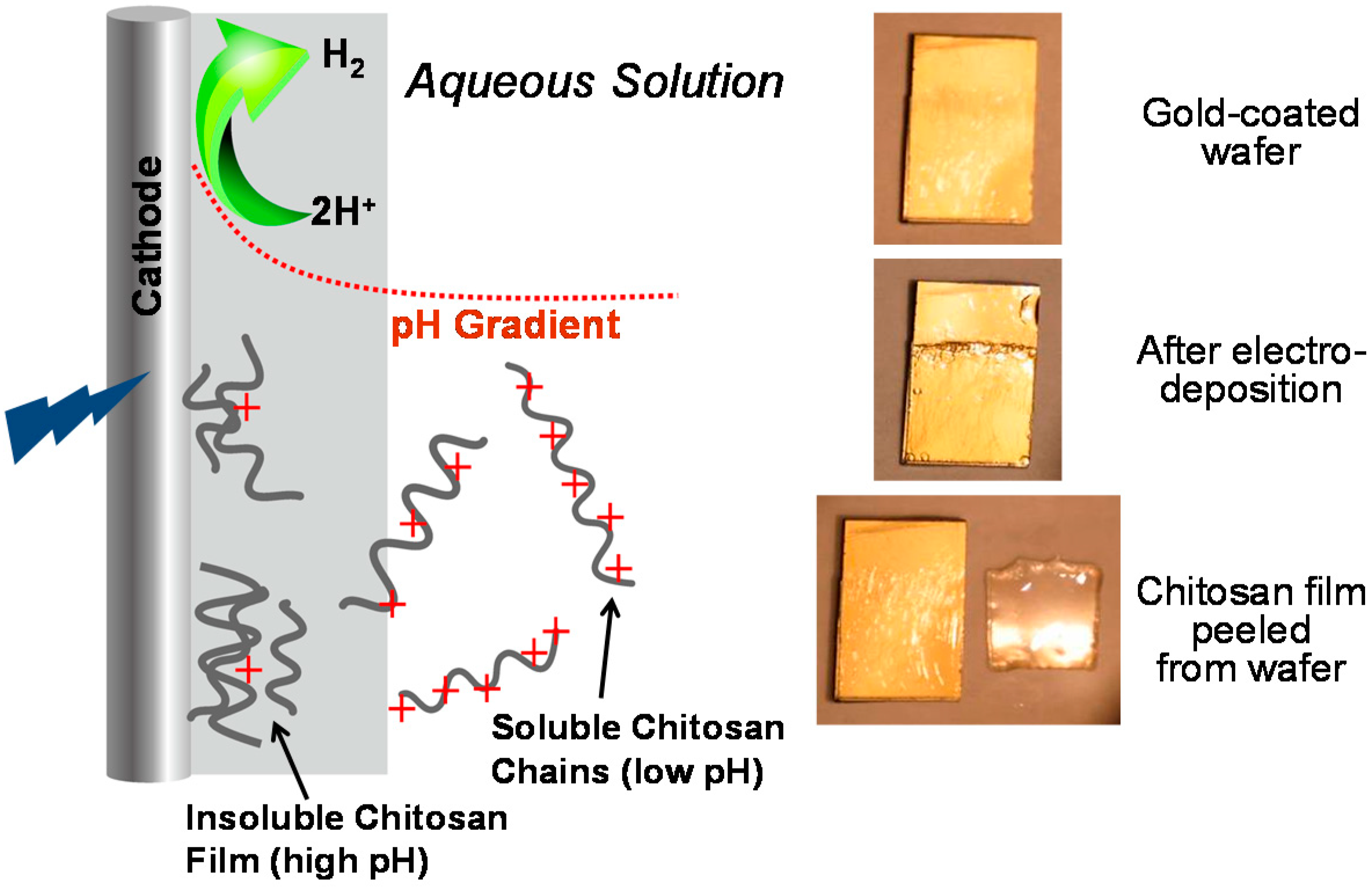
3.4.2. Spatiotemporal Controllability

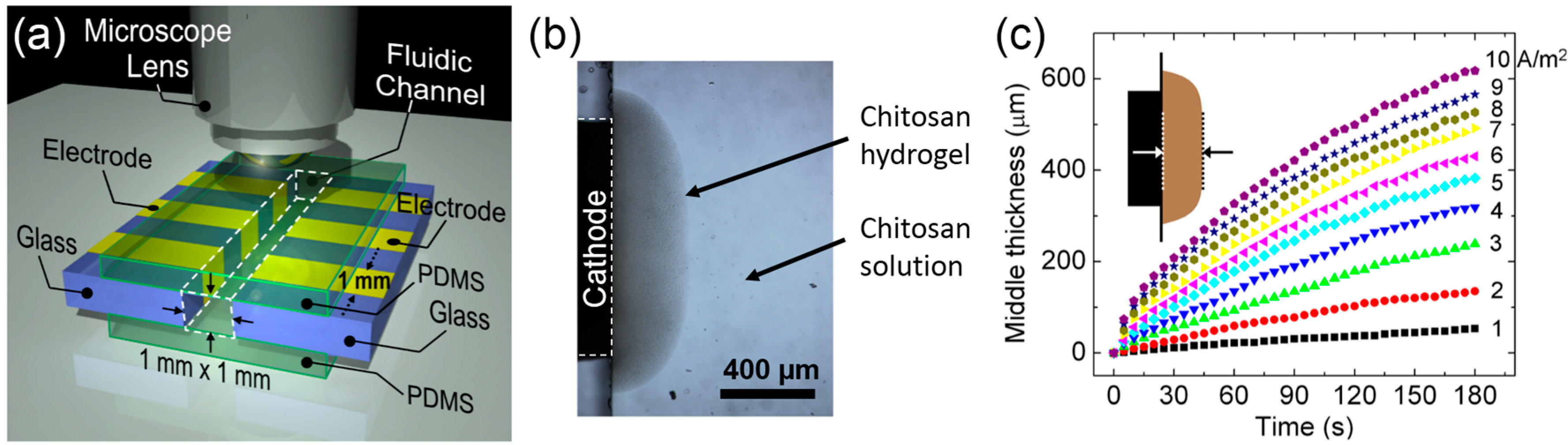
3.4.3. Electrodeposition as a Moving Front
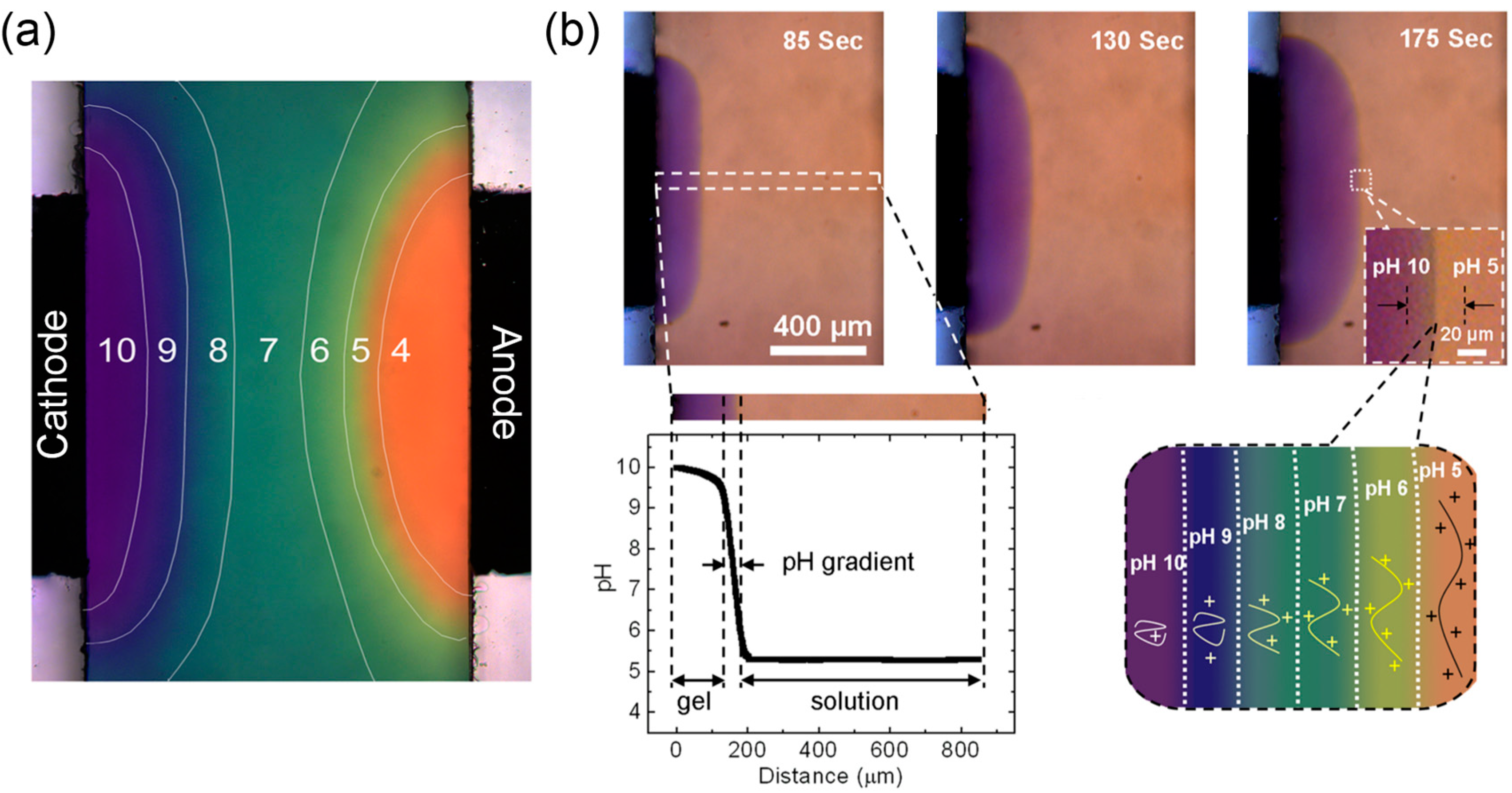
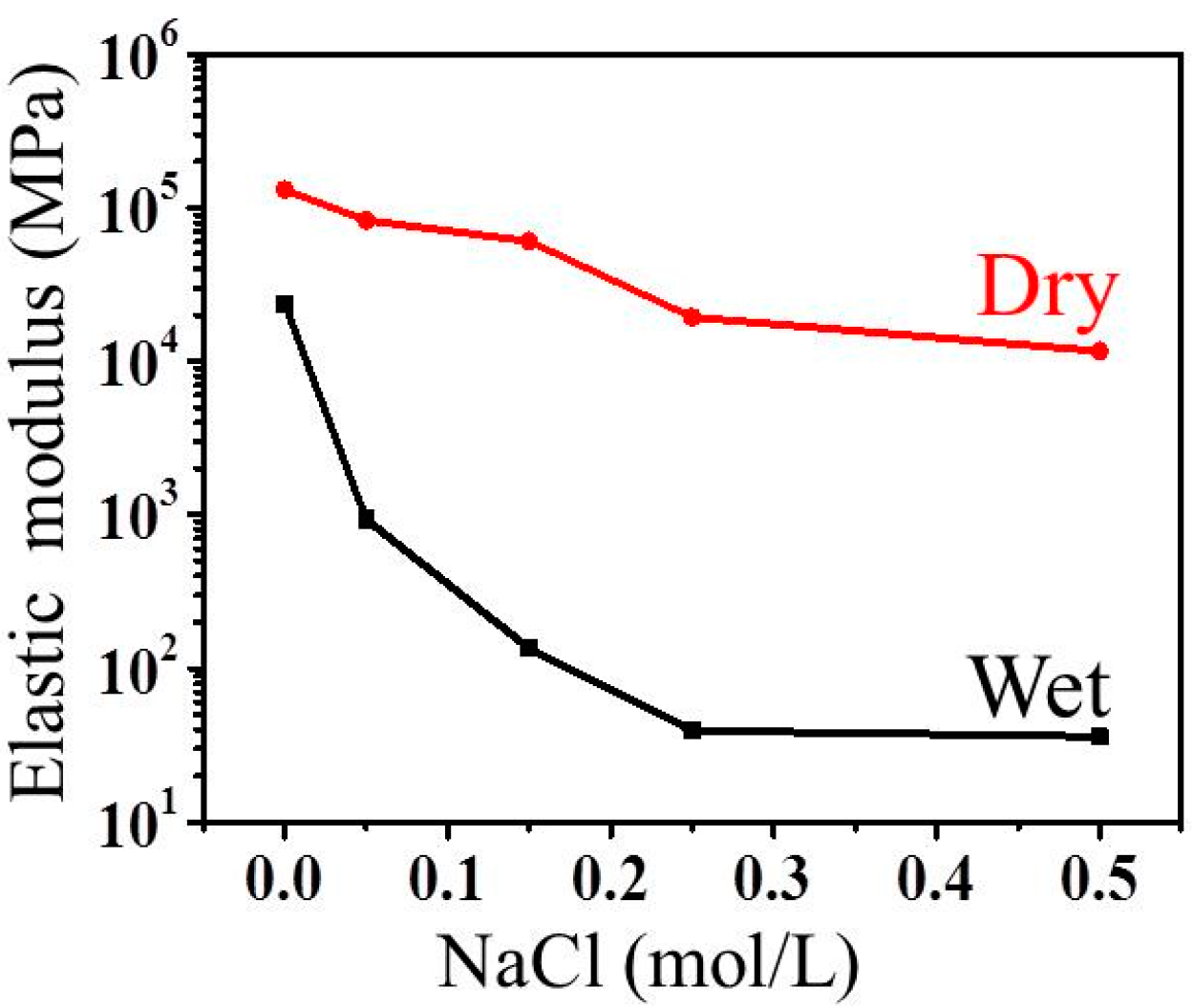
3.4.4. Structure and Properties of Cathodically-deposited Chitosan

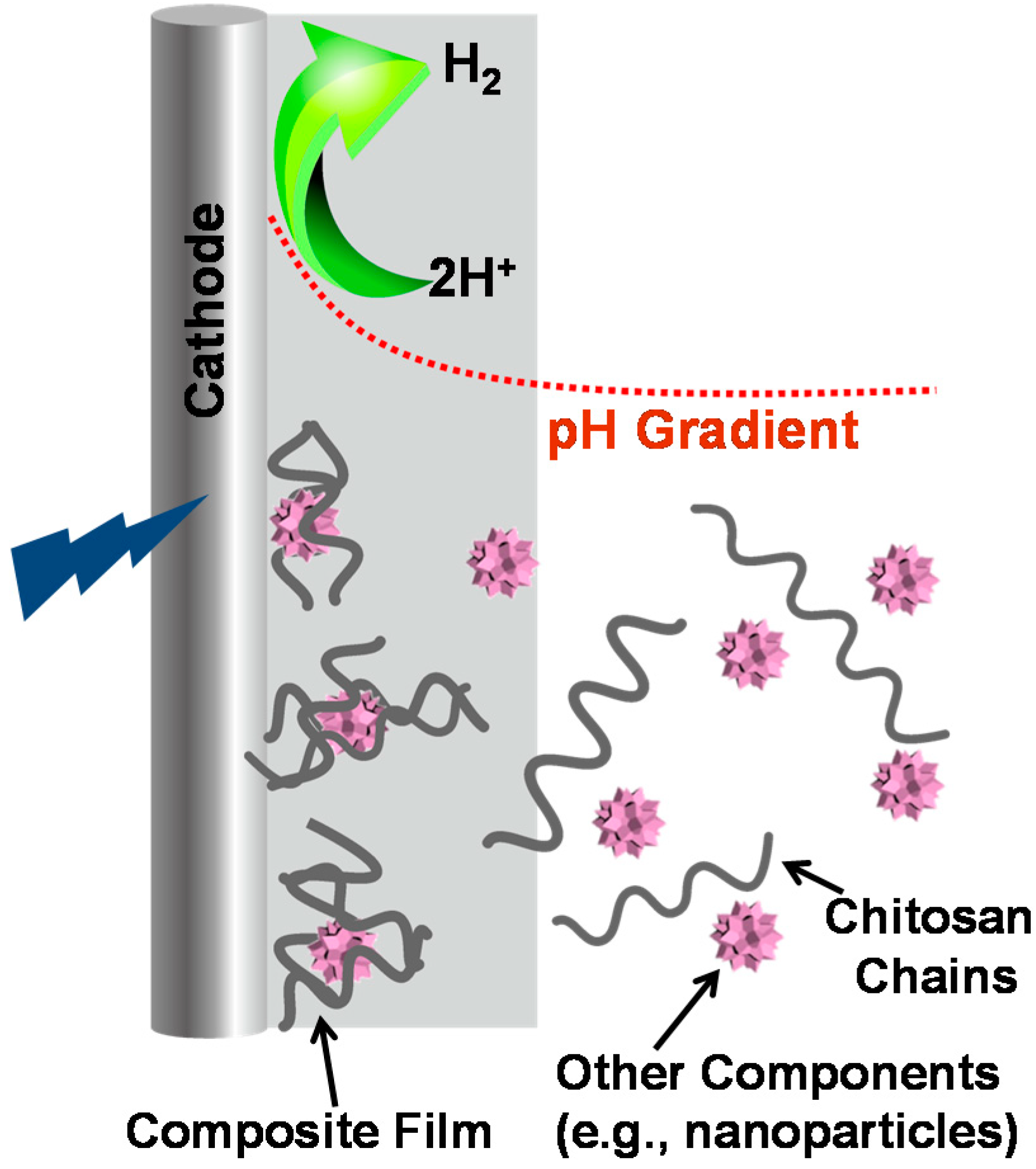
3.4.5. Co-Deposition
3.4.6. Integration with Other Assembly Methods
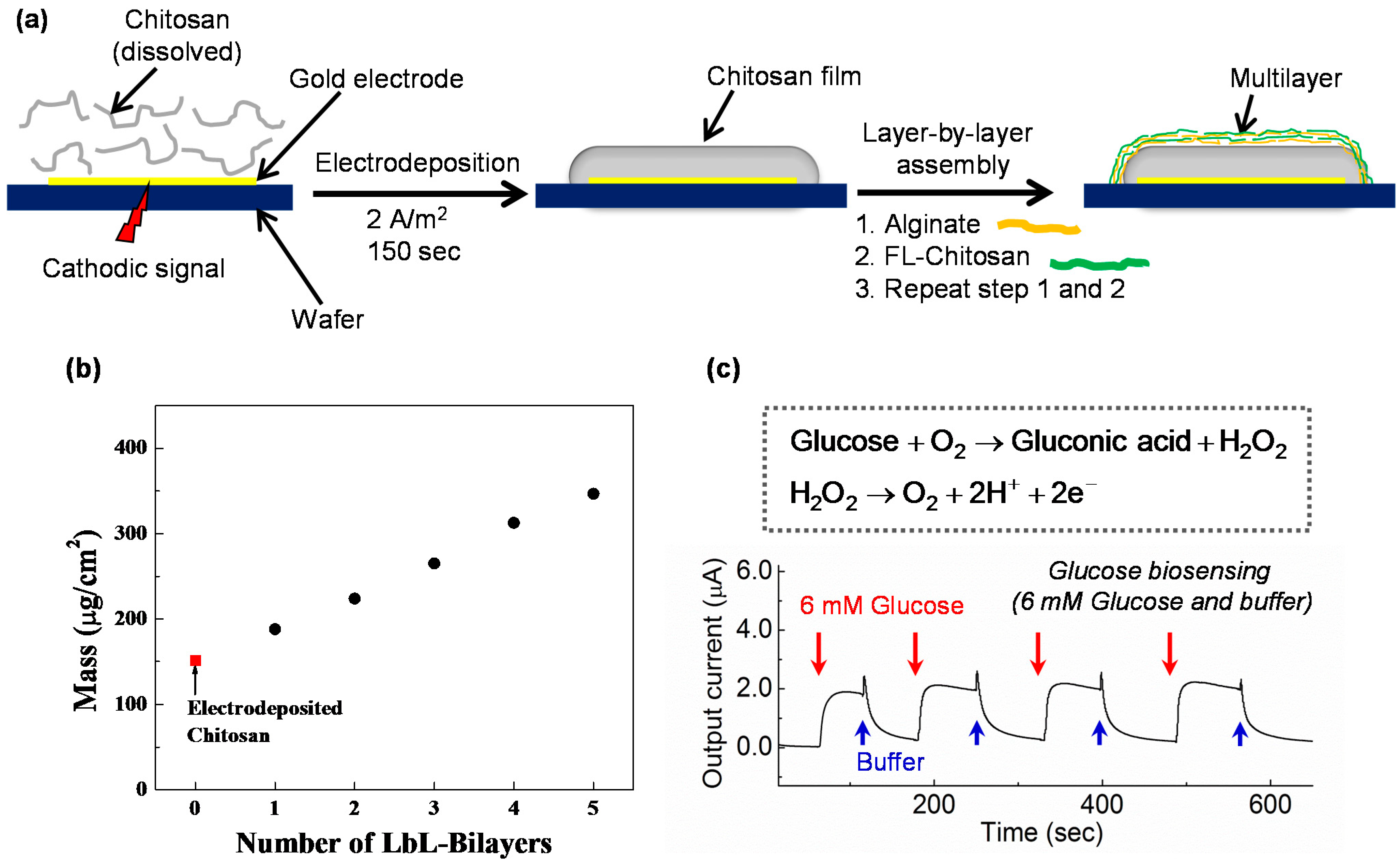
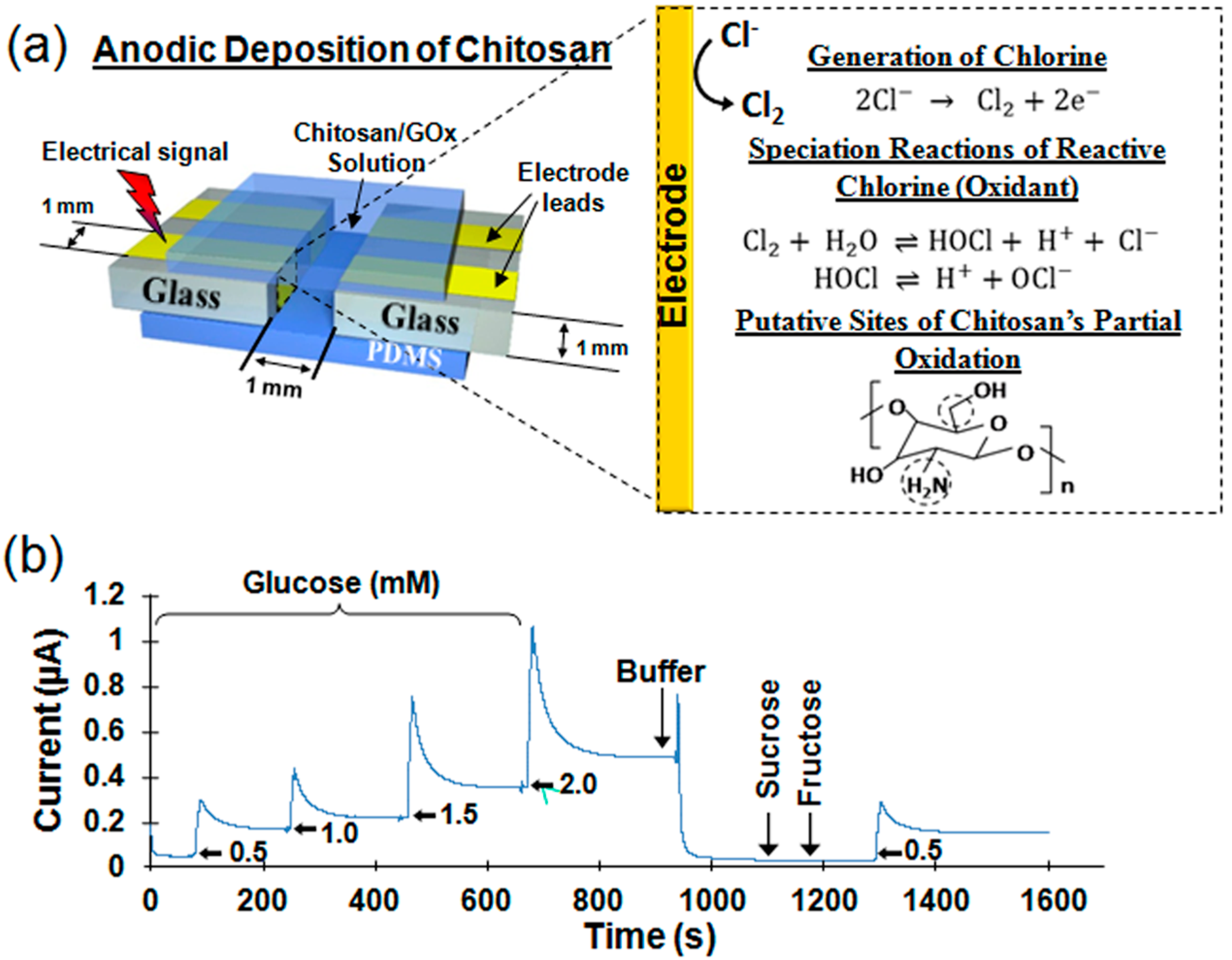
3.5. Chitosan’s Anodic Electrodeposition
3.5.1. Mechanism
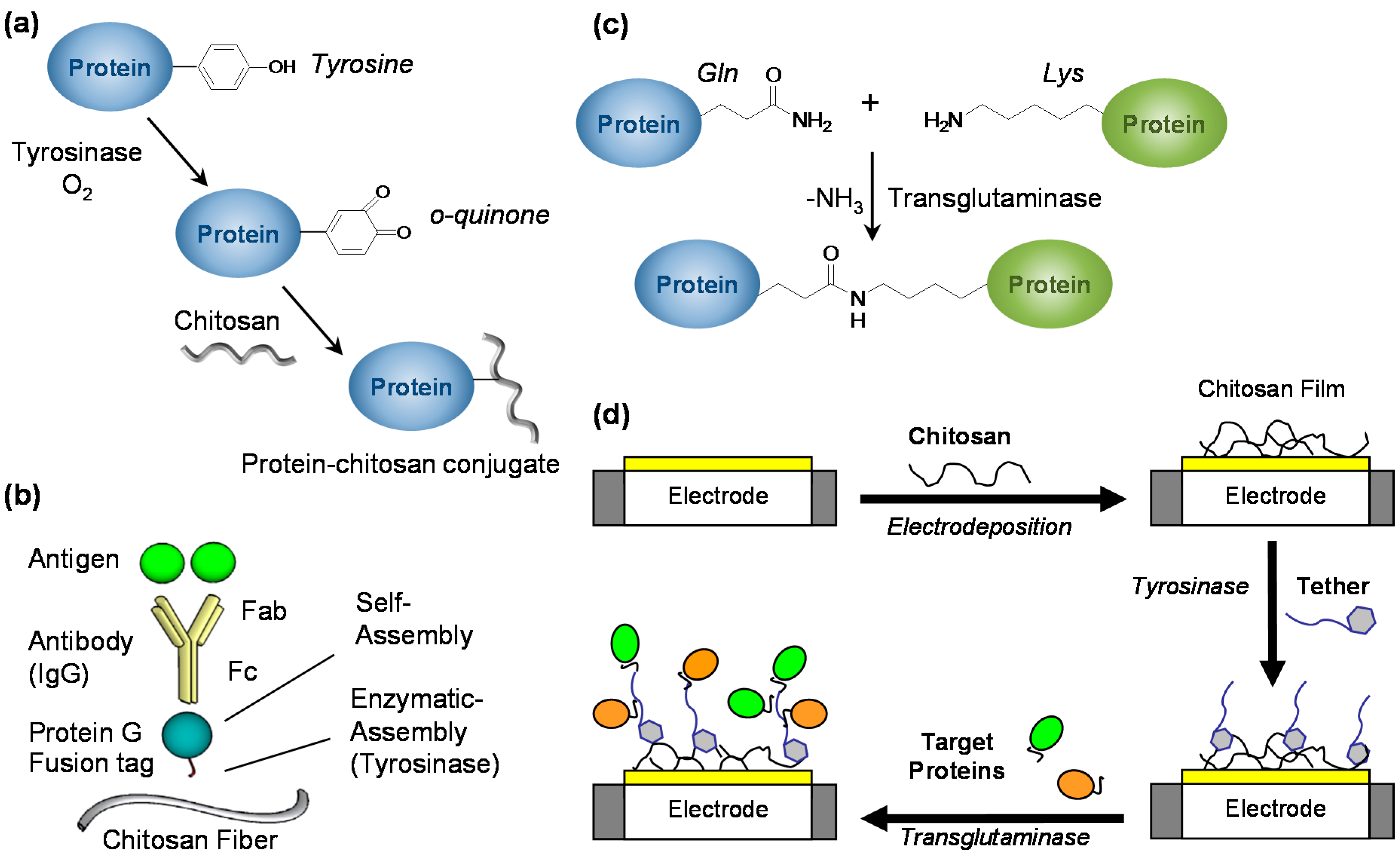
3.6. Biofunctionalizing Electrodeposited Chitosan Films
| Method | Details |
|---|---|
| Chemical conjugation | Glutaraldehyde |
| Carbodiimide | |
| Epoxy | |
| Partial oxidation | |
| Non-covalent binding | Electrostatic |
| (Strept)avidin-biotin | |
| Metal chelation His-tagged protein | |
| Electrochemical | Electro-click |
| Enzymatic | Tyrosinase |
4. Communication
4.1. The Communication Challenge
4.2. Redox to Connect Bio-device Communication
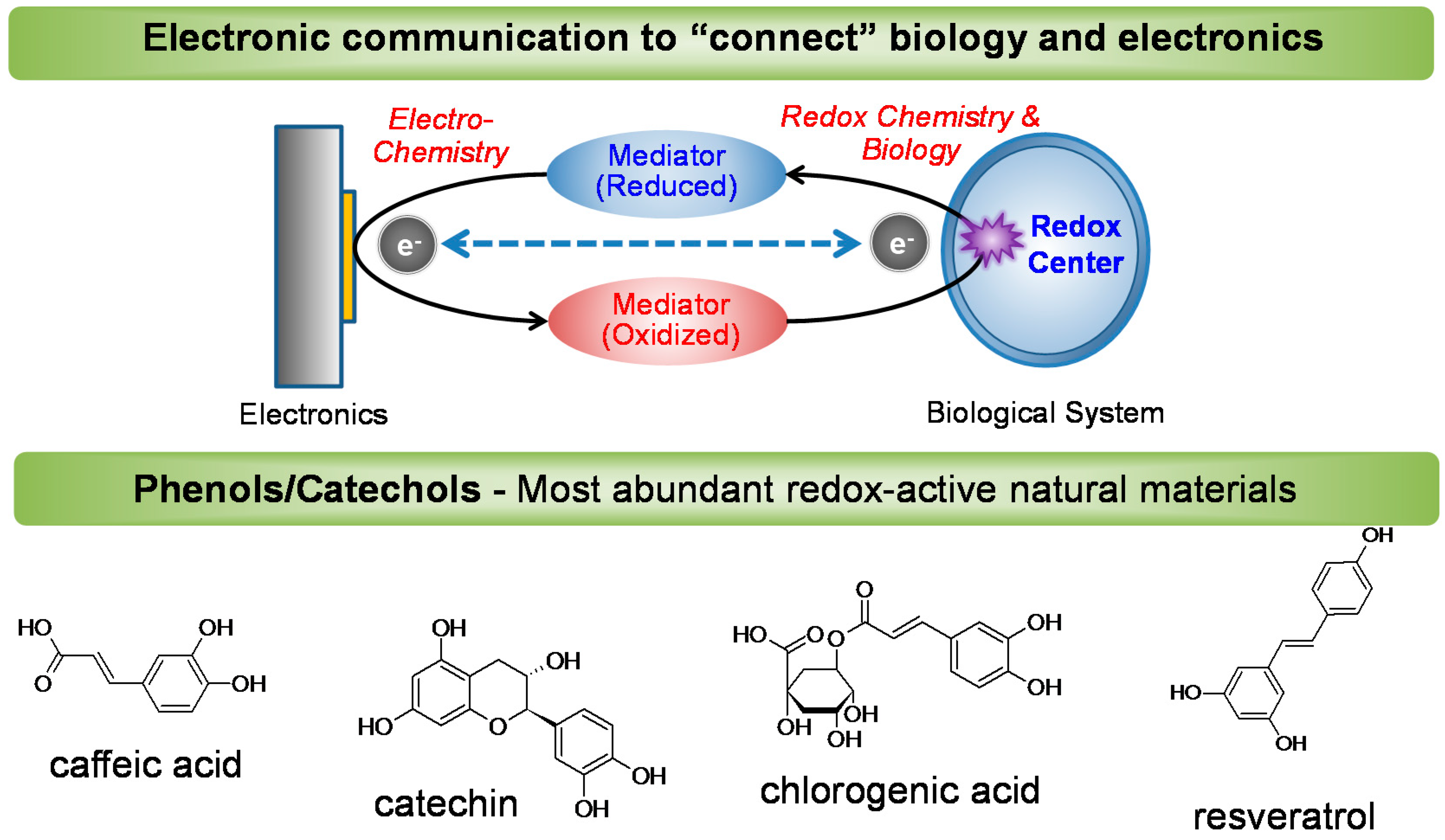
4.3. Catechol-Chitosan Redox-Capacitor
4.3.1. Oxidative Conjugation of Catechols to Chitosan

4.3.2. Redox-Cycling of the Catechol-Modified Chitosan Films

4.4. Information Processing Properties of the Redox-Capacitor
| Property | Details |
|---|---|
| Switching | Oxidative redox-cycling switches the film to an oxidized (discharged) state while reductive redox-cycling switches the film to a reduced (charged) state |
| Amplification | Redox-cycling serves to amplify output currents |
| Partial Rectification | Thermodynamic constraints limit a mediator’s redox-cycling to one direction (either oxidative or reductive) and this enhances mediator currents in one direction while inhibiting mediator currents in the other direction (e.g., large oxidative currents and small reductive currents are observed with the Fc mediator, while the opposite is true for the Ru3+ mediator) |
| Gating | Because the catechol-chitosan film is non-conducting, a mediator is required to charge and discharge the film and thus charging/discharging is controlled by the mediator’s redox potential (i.e., the mediator’s E° serves to gate film charging and discharging) |
| Steady Oscillating Inputs/Outputs | If oscillating electrode potentials are imposed to sequentially engage oxidative and reductive redox-cycling then oscillating output currents can be generated to yield a pattern that remains nearly steady over time (oscillating inputs and outputs are commonly used in signal processing) |
| Communicate with Biological Systems | The catechol-chitosan film can accept electrons from common biological reductants (NADPH and ascorbate) and donate electrons to common biological oxidants (e.g., O2) and thus can “communicate” with biology |
4.5. Enzymatic Charging of the Redox-Capacitor
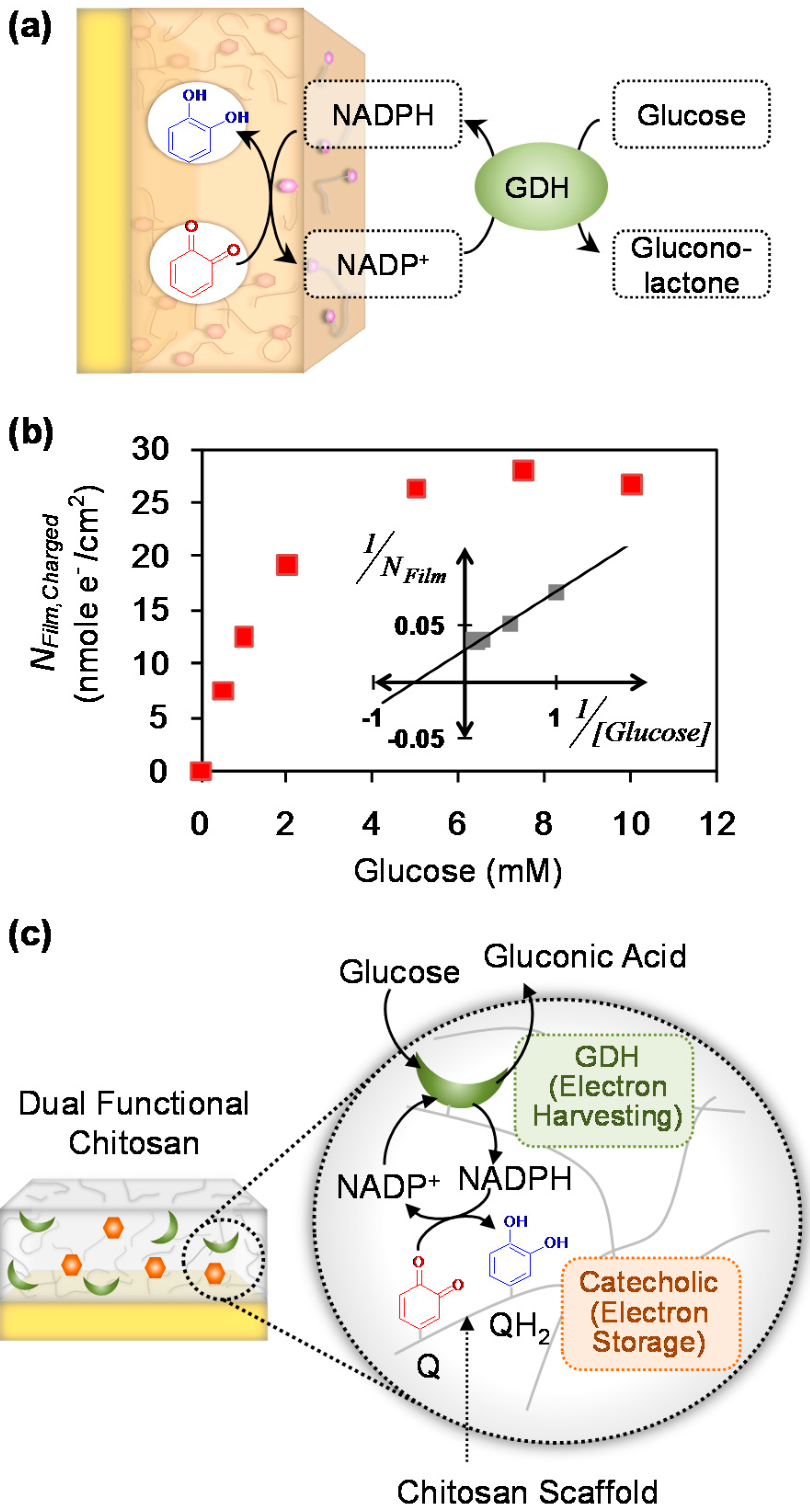
4.6. Accessing Global, Systems-Level Redox Information

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seuss, S.; Boccaccini, A.R. Electrophoretic deposition of biological macromolecules, drugs, and cells. Biomacromolecules 2013, 14, 3355–3369. [Google Scholar] [CrossRef] [PubMed]
- Meini, N.; Ripert, M.; Chaix, C.; Farre, C.; de Crozals, G.; Kherrat, R.; Jaffrezic-Renault, N. Label-free electrochemical monitoring of protein addressing through electroactivated “click” chemistry on gold electrodes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Dinolfo, P.H.; Chidsey, C.E.D.; Collman, J.P. Selective functionalization of independently addressed microelectrodes by electrochemical activation and deactivation of a coupling catalyst. J. Am. Chem. Soc. 2006, 128, 1794–1795. [Google Scholar] [CrossRef]
- Koev, S.T.; Dykstra, P.H.; Luo, X.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Chitosan: An integrative biomaterial for lab-on-a-chip devices. Lab Chip 2010, 10, 3026–3042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Bentley, W.E.; Payne, G.F. Biofabrication to build the biology–device interface. Biofabrication 2010, 2. [Google Scholar] [CrossRef]
- Gordonov, T.; Kim, E.; Cheng, Y.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.; Yin, J.J.; Payne, G.F.; Bentley, W.E. Electronic modulation of biochemical signal generation. Nat. Nanotechnol. 2014, 9, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nano-fabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- Kumar, M. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Kumar, M.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Tommeraas, K.; Varum, K.M.; Ostgaard, K. Electrophoretic light scattering studies of chitosans with different degrees of N-acetylation. Biomacromolecules 2001, 2, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Varum, K.M.; Ottoy, M.H.; Smidsrod, O. Water-solubility of partially N-acetylated chitosans as a function of PH: Effect of chemical composition and depolymerization. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Smidsrod, O. Hydrogen-ion titration of chitosans with varying degrees of N-acetylation by monitoring induced 1H-NMR chemical-shifts. Carbohydr. Polym. 1995, 26, 303–305. [Google Scholar] [CrossRef]
- Filion, D.; Lavertu, M.; Buschmann, M.D. Ionization and solubility of chitosan solutions related to thermosensitive chitosan/glycerol-phosphate systems. Biomacromolecules 2007, 8, 3224–3234. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Fletcher, A.P. Dissociation constants of 2-amino-2-deoxy-d-glucopyranose. J. Chem. Soc. B 1969, 178–181. [Google Scholar]
- Sorlier, P.; Rochas, C.; Morfin, I.; Viton, C.; Domard, A. Light scattering studies of the solution properties of chitosans of varying degrees of acetylation. Biomacromolecules 2003, 4, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Viton, C.; Domard, A. Relation between solution properties and degree of acetylation of chitosan: Role of aging. Biomacromolecules 2002, 3, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Pichot, C.; Delair, T.; Viton, C.; Domard, A. Static light scattering studies on chitosan solutions: From macromolecular chains to colloidal dispersions. Langmuir 2003, 19, 9896–9903. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Alcouffe, P.; Rochas, C.; David, L.; Domard, A. Continuum of structural organization from chitosan solutions to derived physical forms. Biomacromolecules 2010, 11, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.C.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amedee, J.; Montembault, A.; David, L.; et al. Physicochemical modulation of chitosan-based hydrogels induces different biological responses: Interest for tissue engineering. J. Biomed. Mater. Res. A 2014, 102, 3666–3676. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Bezrodnykh, E.A.; Abramchuk, S.S.; Muranov, A.V.; Sinitsyna, O.V.; Khokhlov, A.R.; Tikhonov, V.E. Short chain chitosan solutions: Self-assembly and aggregates disruption effects. J. Polym. Res. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Maki, Y.; Furusawa, K.; Yasuraoka, S.; Okamura, H.; Hosoya, N.; Sunaga, M.; Dobashi, T.; Sugimoto, Y.; Wakabayashi, K. Universality and specificity in molecular orientation in anisotropic gels prepared by diffusion method. Carbohydr. Polym. 2014, 108, 118–126. [Google Scholar] [CrossRef]
- Payne, G.F.; Raghavan, S.R. Chitosan: A soft interconnect for hierarchical assembly of nano-scale components. Soft Matter 2007, 3, 521–527. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Kulikov, S.N.; Vyshivannaya, O.V.; Bezrodnykh, E.A.; Yamskov, I.A.; Tikhonov, V.E. Influence of glucosamine on oligochitosan solubility and antibacterial activity. Carbohydr. Res. 2013, 381, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Pigaleva, M.A.; Portnov, I.V.; Rudov, A.A.; Blagodatskikh, I.V.; Grigoriev, T.E.; Gallyamov, M.O.; Potemkin, I.I. Stabilization of chitosan aggregates at the nanoscale in solutions in carbonic acid. Macromolecules 2014, 47, 5749–5758. [Google Scholar] [CrossRef]
- Rybtchinski, B. Adaptive supramolecular nanomaterials based on strong noncovalent interactions. ACS Nano 2011, 5, 6791–6818. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.S.; Kraft, M.L. Chemistry. Synchronized self-assembly. Science 2008, 320, 620–621. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Terrell, J.L.; Fernandes, R.; Dowling, M.B.; Payne, G.F.; Raghavan, S.R.; Bentley, W.E. Encapsulated fusion protein confers “sense and respond” activity to chitosan–alginate capsules to manipulate bacterial quorum sensing. Biotechnol. Bioeng. 2013, 110, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Wan, A.C.A.; Yim, E.K.F.; Leong, K.W. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release 2005, 104, 347–358. [Google Scholar] [CrossRef]
- Albarelli, J.Q.; Luna, M.T.; Vieira, R.S.; Beppu, M.M. Evaluation of glass beads coated with chitosan for the adsorption of copper(ii) ions from aqueous solution. Adsorpt. Sci. Technol. 2012, 30, 227–240. [Google Scholar] [CrossRef]
- Jouannin, C.; Vincent, C.; Dez, I.; Gaumont, A.C.; Vincent, T.; Guibal, E. Highly porous catalytic materials with Pd and ionic liquid supported on chitosan. J. Appl. Polym. Sci. 2013, 128, 3122–3130. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Kim, Y.K.; Arote, R.; Nah, J.W.; Cho, M.H.; Choi, Y.J.; Akaike, T.; Cho, C.S. Chitosan-graft-polyethylenimine as a gene carrier. J. Control. Release 2007, 117, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Cosani, A.; Terbojevich, M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydr. Polym. 1999, 39, 361–367. [Google Scholar] [CrossRef]
- Kato, Y.; Kaminaga, J.; Matsuo, R.; Isogai, A. Tempo-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan. Carbohydr. Polym. 2004, 58, 421–426. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, S.; Coma, V. Advances on selective C-6 oxidation of chitosan by TEMPO. Biomacromolecules 2008, 9, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Bragd, P.L.; van Bekkum, H.; Besemer, A.C. Tempo-mediated oxidation of polysaccharides: Survey of methods and applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Christensen, B.E.; Aasprong, E.; Stokke, B.T. Gelation of periodate oxidised scleroglucan (scleraldehyde). Carbohydr. Polym. 2001, 46, 241–248. [Google Scholar] [CrossRef]
- Isogai, T.; Saito, T.; Isogai, A. Tempo electromediated oxidation of some polysaccharides including regenerated cellulose fiber. Biomacromolecules 2010, 11, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.S.; Cestari, A.R.; Airoldi, C.; Loh, W. Polysaccharide-based hydrogels: Preparation, characterization, and drug interaction behaviour. Biomacromolecules 2008, 9, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Veelaert, S.; deWit, D.; Gotlieb, K.F.; Verhe, R. The gelation of dialdehyde starch. Carbohydr. Polym. 1997, 32, 131–139. [Google Scholar] [CrossRef]
- Birge, R.R.; Gillespie, N.B.; Izaguirre, E.W.; Kusnetzow, A.; Lawrence, A.F.; Singh, D.; Song, Q.W.; Schmidt, E.; Stuart, J.A.; Seetharaman, S.; et al. Biomolecular electronics: Protein-based associative processors and volumetric memories. J. Phys. Chem. B 1999, 103, 10746–10766. [Google Scholar] [CrossRef]
- Willner, I.; Katz, E. Integration of layered redox proteins and conductive supports for bioelectronic applications. Angew. Chem. Int. Ed. 2000, 39, 1180–1218. [Google Scholar] [CrossRef]
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B. Biomaterials integrated with electronic elements: En route to bioelectronics. Trends Biotechnol. 2001, 19, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Life’s lessons in design. Nature 2001, 409, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Natural strategies for the molecular engineer. Nanotechnology 2002, 13, R15–R28. [Google Scholar] [CrossRef]
- Ball, P. Synthetic biology for nanotechnology. Nanotechnology 2005, 16, R1–R8. [Google Scholar] [CrossRef]
- Yi, H.M.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Luo, X.; Yi, H.; Valentine, T.M.; Payne, G.F.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W. Chitosan-mediated in situ biomolecule assembly in completely packaged microfluidic devices. Lab Chip 2006, 6, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.A.; Koev, S.T.; Schleunitz, A.; Yi, H.M.; Hodzic, V.; Bentley, W.E.; Payne, G.F.; Rubloff, G.W.; Ghodssi, R. A fabrication platform for electrically mediated optically active biofunctionalized sites in biomems. Lab Chip 2005, 5, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.F.; Kim, E.; Cheng, Y.; Wu, H.C.; Ghodssi, R.; Rubloff, G.W.; Raghavan, S.R.; Culver, J.N.; Bentley, W.E. Accessing biology’s toolbox for the mesoscale biofabrication of soft matter. Soft Matter 2013, 9, 6019–6032. [Google Scholar] [CrossRef]
- Wu, L.Q.; Payne, G.F. Biofabrication: Using biological materials and biocatalysts to construct nanostructured assemblies. Trends Biotechnol. 2004, 22, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Bryksin, A.V.; Brown, A.C.; Baksh, M.M.; Finn, M.G.; Barker, T.H. Learning from nature–novel synthetic biology approaches for biomaterial design. Acta Biomater. 2014, 10, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; Gadre, A.P.; Yi, H.M.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater. Res. A 2003, 66, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Xu, J.J.; Du, Y.; Chen, H.Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Xu, J.J.; Wang, J.L.; Chen, H.Y. Electrochemically deposited nanocomposite of chitosan and carbon nanotubes for biosensor application. Chem. Commun. 2005, 2169–2171. [Google Scholar]
- Pang, X.; Zhitomirsky, I. Electrodeposition of composite hydroxyapatite–chitosan films. Mater. Chem. Phys. 2005, 94, 245–251. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Park, J.J.; Rubloff, G.W.; Tarlov, M.J. Electrochemical study of chitosan films deposited from solution at reducing potentials. Electrochim. Acta 2006, 51, 5324–5333. [Google Scholar] [CrossRef]
- Fernandes, R.; Wu, L.Q.; Chen, T.H.; Yi, H.M.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electrochemically induced deposition of a polysaccharide hydrogel onto a patterned surface. Langmuir 2003, 19, 4058–4062. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Xie, Q.J.; Fu, Y.C.; Su, Z.H.; Jia, X.; Yao, S.Z. Electrodeposition of carbon nanotubes–chitosan–glucose oxidase biosensing composite films triggered by reduction of p-benzoquinone or H2O2. J. Phys. Chem. B 2007, 111, 11276–11284. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Q.; Payne, G.F.; Shi, X.-W.; Du, Y. Electrodeposition of a biopolymeric hydrogel in track-etched micropores. Soft Matter 2013, 9, 2131–2135. [Google Scholar] [CrossRef]
- Altomare, L.; Draghi, L.; Chiesa, R.; de Nardo, L. Morphology tuning of chitosan films via electrochemical deposition. Mater. Lett. 2012, 78, 18–21. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of chitosan in different alcohols. J. Coat. Technol. Res. 2014, 11, 739–746. [Google Scholar] [CrossRef]
- Wu, L.Q.; Yi, H.M.; Li, S.; Rubloff, G.W.; Bentley, W.E.; Ghodssi, R.; Payne, G.F. Spatially selective deposition of a reactive polysaccharide layer onto a patterned template. Langmuir 2003, 19, 519–524. [Google Scholar] [CrossRef]
- Buckhout-White, S.L.; Rubloff, G.W. Spatial resolution in chitosan-based programmable biomolecular scaffolds. Soft Matter 2009, 5, 3677–3681. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Betz, J.; Buckhout-White, S.; Bekdash, O.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter 2010, 6, 3177–3183. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Mechanism of anodic electrodeposition of calcium alginate. Soft Matter 2011, 7, 5677–5684. [Google Scholar] [CrossRef]
- Dobashi, T.; Tomita, N.; Maki, Y.; Chang, C.P.; Yamamoto, T. An analysis of anisotropic gel forming process of chitosan. Carbohydr. Polym. 2011, 84, 709–712. [Google Scholar] [CrossRef]
- Yan, K.; Ding, F.Y.; Bentley, W.E.; Deng, H.B.; Du, Y.M.; Payne, G.F.; Shi, X.W. Coding for hydrogel organization through signal guided self-assembly. Soft Matter 2014, 10, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Chatzipirpiridis, G.; Sivaraman, K.M.; Ergeneman, O.; Nelson, B.J.; Pané, S. Chitosan electrodeposition for microrobotic drug delivery. Adv. Healthc. Mater. 2013, 2, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gray, K.M.; David, L.; Royaud, I.; Payne, G.F.; Rubloff, G.W. Characterization of the cathodic electrodeposition of semicrystalline chitosan hydrogel. Mater. Lett. 2012, 87, 97–100. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Gray, K.M.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Wang, Q.; Payne, G.F. Electrodeposition of a weak polyelectrolyte hydrogel: Remarkable effects of salt on kinetics, structure and properties. Soft Matter 2013, 9, 2703–2710. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yan, K.; Bentley, W.E.; Deng, H.; Du, Y.; Payne, G.F.; Shi, X.W. Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide. ACS Appl. Mater. Interfaces 2014, 6, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.J.; Li, X.F.; Long, Y.H.; Wu, J.J.; Liang, S.M.; Zhang, X.L.; Zhao, N.; Xu, J. Multi-membrane hydrogel fabricated by facile dynamic self-assembly. Soft Matter 2009, 5, 1987–1989. [Google Scholar] [CrossRef]
- Ladet, S.G.; Tahiri, K.; Montembault, A.S.; Domard, A.J.; Corvol, M.T.M. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials 2011, 32, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhao, Y.T.; Duan, J.J.; Wang, Z.G.; Chen, Y.; Zhang, L.N. Fast contact of solid–liquid interface created high strength multi-layered cellulose hydrogels with controllable size. ACS Appl. Mater. Interfaces 2014, 6, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Luo, X.L.; Du, Y.; Chen, H.Y. Application of MnO2 nanoparticles as an eliminator of ascorbate interference to amperometric glucose biosensors. Electrochem. Commun. 2004, 6, 1169–1173. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Zhang, Q.; Yang, G.J.; Chen, H.Y. Electrochemically deposited chitosan hydrogel for horseradish peroxidase immobilization through gold nanoparticles self-assembly. Biosens. Bioelectron. 2005, 21, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Ji, C. Electro-induced covalent cross-linking of chitosan and formation of chitosan hydrogel films: Its application as an enzyme immobilization matrix for use in a phenol sensor. Anal. Chem. 2010, 82, 5275–5281. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chen, R.L.C.; Cheng, T.J.; Wittstock, G. Localized deposition of chitosan as matrix for enzyme immobilization. Electroanalysis 2009, 21, 804–810. [Google Scholar]
- Li, Y.; Pang, X.; Epand, R.F.; Zhitomirsky, I. Electrodeposition of chitosan–hemoglobin films. Mater. Lett. 2011, 65, 1463–1465. [Google Scholar] [CrossRef]
- Guo, M.Q.; Fang, H.D.; Wang, R.; Yang, Z.Q.; Xu, X.H. Electrodeposition of chitosan–glucose oxidase biocomposite onto Pt–Pb nanoparticles modified stainless steel needle electrode for amperometric glucose biosensor. J. Mater. Sci. Mater. Med. 2011, 22, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, J.J.; Chen, H.Y. Fabrication, characterization of Fe3O4 multilayer film and its application in promoting direct electron transfer of hemoglobin. Electrochem. Commun. 2006, 8, 148–154. [Google Scholar] [CrossRef]
- Bai, Y.H.; Du, Y.; Xu, J.J.; Chen, H.Y. Choline biosensors based on a bi-electrocatalytic property of MnO2 nanoparticles modified electrodes to H2O2. Electrochem. Commun. 2007, 9, 2611–2616. [Google Scholar] [CrossRef]
- Bai, Y.H.; Xu, J.J.; Chen, H.Y. Selective sensing of cysteine on manganese dioxide nanowires and chitosan modified glassy carbon electrodes. Biosens. Bioelectron. 2009, 24, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Zhang, H.; Xu, J.J.; Chen, H.Y. Relationship between nanostructure and electrochemical/biosensing properties of MnO2 nanomaterials for H2O2/choline. J. Phys. Chem. C 2008, 112, 18984–18990. [Google Scholar] [CrossRef]
- Tangkuaram, T.; Ponchio, C.; Kangkasomboon, T.; Katikawong, P.; Veerasai, W. Design and development of a highly stable hydrogen peroxide biosensor on screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan. Biosens. Bioelectron. 2007, 22, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Ding, J.W.; Cai, J.; Zhang, A.D. Determination of carbaryl pesticide using amperometric acetylcholinesterase sensor formed by electrochemically deposited chitosan. Colloids Surf. B Biointerfaces 2007, 58, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Ding, J.W.; Cai, J.; Zhang, A.D. Electrochemical thiocholine inhibition sensor based on biocatalytic growth of Au nanoparticles using chitosan as template. Sens. Actuators B Chem. 2007, 127, 317–322. [Google Scholar] [CrossRef]
- Du, D.; Ding, J.W.; Cai, J.; Zhang, A.D. One-step electrochemically deposited interface of chitosan-gold nanoparticles for acetylcholinesterase biosensor design. J. Electroanal. Chem. 2007, 605, 53–60. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Chen, W.; Zhang, S.S. A simple strategy for one-step construction of bienzyme biosensor by in situ formation of biocomposite film through electrodeposition. Biosens. Bioelectron. 2009, 24, 3030–3035. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Fan, L.X.; Wang, R.; Qiu, J.D. One-step electrochemically deposited nanocomposite film of Cs–Fc/MWNTs/GOD for glucose biosensor application. Electroanalysis 2009, 21, 1685–1691. [Google Scholar] [CrossRef]
- Liang, R.P.; Peng, H.Z.; Qiu, J.D. Fabrication, characterization, and application of potentiometric immunosensor based on biocompatible and controllable three-dimensional porous chitosan membranes. J. Colloid Interface Sci. 2008, 320, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 3815–3821. [Google Scholar] [CrossRef]
- Zhu, C.; Lee, J.H.; Raghavan, S.R.; Payne, G.F. Bioinspired vesicle restraint and mobilization using a biopolymer scaffold. Langmuir 2006, 22, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, L.Q.; Wang, X.; Lee, J.H.; English, D.S.; Ghodssi, R.; Raghavan, S.R.; Payne, G.F. Reversible vesicle restraint in response to spatiotemporally controlled electrical signals: A bridge between electrical and chemical signaling modes. Langmuir 2007, 23, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Suzuki, M.; Abd El-Moneim, A. Synthesis of MnO2-chitosan nanocomposite by one-step electrodeposition for electrochemical energy storage application. J. Power Sources 2014, 246, 68–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, T.; Ma, K.N.; Cai, X.J.; Zhou, Y.; Wang, Y.N. Low temperature electrophoretic deposition of porous chitosan/silk fibroin composite coating for titanium biofunctionalization. J. Mater. Chem. 2011, 21, 7705–7713. [Google Scholar] [CrossRef]
- Pishbin, F.; Mourino, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of gentamicin-loaded bioactive glass/chitosan composite coatings for orthopaedic implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [PubMed]
- Raddaha, N.S.; Cordero-Arias, L.; Cabanas-Polo, S.; Virtanen, S.; Roether, J.A.; Boccaccini, A.R. Electrophoretic deposition of chitosan/h-BN and chitosan/h-BN/TiO2 composite coatings on stainless steel (316L) substrates. Materials 2014, 7, 1814–1829. [Google Scholar] [CrossRef]
- Santos, R.M.; Rodrigues, M.S.; Laranjinha, J.; Barbosa, R.M. Biomimetic sensor based on hemin/carbon nanotubes/chitosan modified microelectrode for nitric oxide measurement in the brain. Biosens. Bioelectron. 2013, 44, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Huang, Y.X.; Sun, X.F.; Sheng, G.P.; Zhao, F.; Wang, S.G.; Yu, H.Q. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Geng, Z.H.; Guo, M.M.; Chen, Y.J.; Guo, X.C.; Wang, X. Electroaddressing of ZnS quantum dots by codeposition with chitosan to construct fluorescent and patterned device surface. ACS Appl. Mater. Interfaces 2014, 6, 15510–15515. [Google Scholar]
- Liu, B.Z.; Deng, Y.H.; Hu, X.B.; Gao, Z.Q.; Sun, C. Electrochemical sensing of trichloroacetic acid based on silver nanoparticles doped chitosan hydrogel film prepared with controllable electrodeposition. Electrochim. Acta 2012, 76, 410–415. [Google Scholar] [CrossRef]
- Li, S.S.; Du, D.; Huang, J.; Tu, H.Y.; Yang, Y.Q.; Zhang, A.D. One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/aunps hybrid film and its application in the selective determination of p-nitrophenol. Analyst 2013, 138, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.H.; Strickler, J.R.; Chang, W.J.; Gunasekaran, S. Nickel nanoparticle–chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Thongkaew, C.; Weiss, J. Theoretical and practical considerations in electrostatic depositioning of charged polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Caruso, F.; Trau, D.; Mohwald, H.; Renneberg, R. Enzyme encapsulation in layer-by-layer engineered polymer multilayer capsules. Langmuir 2000, 16, 1485–1488. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F. Coupling electrodeposition with layer-by-layer assembly to address proteins within microfluidic channels. Adv. Mater. 2011, 23, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling concept combining chitosan and alginate—Proof of principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.H.; Cao, Z.J.; Xie, Q.J.; Fu, Y.C.; Tan, Y.M.; Ma, M.; Yao, S.Z. One-pot electrodeposition of 3-aminopropyltriethoxysilane-chitosan hybrid gel film to immobilize glucose oxidase for biosensing. Sens. Actuators B Chem. 2011, 157, 282–289. [Google Scholar] [CrossRef]
- Qiu, J.D.; Wang, R.; Liang, R.P.; Xia, X.H. Electrochemically deposited nanocomposite film of CS-Fc/Au NPs/GOx for glucose biosensor application. Biosens. Bioelectron. 2009, 24, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhang, X.Q.; Gu, J.M.; Yang, H.T.; Nie, J.; Ma, G.P. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef]
- Cheong, M.; Zhitomirsky, I. Electrodeposition of alginic acid and composite films. Colloids Surf. Physicochem. Eng. Aspects 2008, 328, 73–78. [Google Scholar] [CrossRef]
- Gray, K.M.; Liba, B.D.; Wang, Y.; Cheng, Y.; Rubloff, G.W.; Bentley, W.E.; Montembault, A.; Royaud, I.; David, L.; Payne, G.F. Electrodeposition of a biopolymeric hydrogel: Potential for one-step protein electroaddressing. Biomacromolecules 2012, 13, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Yang, X.H.; Gaskell, K.J.; Liu, Y.; Kobatake, E.; Bentley, W.E.; Payne, G.F. Reagentless protein assembly triggered by localized electrical signals. Adv. Mater. 2009, 21, 984–988. [Google Scholar] [CrossRef]
- Bechtold, T.; Turcanu, A.; Campese, R.; Maier, P.; Schrott, W. On-site formation of hypochlorite for indigo oxidation–scale-up and full scale operation of an electrolyser for denim bleach processes. J. Appl. Electrochem. 2006, 36, 287–293. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kelsall, G.H. Models of hypochlorite production in electrochemical reactors with plate and porous anodes. J. Appl. Electrochem. 2007, 37, 1203–1217. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Vissers, M.C.M.; Winterbourn, C.C. Living with a killer: The effects of hypochlorous acid on mammalian cells. Iubmb Life 2000, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Prutz, W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 1996, 332, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.J.; Crowley, J.R.; Hsu, F.-F.; Thukkani, A.K.; Ford, D.A. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens. J. Biol. Chem. 2001, 276, 23733–23741. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Shi, X.W.; Tsao, C.Y.; Lewandowski, A.T.; Fernandes, R.; Hung, C.W.; DeShong, P.; Kobatake, E.; Valdes, J.J.; Payne, G.F.; et al. Biofabrication of antibodies and antigens via IgG-binding domain engineered with activatable pentatyrosine pro-tag. Biotechnol. Bioeng. 2009, 103, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Luo, X.; Tsao, C.Y.; Payne, G.F.; Ghodssi, R.; Rubloff, G.W.; Bentley, W.E. Biological nanofactories facilitate spatially selective capture and manipulation of quorum sensing bacteria in a biomems device. Lab Chip 2010, 10, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Tsao, C.-Y.; Yang, X.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels. Adv. Funct. Mater. 2009, 19, 2074–2080. [Google Scholar] [CrossRef]
- Terrell, J.L.; Gordonov, T.; Cheng, Y.; Wu, H.C.; Sampey, D.; Luo, X.L.; Tsao, C.Y.; Ghodssi, R.; Rubloff, G.W.; Payne, G.F.; et al. Integrated biofabrication for electro-addressed in-film bioprocessing. Biotechnol. J. 2012, 7, 428–439. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Payne, G.F.; Rubloff, G.W. Biofabrication: Programmable assembly of polysaccharide hydrogels in microfluidics as biocompatible scaffolds. J. Mater. Chem. 2012, 22, 7659–7666. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Tsao, C.Y.; Wu, H.C.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation. Lab Chip 2011, 11, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tsao, C.-Y.; Wu, H.-C.; Luo, X.; Terrell, J.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Electroaddressing functionalized polysaccharides as model biofilms for interrogating cell signaling. Adv. Funct. Mater. 2012, 22, 519–528. [Google Scholar] [CrossRef]
- Yang, X.H.; Kim, E.; Liu, Y.; Shi, X.W.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Pancer, Z.; Payne, G.F. In-film bioprocessing and immunoanalysis with electroaddressable stimuli-responsive polysaccharides. Adv. Funct. Mater. 2010, 20, 1645–1652. [Google Scholar] [CrossRef]
- Liu, Y.; Terrell, J.L.; Tsao, C.Y.; Wu, H.C.; Javvaji, V.; Kim, E.; Cheng, Y.; Wang, Y.F.; Ulijn, R.V.; Raghavan, S.R.; et al. Biofabricating multifunctional soft matter with enzymes and stimuli-responsive materials. Adv. Funct. Mater. 2012, 22, 3004–3012. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Del. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.A.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Shakya, A.K.; Sami, H.; Srivastava, A.; Kumar, A. Stability of responsive polymer–protein bioconjugates. Prog. Polym. Sci. 2010, 35, 459–486. [Google Scholar] [CrossRef]
- Tseng, T.T.C.; Chang, C.F.; Chan, W.C. Fabrication of implantable, enzyme-immobilized glutamate sensors for the monitoring of glutamate concentration changes in vitro and in vivo. Molecules 2014, 19, 7341–7355. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Duhalt, R.; Tinoco, R.; D’Antonio, P.; Topoleski, L.D.T.; Payne, G.F. Enzyme conjugation to the polysaccharide chitosan: Smart biocatalysts and biocatalytic hydrogels. Bioconj. Chem. 2001, 12, 301–306. [Google Scholar] [CrossRef]
- Seo, H.; Itoyama, K.; Morimoto, K.; Takagishi, T.; Oka, M.; Hayashi, T. Spacer effects on enzymatic activity of bromelain immobilized onto porous chitosan beads. Eur. Polym. J. 1998, 34, 917–922. [Google Scholar] [CrossRef]
- Juang, R.S.; Wu, F.C.; Tseng, R.L. Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresour. Technol. 2001, 80, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.W.; Wang, F.; Zhang, H. Preparation of PVA/chitosan lipase membrane reactor and its application in synthesis of monoglyceride. J. Mol. Catal. B Enzym. 2002, 18, 325–331. [Google Scholar] [CrossRef]
- Shi, X.W.; Qiu, L.; Nie, Z.; Xiao, L.; Payne, G.F.; Du, Y.M. Protein addressing on patterned microchip by coupling chitosan electrodeposition and “electro-click” chemistry. Biofabrication 2013, 5. [Google Scholar] [CrossRef]
- Shi, X.W.; Liu, Y.; Lewandowski, A.T.; Wu, L.Q.; Wu, H.C.; Ghodssi, R.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F. Chitosan biotinylation and electrodeposition for selective protein assembly. Macromol. Biosci. 2008, 8, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Custodio, C.A.; Miguel-Arranz, V.S.; Gropeanu, R.A.; Gropeanu, M.; Wirkner, M.; Reis, R.L.; Mano, J.F.; del Campo, A. Photopatterned antibodies for selective cell attachment. Langmuir 2014, 30, 10066–10071. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Wu, H.C.; Liu, Y.; Tsao, C.Y.; Wang, K.; Kobatake, E.; Bentley, W.E.; Payne, G.F. Chitosan fibers: Versatile platform for nickel-mediated protein assembly. Biomacromolecules 2008, 9, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Rollett, A.; Thallinger, B.; Ohradanova-Repic, A.; Machacek, C.; Walenta, E.; Cavaco-Paulo, A.; Birner-Gruenberger, R.; Bogner-Strauss, J.G.; Stockinger, H.; Guebitz, G.M. Enzymatic synthesis of antibody-human serum albumin conjugate for targeted drug delivery using tyrosinase from agaricus bisporus. RSC Adv. 2013, 3, 1460–1467. [Google Scholar] [CrossRef]
- Yang, X.H.; Liu, Y.; Payne, G.F. Crosslinking lessons from biology: Enlisting enzymes for macromolecular assembly. J. Adhes. 2009, 85, 576–589. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic hydrogelation of small molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Stayner, R.S.; Min, D.J.; Kiser, P.F.; Stewart, R.J. Site-specific cross-linking of proteins through tyrosine hexahistidine tags. Bioconj. Chem. 2005, 16, 1617–1623. [Google Scholar] [CrossRef]
- Jus, S.; Kokol, V.; Guebitz, G.M. Tyrosinase-catalysed coating of wool fibres with different protein-based biomaterials. J. Biomater. Sci. Polym. Ed. 2009, 20, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Jus, S.; Stachel, I.; Fairhead, M.; Meyer, M.; Thony-Meyer, L.; Guebitz, G.M. Enzymatic cross-linking of gelatine with laccase and tyrosinase. Biocatal. Biotransform. 2012, 30, 86–95. [Google Scholar] [CrossRef]
- Bakota, E.L.; Aulisa, L.; Galler, K.M.; Hartgerink, J.D. Enzymatic cross-linking of a nanofibrous peptide hydrogel. Biomacromolecules 2011, 12, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Cross, H.F.; She, J.K.; Cavalli, G.; Martins, H.F.; Neylon, C. Covalent attachment of proteins to solid supports and surfaces via sortase-mediated ligation. PLoS One 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, M.; Todd, S.J.; Hirst, A.R.; McDonald, T.O.; Ulijn, R.V. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2013, 1, 11–39. [Google Scholar] [CrossRef]
- Sato, H.; Hayashi, E.; Yamada, N.; Yatagai, M.; Takahara, Y. Further studies on the site-specific protein modification by microbial transglutaminase. Bioconj. Chem. 2001, 12, 701–710. [Google Scholar] [CrossRef]
- Tanaka, T.; Kamiya, N.; Nagamune, T. Peptidyl linkers for protein heterodimerization catalyzed by microbial transglutaminase. Bioconj. Chem. 2004, 15, 491–497. [Google Scholar] [CrossRef]
- Tanaka, T.; Kamiya, N.; Nagamune, T. N-terminal glycine-specific protein conjugation catalyzed by microbial transglutaminase. FEBS Lett. 2005, 579, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yamamoto, T.; Tsukiji, S.; Nagamune, T. Site-specific protein modification on living cells catalyzed by sortase. ChemBioChem 2008, 9, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Doi, S.; Tominaga, J.; Ichinose, H.; Goto, M. Transglutaminase-mediated protein immobilization to casein nanolayers created on a plastic surface. Biomacromolecules 2005, 6, 35–38. [Google Scholar] [CrossRef]
- Kamiya, N.; Tanaka, T.; Suzuki, T.; Takazawa, T.; Takeda, S.; Watanabe, K.; Nagamune, T. S-peptide as a potent peptidyl linker for protein cross-linking by microbial transglutaminase from streptomyces mobaraensis. Bioconj. Chem. 2003, 14, 351–357. [Google Scholar] [CrossRef]
- Shao, L.H.; Kumar, G.; Lenhart, J.L.; Smith, P.J.; Payne, G.F. Enzymatic modification of the synthetic polymer polyhydroxystyrene. Enzyme Microb. Technol. 1999, 25, 660–668. [Google Scholar] [CrossRef]
- Nyanhongo, G.S.; Prasetyo, E.N.; Acero, E.H.; Guebitz, G.M. Engineering strategies for successful development of functional polymers using oxidative enzymes. Chem. Eng. Technol. 2012, 35, 1359–1372. [Google Scholar] [CrossRef]
- Vachoud, L.; Chen, T.H.; Payne, G.F.; Vazquez-Duhalt, R. Peroxidase catalyzed grafting of gallate esters onto the polysaccharide chitosan. Enzyme Microb. Technol. 2001, 29, 380–385. [Google Scholar] [CrossRef]
- Aberg, C.M.; Chen, T.H.; Olumide, A.; Raghavan, S.R.; Payne, G.F. Enzymatic grafting of peptides from casein hydrolysate to chitosan. Potential for value-added byproducts from food-processing wastes. J. Agric. Food Chem. 2004, 52, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Vazquez-Duhalt, R.; Wu, C.F.; Bentley, W.E.; Payne, G.F. Combinatorial screening for enzyme-mediated coupling. Tyrosinase-catalyzed coupling to create protein—Chitosan conjugates. Biomacromolecules 2001, 2, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Demolliens, A.; Boucher, C.; Durocher, Y.; Jolicoeur, M.; Buschmann, M.D.; de Crescenzo, G. Tyrosinase-catalyzed synthesis of a universal coil-chitosan bioconjugate for protein immobilization. Bioconj. Chem. 2008, 19, 1849–1854. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Embree, H.D.; Wu, L.Q.; Payne, G.F. In vitro protein-polysaccharide conjugation: Tyrosinase-catalyzed conjugation of gelatin and chitosan. Biopolymers 2002, 64, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, M.L.; Cui, L.; Yuan, J.G.; Wang, Q.; Fan, X.R. Modification of Bombyx mori silk fabrics by tyrosinase-catalyzed grafting of chitosan. Eng. Life Sci. 2014, 14, 211–217. [Google Scholar] [CrossRef]
- Sampaio, S.; Taddei, P.; Monti, P.; Buchert, J.; Freddi, G. Enzymatic grafting of chitosan onto bombyx mori silk fibroin: Kinetic and IR vibrational studies. J. Biotechnol. 2005, 116, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Anghileri, A.; Lantto, R.; Kruus, K.; Arosio, C.; Freddi, G. Tyrosinase-catalyzed grafting of sericin peptides onto chitosan and production of protein-polysaccharide bioconjugates. J. Biotechnol. 2007, 127, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Freddi, G.; Anghileri, A.; Sampaio, S.; Buchert, J.; Monti, P.; Taddei, P. Tyrosinase-catalyzed modification of Bombyx mori silk fibroin: Grafting of chitosan under heterogeneous reaction conditions. J. Biotechnol. 2006, 125, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Lee, K.H.; Ki, C.S.; Nahm, J.H.; Park, Y.H. Silk fibroin/chitosan conjugate crosslinked by tyrosinase. Macromol. Res. 2004, 12, 534–539. [Google Scholar] [CrossRef]
- Kang, G.D.; Lee, K.H.; Ki, C.S.; Park, Y.H. Crosslinking reaction of phenolic side chains in silk fibroin by tyrosinase. Fibers Polym. 2004, 5, 234–238. [Google Scholar] [CrossRef]
- Lewandowski, A.T.; Small, D.A.; Chen, T.H.; Payne, G.F.; Bentley, W.E. Tyrosine-based “activatable pro-tag”: Enzyme-catalyzed protein capture and release. Biotechnol. Bioeng. 2006, 93, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.T.; Bentley, W.E.; Yi, H.M.; Rubloff, G.W.; Payne, G.F.; Ghodssi, R. Towards area-based in vitro metabolic engineering: Assembly of pfs enzyme onto patterned microfabricated chips. Biotechnol. Prog. 2008, 24, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Lewandowski, A.T.; Yi, H.M.; Payne, G.F.; Ghodssi, R.; Bentley, W.E.; Rubloff, G.W. Programmable assembly of a metabolic pathway enzyme in a pre-packaged reusable biomems device. Lab Chip 2008, 8, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Tsao, C.Y.; Hashimoto, Y.; Wang, L.; Wood, T.K.; Payne, G.F.; Bentley, W.E. Magnetic nanofactories: Localized synthesis and delivery of quorum-sensing signaling molecule autoinducer-2 to bacterial cell surfaces. Metab. Eng. 2007, 9, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Minamihata, K.; Goto, M.; Kamiya, N. Site-specific protein cross-linking by peroxidase-catalyzed activation of a tyrosine-containing peptide tag. Bioconj. Chem. 2011, 22, 74–81. [Google Scholar] [CrossRef]
- Chen, T.H.; Small, D.A.; Wu, L.Q.; Rubloff, G.W.; Ghodssi, R.; Vazquez-Duhalt, R.; Bentley, W.E.; Payne, G.F. Nature-inspired creation of protein-polysaccharide conjugate and its subsequent assembly onto a patterned surface. Langmuir 2003, 19, 9382–9386. [Google Scholar] [CrossRef]
- Lewandowski, A.T.; Yi, H.M.; Luo, X.L.; Payne, G.F.; Ghodssi, R.; Rubloff, G.W.; Bentley, W.E. Protein assembly onto patterned microfabricated devices through enzymatic activation of fusion pro-tag. Biotechnol. Bioeng. 2008, 99, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Shi, X.W.; Liu, Y.; Bentley, W.E.; Payne, G.F. Orthogonal enzymatic reactions for the assembly of proteins at electrode addresses. Langmuir 2009, 25, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Kim, E.; Wu, L.Q.; Liu, Y.; Bentley, W.E.; Payne, G.F. Biomimetic fabrication of information-rich phenolic-chitosan films. Soft Matter 2011, 7, 9601–9615. [Google Scholar] [CrossRef]
- Sedo, J.; Saiz-Poseu, J.; Busque, F.; Ruiz-Molina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Falentin-Daudre, C.; Jerome, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Bentley, W.E.; Payne, G.F. Nature’s other self-assemblers. Science 2013, 341, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Page, S.E.; Sander, M.; Arnold, W.A.; McNeill, K. Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ. Sci. Technol. 2012, 46, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.; Christl, I.; Hoffmann, M.; Kretzschmar, R. Reduction and reoxidation of humic acid: Influence on speciation of cadmium and silver. Environ. Sci. Technol. 2012, 46, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bruggeman, P.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef]
- Kim, Y.J.; Wu, W.; Chun, S.E.; Whitacre, J.F.; Bettinger, C.J. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc. Natl. Acad. Sci. USA 2013, 110, 20912–20917. [Google Scholar] [CrossRef] [PubMed]
- Muskovich, M.; Bettinger, C.J. Biomaterials-based electronics: Polymers and interfaces for biology and medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef] [PubMed]
- McGinness, J.; Corry, P.; Proctor, P. Amorphous semiconductor switching in melanins. Science 1974, 183, 853–855. [Google Scholar] [CrossRef] [PubMed]
- McGinness, J.E. Mobility gaps: A mechanism for band gaps in melanins. Science 1972, 177, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G.; Inganas, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Bristow, J.F.; Smith, P.J.; Payne, G.F. Enzymatic gelation of the natural polymer chitosan. Polymer 2000, 41, 2157–2168. [Google Scholar] [CrossRef]
- Kumar, G.; Smith, P.J.; Payne, G.F. Enzymatic grafting of a natural product onto chitosan to confer water solubility under basic conditions. Biotechnol. Bioeng. 1999, 63, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; Embree, H.D.; Balgley, B.M.; Smith, P.J.; Payne, G.F. Utilizing renewable resources to create functional polymers: Chitosan-based associative thickener. Environ. Sci. Technol. 2002, 36, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, B.C.; Javvaji, V.; Kim, E.; Lee, M.E.; Raghavan, S.R.; Wang, Q.; Payne, G.F. Tyrosinase-mediated grafting and crosslinking of natural phenols confers functional properties to chitosan. Biochem. Eng. J. 2014, 89, 21–27. [Google Scholar] [CrossRef]
- Wu, L.Q.; Lee, K.; Wang, X.; English, D.S.; Losert, W.; Payne, G.F. Chitosan-mediated and spatially selective electrodeposition of nanoscale particles. Langmuir 2005, 21, 3641–3646. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; McDermott, M.K.; Zhu, C.; Ghodssi, R.; Payne, G.E. Mimicking biological phenol reaction cascades to confer mechanical function. Adv. Funct. Mater. 2006, 16, 1967–1974. [Google Scholar] [CrossRef]
- Sun, W.Q.; Payne, G.F.; Moas, M.; Chu, J.H.; Wallace, K.K. Tyrosinase reaction chitosan adsorption for removing phenols from waste-water. Biotechnol. Prog. 1992, 8, 179–186. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Muzzarelli, R.A.A. Reactivity of quinones towards chitosan. Trends Glycosci. Glycotechnol. 2002, 14, 223–229. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Littarrua, G.; Muzzarelli, C.; Tosi, G. Selective reactivity of biochemically relevant quinones towards chitosans. Carbohydr. Polym. 2003, 53, 109–115. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Sedó, J.; García, B.; Benaiges, C.; Parella, T.; Alibés, R.; Hernando, J.; Busqué, F.; Ruiz-Molina, D. Versatile nanostructured materials via direct reaction of functionalized catechols. Adv. Mater. 2013, 25, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L.; Whitney, D.L.; Sheikh, A. Mass spectrometric profiling of glucosamine, glucosamine polymers and their catecholamine adducts—Model reactions and cuticular hydrolysates of toxorhynchites amboinensis (culicidae) pupae. Insect Biochem. Mol. Biol. 1999, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Y.; Shi, X.-W.; Yang, X.; Bentley, W.E.; Payne, G.F. Biomimetic approach to confer redox activity to thin chitosan films. Adv. Funct. Mater. 2010, 20, 2683–2694. [Google Scholar] [CrossRef]
- Wu, L.Q.; Ghodssi, R.; Elabd, Y.A.; Payne, G.F. Biomimetic pattern transfer. Adv. Funct. Mater. 2005, 15, 189–195. [Google Scholar] [CrossRef]
- Liba, B.D.; Kim, E.; Martin, A.N.; Liu, Y.; Bentley, W.E.; Payne, G.F. Biofabricated film with enzymatic and redox-capacitor functionalities to harvest and store electrons. Biofabrication 2013, 5. [Google Scholar] [CrossRef]
- Kim, E.; Leverage, W.T.; Liu, Y.; White, I.M.; Bentley, W.E.; Payne, G.F. Redox-capacitor to connect electrochemistry to redox-biology. Analyst 2014, 139, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, E.; White, I.M.; Bentley, W.E.; Payne, G.F. Information processing through a bio-based redox capacitor: Signatures for redox-cycling. Bioelectrochemistry 2014, 98, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Gordonov, T.; Bentley, W.E.; Payne, G.F. Amplified and in situ detection of redox-active metabolite using a biobased redox capacitor. Anal. Chem. 2013, 85, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoav, H.; Winkler, T.E.; Kim, E.; Chocron, S.E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Redox cycling-based amplifying electrochemical sensor for in situ clozapine antipsychotic treatment monitoring. Electrochim. Acta 2014, 130, 497–503. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Leverage, W.T.; Yin, J.-J.; White, I.M.; Bentley, W.E.; Payne, G.F. Context-dependent redox properties of natural phenolic materials. Biomacromolecules 2014, 15, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Y.; Baker, C.J.; Owens, R.; Xiao, S.Y.; Bentley, W.E.; Payne, G.F. Redox-cycling and H2O2 generation by fabricated catecholic films in the absence of enzymes. Biomacromolecules 2011, 12, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Y.; Bentley, W.E.; Payne, G.F. Redox capacitor to establish bio-device redox-connectivity. Adv. Funct. Mater. 2012, 22, 1409–1416. [Google Scholar] [CrossRef]
- Dietrich, L.E.P.; Teal, T.K.; Price-Whelan, A.; Newman, D.K. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 2008, 321, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Koley, D.; Ramsey, M.M.; Bard, A.J.; Whiteley, M. Discovery of a biofilm electrocline using real-time 3d metabolite analysis. Proc. Natl. Acad. Sci. USA 2011, 108, 19996–20001. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Recinos, D.A.; Sekedat, M.D.; Hernandez, A.; Cohen, T.S.; Sakhtah, H.; Prince, A.S.; Price-Whelan, A.; Dietrich, L.E.P. Redundant phenazine operons in pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc. Natl. Acad. Sci. USA 2012, 109, 19420–19425. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.E.P.; Kiley, P.J. A shared mechanism of soxr activation by redox-cycling compounds. Mol. Microbiol. 2011, 79, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Jamieson, H.A.; Hilmer, S.N.; Muller, M.; le Couteur, D.G. The effect of pseudomonas aeruginosa virulence factor, pyocyanin, on the liver sinusoidal endothelial cell. J. Gastroenterol. Hepatol. 2007, 22, 1350–1351. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Newman, D.K. Extracellular electron transfer. Cell. Mol. Life Sci. 2001, 58, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.M.; Shih, H.W.; Wen, S.R.; Cheng, M.W.; Hwang, H.H.; Chiu, S.H. Proteomic analysis of agrobacterium tumefaciens response to the vir gene inducer acetosyringone. Proteomics 2006, 6, 4130–4136. [Google Scholar] [CrossRef]
- Baker, C.J.; Whitaker, B.D.; Roberts, D.P.; Mock, N.M.; Rice, C.P.; Deahl, K.L.; Aver'yanov, A.A. Induction of redox sensitive extracellular phenolics during plant-bacterial interactions. Physiol. Mol. Plant Pathol. 2005, 66, 90–98. [Google Scholar] [CrossRef]
- Kim, E.; Gordonov, T.; Liu, Y.; Bentley, W.E.; Payne, G.F. Reverse engineering to suggest biologically relevant redox activities of phenolic materials. ACS Chem. Biol. 2013, 8, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Hayafune, M.; Berisio, R.; Marchetti, R.; Silipo, A.; Kayama, M.; Desaki, Y.; Arima, S.; Squeglia, F.; Ruggiero, A.; Tokuyasu, K.; et al. Chitin-induced activation of immune signaling by the rice receptor cebip relies on a unique sandwich-type dimerization. Proc. Natl. Acad. Sci. USA 2014, 111, E404–E413. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, B.H.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.W.; Shen, J.; et al. Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface. Polymers 2015, 7, 1-46. https://doi.org/10.3390/polym7010001
Kim E, Xiong Y, Cheng Y, Wu H-C, Liu Y, Morrow BH, Ben-Yoav H, Ghodssi R, Rubloff GW, Shen J, et al. Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface. Polymers. 2015; 7(1):1-46. https://doi.org/10.3390/polym7010001
Chicago/Turabian StyleKim, Eunkyoung, Yuan Xiong, Yi Cheng, Hsuan-Chen Wu, Yi Liu, Brian H. Morrow, Hadar Ben-Yoav, Reza Ghodssi, Gary W. Rubloff, Jana Shen, and et al. 2015. "Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface" Polymers 7, no. 1: 1-46. https://doi.org/10.3390/polym7010001
APA StyleKim, E., Xiong, Y., Cheng, Y., Wu, H.-C., Liu, Y., Morrow, B. H., Ben-Yoav, H., Ghodssi, R., Rubloff, G. W., Shen, J., Bentley, W. E., Shi, X., & Payne, G. F. (2015). Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface. Polymers, 7(1), 1-46. https://doi.org/10.3390/polym7010001