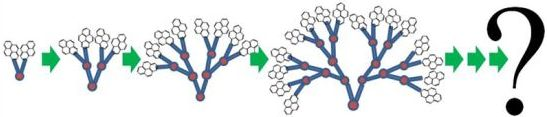
Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation
Abstract
:1. Introduction
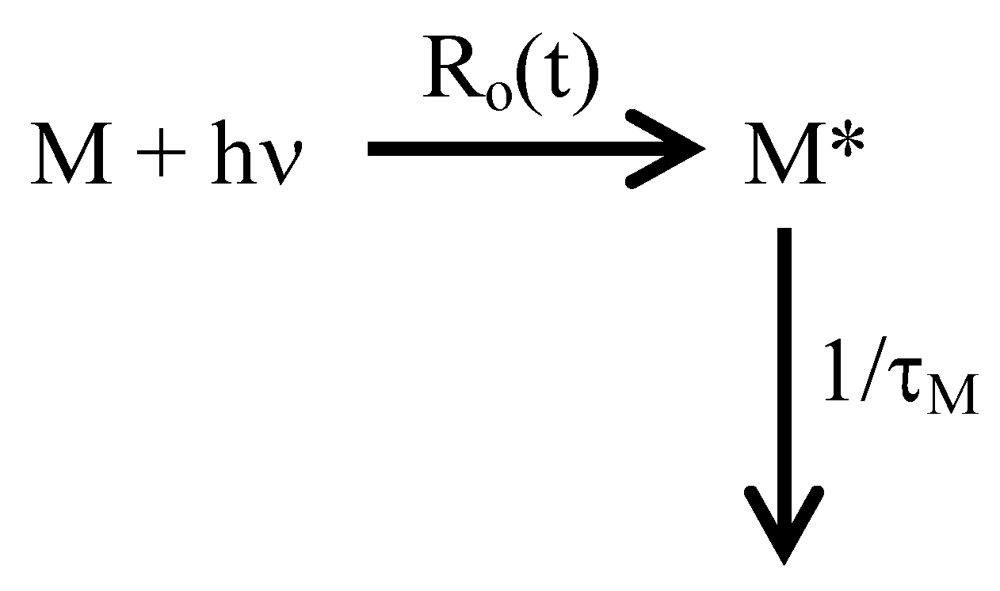
2. Fluorescence and Pyrene Excimer Formation





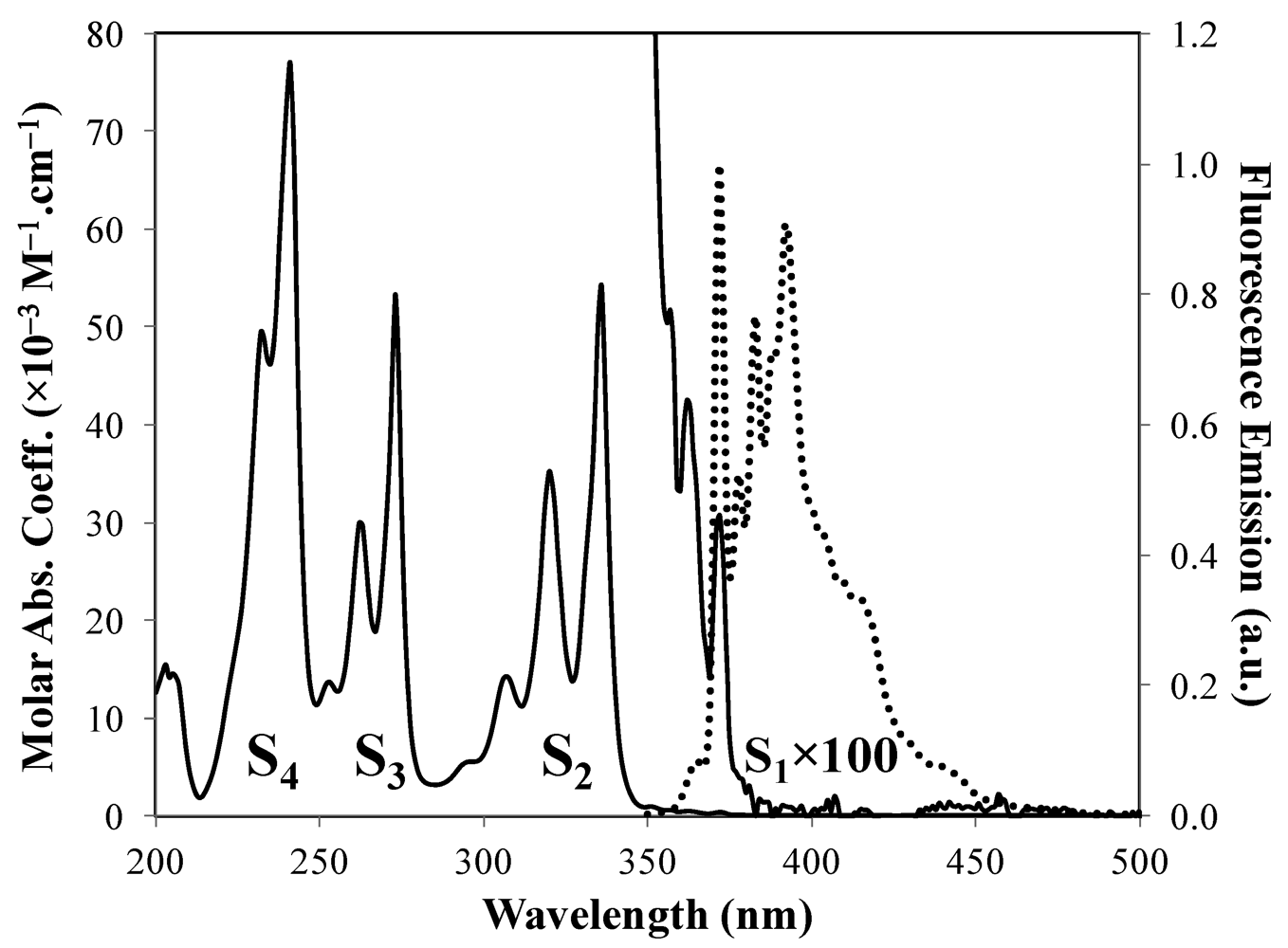
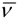 is the wavenumber, and IF is the fluorescence intensity. Based on Equation (6), krad is small for pyrene so that the lifetime of pyrene is large since τM−1 = krad + knrad. Indeed, pyrene and its derivatives have lifetimes of several hundreds of nanoseconds, much longer than any other aromatic fluorophore. The long lifetime of pyrene provides a long time window through which photophysical phenomena occurring over a few tens of picoseconds to several hundreds of nanoseconds can be probed, a dynamic range covering five orders of magnitude.
is the wavenumber, and IF is the fluorescence intensity. Based on Equation (6), krad is small for pyrene so that the lifetime of pyrene is large since τM−1 = krad + knrad. Indeed, pyrene and its derivatives have lifetimes of several hundreds of nanoseconds, much longer than any other aromatic fluorophore. The long lifetime of pyrene provides a long time window through which photophysical phenomena occurring over a few tens of picoseconds to several hundreds of nanoseconds can be probed, a dynamic range covering five orders of magnitude.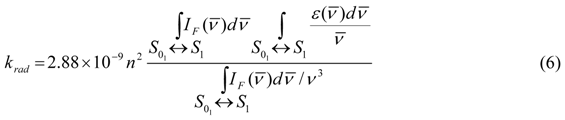
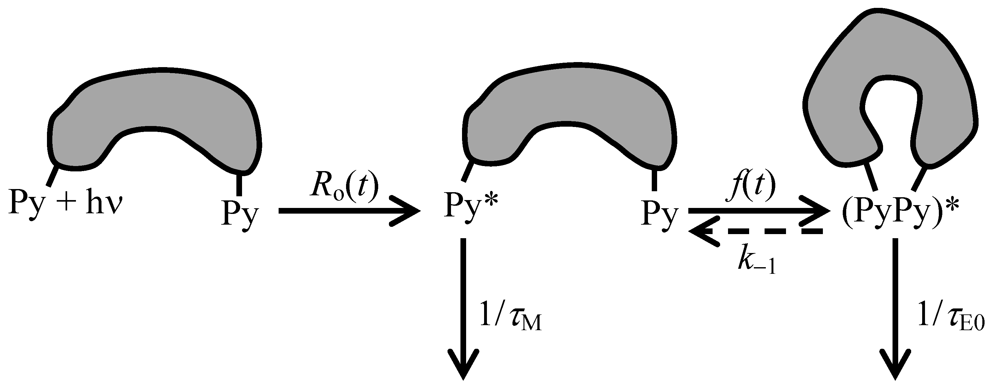
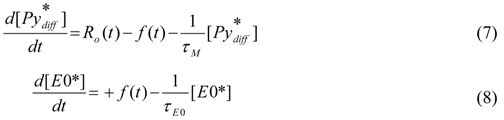
 in Equation (7) due to excimer formation results in an equivalent increase in [E0*] in Equation (7). Integration of Equations (7) and (8) yields the time-dependent concentrations of
in Equation (7) due to excimer formation results in an equivalent increase in [E0*] in Equation (7). Integration of Equations (7) and (8) yields the time-dependent concentrations of  (t) and [E0*](t). How this derivation is mathematically handled with the specific application to pyrene-labeled dendrimers is the topic of the next section.
(t) and [E0*](t). How this derivation is mathematically handled with the specific application to pyrene-labeled dendrimers is the topic of the next section.3. Analysis of the Kinetics of Excimer Formation for Pyrene-Labeled Dendrimers
 in Scheme 2 and in Equations (7) and (8)) [139]. In that case, the kinetics of excimer formation are simple enough and the dissociation rate constant can actually be recovered from a more detailed analysis than that proposed in Equations (7) and (8). Whereas
in Scheme 2 and in Equations (7) and (8)) [139]. In that case, the kinetics of excimer formation are simple enough and the dissociation rate constant can actually be recovered from a more detailed analysis than that proposed in Equations (7) and (8). Whereas  (t) would be found to decay monoexponentially if Equation (7) was integrated assuming a function Ro(t) = Ro × δ(t) (i.e., instantaneous excitation of pyrene), the complete treatment of the kinetics of excimer formation that includes the dissociation rate constant k−1 in Scheme 2 is described by J.B. Birks yielding a biexponential decay for
(t) would be found to decay monoexponentially if Equation (7) was integrated assuming a function Ro(t) = Ro × δ(t) (i.e., instantaneous excitation of pyrene), the complete treatment of the kinetics of excimer formation that includes the dissociation rate constant k−1 in Scheme 2 is described by J.B. Birks yielding a biexponential decay for  (t) [139]. The contribution to the overall decay of the exponential having the shorter decay time is usually rather small (less than 15% of the total pre-exponential weight) so that the decay of
(t) [139]. The contribution to the overall decay of the exponential having the shorter decay time is usually rather small (less than 15% of the total pre-exponential weight) so that the decay of  (t) can be considered to be essentially monoexponential as would be expected for a process where k−1 is small compared to τE−1 in Scheme 2 (i.e., the excimer fluoresces more quickly than it can dissociate).
(t) can be considered to be essentially monoexponential as would be expected for a process where k−1 is small compared to τE−1 in Scheme 2 (i.e., the excimer fluoresces more quickly than it can dissociate).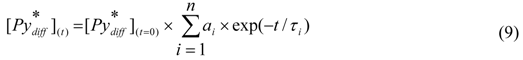
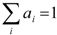 ). Since
). Since  (t) is known, its expression can be used in Equation (7) to determine f(t) which for times t > 0 is given by Equation (10).
(t) is known, its expression can be used in Equation (7) to determine f(t) which for times t > 0 is given by Equation (10).
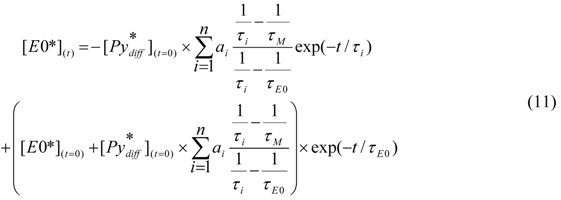
 (t) given in Equation (12) is added to Equation (9) to yield Equation (13).
(t) given in Equation (12) is added to Equation (9) to yield Equation (13).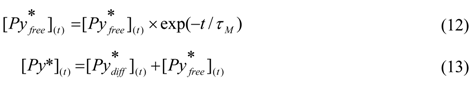


 (t), and [E0*](t) were obtained by making no assumption about the nature of f(t), this type of mathematical treatment used to fit the fuorescence decays has been referred to as the Model Free (MF) analysis [126,176,177].
(t), and [E0*](t) were obtained by making no assumption about the nature of f(t), this type of mathematical treatment used to fit the fuorescence decays has been referred to as the Model Free (MF) analysis [126,176,177]. in Equation (9) and
in Equation (9) and 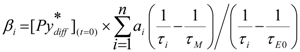 and
and 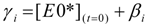 in Equation (11) are being handled because αi, βi, and γi are not treated as floating parameters in the optimization, but rather are fitted as a function of the decay times τi and τE0 and the pre-exponential factors ai. As it turns out, analyzing the fluorescence decays of the pyrene monomer and excimer in this manner retrieves the parameters ai, the decay times τi and τE0, and the molar fractions fdiff, ffree, fE0, and fD of the pyrene species
in Equation (11) are being handled because αi, βi, and γi are not treated as floating parameters in the optimization, but rather are fitted as a function of the decay times τi and τE0 and the pre-exponential factors ai. As it turns out, analyzing the fluorescence decays of the pyrene monomer and excimer in this manner retrieves the parameters ai, the decay times τi and τE0, and the molar fractions fdiff, ffree, fE0, and fD of the pyrene species  ,
,  , E0* and D* found in solution with improved accuracy [126,150,176,177] compared to more traditional analyses [74,108,160]. Furthermore, it also enables the calculation of the ratio
, E0* and D* found in solution with improved accuracy [126,150,176,177] compared to more traditional analyses [74,108,160]. Furthermore, it also enables the calculation of the ratio  given in Equation (16) which constitutes an absolute measure of the IE/IM ratio obtained by steady-state fluorescence.
given in Equation (16) which constitutes an absolute measure of the IE/IM ratio obtained by steady-state fluorescence.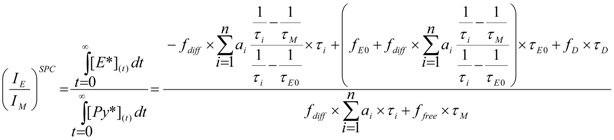 (16)
(16)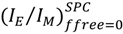 . In turn,
. In turn, 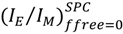 is expected to be proportional to the average rate constant of pyrene excimer formation <k> defined hereafter, as has been found in a number of studies [73,74,78,125,126,176]. A discrepancy between
is expected to be proportional to the average rate constant of pyrene excimer formation <k> defined hereafter, as has been found in a number of studies [73,74,78,125,126,176]. A discrepancy between 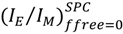 and <k> would imply that something is amiss in the analysis. Discrepancies between the ratios (IE/IM)SPC and (IE/IM)SS and <k> typically indicate that some free pyrene is present in solution that has not been accounted for.
and <k> would imply that something is amiss in the analysis. Discrepancies between the ratios (IE/IM)SPC and (IE/IM)SS and <k> typically indicate that some free pyrene is present in solution that has not been accounted for.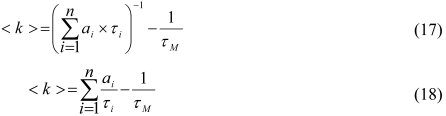
4. Internal Segmental Dynamics of Pyrene-Labeled Dendrimers
 Stewart, G.M., et al. [120] |  Cicchi, S., et al. [44] |
 Baker, L.A., et al. [127] |  Wang, B.-B., et al. [128] |
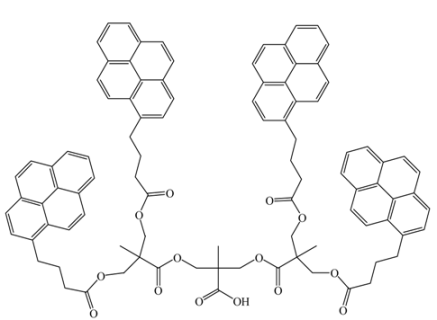 Yip, J., et al. [126] | 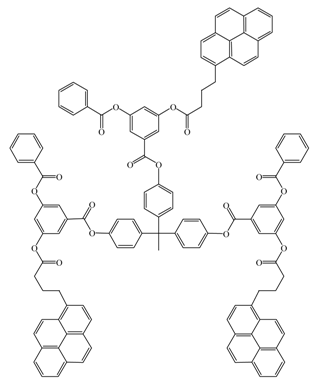 Wilken, R., et al. [134] |
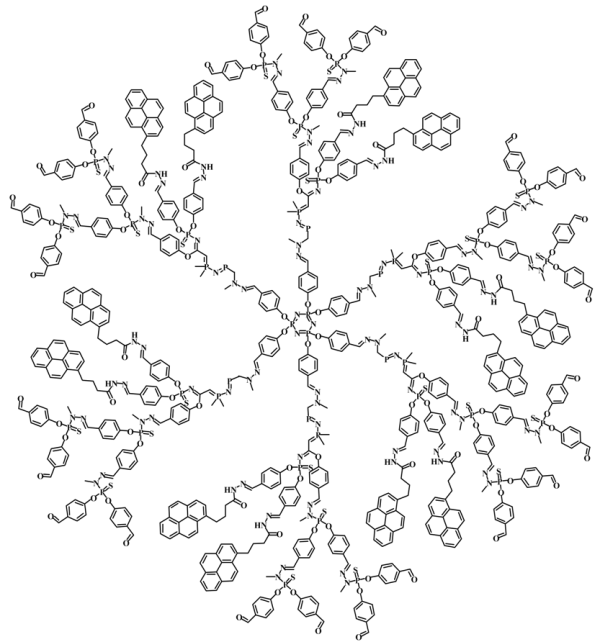 Brauge, L., et al. [130] | |

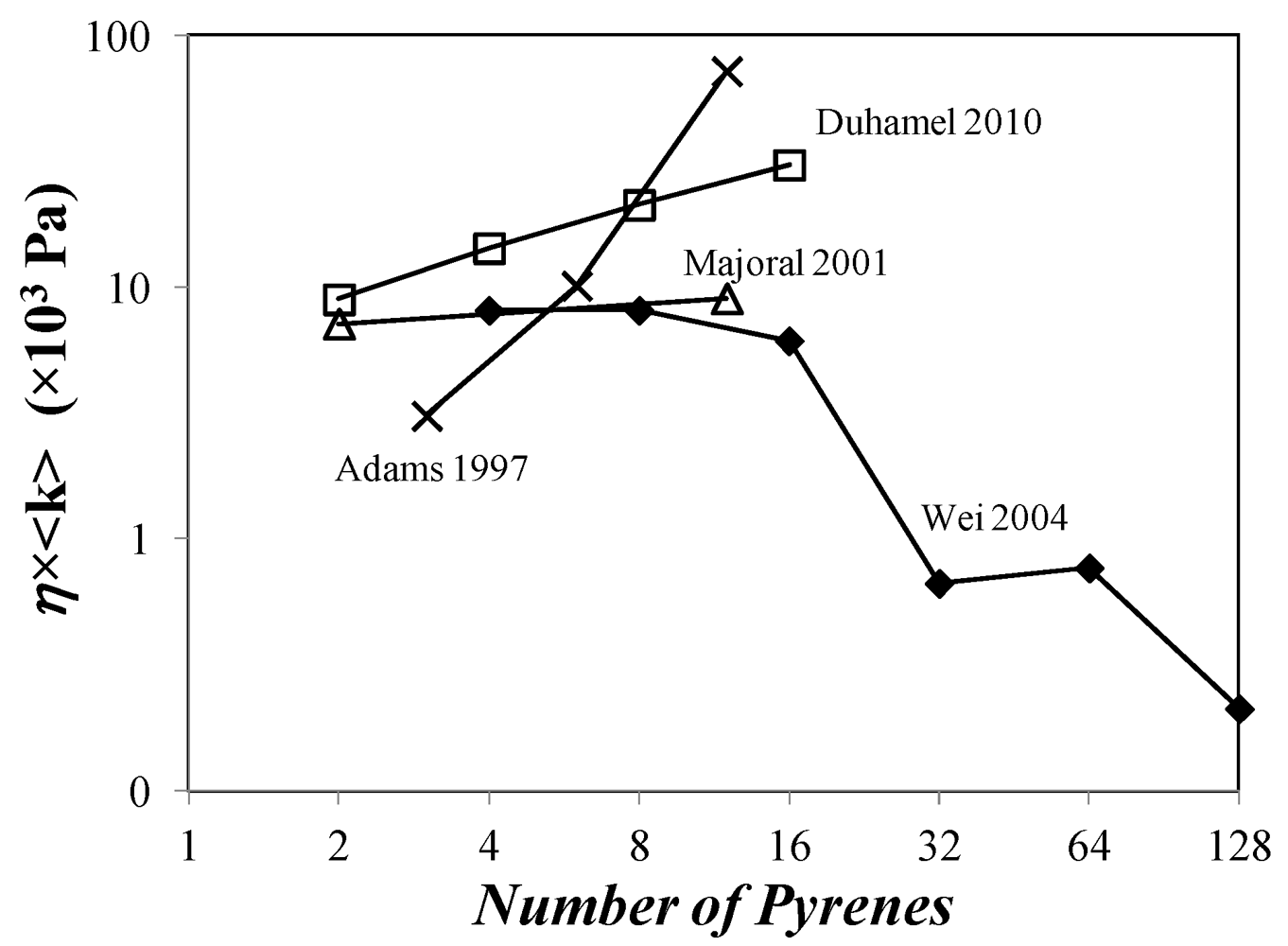
 versus <k> for Reference [126].
versus <k> for Reference [126].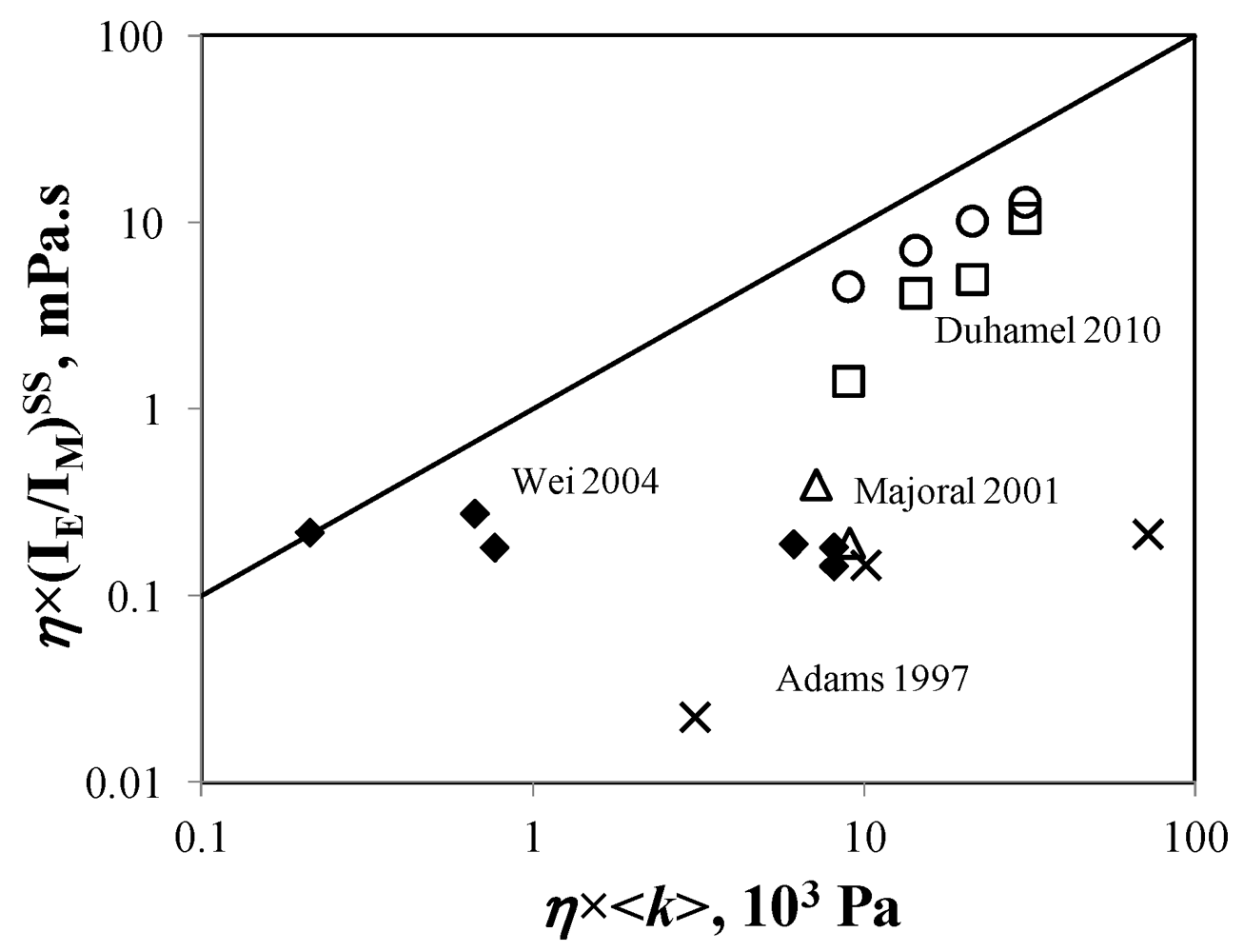
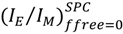 which brought the (IE/IM)SPC ratio back to its expected value. Purification of the 4th generation dendrimer by gel permeation chromatography demonstrated the presence of unattached 1-pyrenebutyric acid and the fluorescence spectrum acquired with the purified dendrimer free of unattached pyrene label yielded the expected (IE/IM)SS and (IE/IM)SPC ratios in agreement with the
which brought the (IE/IM)SPC ratio back to its expected value. Purification of the 4th generation dendrimer by gel permeation chromatography demonstrated the presence of unattached 1-pyrenebutyric acid and the fluorescence spectrum acquired with the purified dendrimer free of unattached pyrene label yielded the expected (IE/IM)SS and (IE/IM)SPC ratios in agreement with the 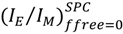 value.
value. 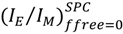 remained constant and equal to its original value. This study demonstrated the ability of the MF analysis to probe quantitatively the presence of minute amounts of unattached pyrene label and perhaps more importantly, to account for it by setting ffree to equal zero in Equation (16) [176]. Indeed plotting
remained constant and equal to its original value. This study demonstrated the ability of the MF analysis to probe quantitatively the presence of minute amounts of unattached pyrene label and perhaps more importantly, to account for it by setting ffree to equal zero in Equation (16) [176]. Indeed plotting 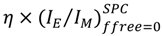 as a function of η × <k> in Figure 4 for the pyrene-labeled dendrimers characterized by Duhamel et al. [126] yielded a perfect straight line of slope unity (see hollow circles in Figure 4).
as a function of η × <k> in Figure 4 for the pyrene-labeled dendrimers characterized by Duhamel et al. [126] yielded a perfect straight line of slope unity (see hollow circles in Figure 4).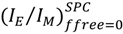 and <k> were found to increase linearly with increasing generation number. Considering that the mobility of the chain ends has been found to be slightly affected by increasing generation number, these trends led to the conclusion that the pyrene groups did not probe the entire volume of the dendrimer while being excited. Also <k> took very large values ranging between 10 and 70 × 107 s−1, much larger than those found for pyrene end-labeled polymers, reflecting the expected flexibility of the backbone of these dendrimers and their large local pyrene concentration.
and <k> were found to increase linearly with increasing generation number. Considering that the mobility of the chain ends has been found to be slightly affected by increasing generation number, these trends led to the conclusion that the pyrene groups did not probe the entire volume of the dendrimer while being excited. Also <k> took very large values ranging between 10 and 70 × 107 s−1, much larger than those found for pyrene end-labeled polymers, reflecting the expected flexibility of the backbone of these dendrimers and their large local pyrene concentration.5. Recommendations for Future Studies on the Internal Segmental Dynamics of Pyrene-Labeled Dendrimers and Conclusions
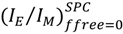 and <k> vary with increasing generation number beginning with dendrimers whose chain ends are fully labeled with pyrenyl pendants. The simpler synthetic protocols associated with the full labeling of the dendrimer terminals should make the preparation of such dendrimers more achievable. In each study, the fluorescence data must be shown to yield internally consistent trends, particularly with respect to
and <k> vary with increasing generation number beginning with dendrimers whose chain ends are fully labeled with pyrenyl pendants. The simpler synthetic protocols associated with the full labeling of the dendrimer terminals should make the preparation of such dendrimers more achievable. In each study, the fluorescence data must be shown to yield internally consistent trends, particularly with respect to 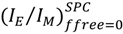 and <k> as was done in the study carried out by Duhamel et al. Only after such precautions have been taken will a clear consensus emerge on how pyrene excimer formation takes place in pyrene end-labeled dendrimers. In turn, the information retrieved about the long range motion of the chain ends of a dendrimer will prove valuable to predict the time scale over which the chain ends are expected to exchange location between the dendrimer interior and its surface.
and <k> as was done in the study carried out by Duhamel et al. Only after such precautions have been taken will a clear consensus emerge on how pyrene excimer formation takes place in pyrene end-labeled dendrimers. In turn, the information retrieved about the long range motion of the chain ends of a dendrimer will prove valuable to predict the time scale over which the chain ends are expected to exchange location between the dendrimer interior and its surface.References
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Maestri, M.; Saudan, C.; Vicinelli, V. Luminescent dendrimers. Recent advances. Top. Curr. Chem. 2003, 228, 159–191. [Google Scholar] [CrossRef]
- D’Ambruoso, G.D.; McGrath, D.V. Energy harvesting in synthetic dendrimer materials. Adv. Polym. Sci. 2008, 214, 87–147. [Google Scholar]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharma. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimer and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Helms, B.; Fréchet, J.M.J. The dendrimer effect in homogeneous catalysis. Adv. Synth. Catal. 2006, 348, 1125–1148. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Dendrimers and other dendritic macromolecules: From building blocks to functional assemblies in nanoscience and nanotechnology. J. Polym. Sci. A 2003, 41, 3713–3725. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Schreiner, C.D.; Moorefield, C.N.; Newkome, G.R. Recent progress and applications for metallodendrimers. New J. Chem. 2007, 31, 1192–1217. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 1978, 2, 155–158. [Google Scholar]
- Mansfield, M.L.; Klushin, L.I. Monte carlo studies of dendrimer molecules. Macromolecules 1993, 26, 4262–4268. [Google Scholar] [CrossRef]
- Murat, M.; Grest, G.S. Molecular dynamic study of dendrimer molecules in solvents of varying quality. Macromolecules 1996, 29, 1278–1285. [Google Scholar] [CrossRef]
- Scherrenberg, R.; Coussens, B.; Vliet, P.V.; Edouard, G.; Brackman, J.; Brabander, E.D.; Mortensen, K. The molecular characteristics of poly(propyleneimine) dendrimers as studied with small-angle neutron scattering, viscosimetry, and molecular dynamics. Macromolecules 1998, 31, 456–461. [Google Scholar]
- Cavallo, L.; Fraternali, F. A molecular dynamics study of the first five generations of poly(propylene imine) dendrimers modified with N-tBoc-L-phenylalinine. Chem. Eur. J. 1998, 4, 927–934. [Google Scholar] [CrossRef]
- Mansfield, M.L. Monte carlo studies of dendrimers. additional results for the diamond lattice model. Macromolecules 2000, 33, 8043–8049. [Google Scholar] [CrossRef]
- Mansfield, M.L.; Jeong, M. Simulation of lattice dendrimers by a monte carlo technique with detailed balance. Macromolecules 2002, 35, 9794–9798. [Google Scholar] [CrossRef]
- Giupponi, G.; Buzza, D.M.A. A monte carlo simulation scheme for nonideal dendrimers satisfying details balance. Macromolecules 2002, 35, 9799–9812. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Meijer, E.W. Molecular characteristics of poly(propylene imine) dendrimers as studied with translational diffusion and viscometry. Colloid Polym. Sci. 2002, 280, 416–423. [Google Scholar] [CrossRef]
- Rathgeber, S.; Monkenbush, M.; Kreitschmann, M.; Urban, V.; Brulet, A. Dynamics of star-burst dendrimers in solution in relation to their structural properties. J. Chem. Phys. 2002, 117, 4047–4062. [Google Scholar] [CrossRef]
- Rathgeber, S.; Pakula, T.; Urban, V. Structure of star-burst dendrimers: A comparison between small angle x-ray scattering and computer simulation results. J. Chem. Phys. 2004, 121, 3840–3853. [Google Scholar]
- De Gennes, P.G.; Hervet, H. Statistics of <<Starburst>> polymers. J. Phys. Lett. 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Lescanec, R.L.; Muthukumar, M. Configurational characteristics and scaling behavior of starburst molecules: A computational study. Macromolecules 1990, 23, 2280–2288. [Google Scholar] [CrossRef]
- Evmenenko, G.; Bauer, B.J.; Kleppinger, R.; Forier, B.; Dehaen, W.; Amis, E.J.; Mischenko, N.; Reynaers, H. The influence of molecular architecture and solvent type of the size and structure of poly(benzyl ether) dendrimers by SANS. Macromol. Chem. Phys. 2001, 202, 891–899. [Google Scholar]
- Rosenfeldt, S.; Dingenouts, N.; Ballauff, M.; Werner, N.; Vögtle, F.; Lindner, P. Distribution of end groups within a dendritic structure: A sans study including contrast variation. Macromolecules 2002, 35, 8098–8105. [Google Scholar]
- Mansfield, M.L. Dendron segregation in model dendrimers. Polymer 1994, 35, 1827–1830. [Google Scholar] [CrossRef]
- Boris, D.; Rubinstein, M. A self-consistent mean field model of a starburst dendrimer: Dense core vs. dense shell. Macromolecules 1996, 29, 7251–7260. [Google Scholar] [CrossRef]
- Lyulin, A.V.; Davies, G.R.; Adolf, D.B. Brownian dynamics simulation of dendrimers under shear flow. Macromolecules 2000, 33, 3294–3304. [Google Scholar] [CrossRef]
- Lyulin, A.V.; Davies, G.R.; Adolf, D.B. Location of terminal groups of dendrimers: Brownian dynamics simulation. Macromolecules 2000, 33, 6899–6900. [Google Scholar] [CrossRef]
- Karatasos, K.; Adolf, D.B.; Davies, G.R. Statistics and dynamics of model dendrimers as studied by molecular dynamics simulations. J. Chem. Phys. 2001, 115, 5310–5318. [Google Scholar]
- Zook, T.C.; Pickett, G.T. Hollow-core dendrimers revisited. Phys. Rev. Lett. 2003. [Google Scholar]
- Harreis, H.M.; Likos, C.N.; Ballauff, M. Can dendrimers be viewed as compact colloids? A simulation study of the fluctuations in a dendrimer of fourth generation. J. Chem. Phys. 2003, 118, 1979–1988. [Google Scholar] [CrossRef]
- Timoshenko, E.G.; Kuznetsov, Y.A.; Connolly, R. Conformations of dendrimers in dilute solutions. J. Chem. Phys. 2002, 117, 9050–9062. [Google Scholar] [CrossRef]
- Han, M.; Chen, P.; Yang, X. Molecular dynamics simulation of PAMAM dendrimer in aqueous solution. Polymer 2005, 46, 3481–3488. [Google Scholar] [CrossRef]
- Lee, I.; Athey, B.D.; Wetzel, A.W.; Meixner, W.; Baker, J.R. Structural molecular dynamics studies on polyamidoamine dendrimers for a therapeutic application: Effect of pH and generation. Macromolecules 2002, 35, 4510–4520. [Google Scholar]
- Ahn, T.-S.; Nantalaksakul, A.; Dasari, R.R.; Al-Kaysi, R.O.; Müller, A.M.; Thayumanavan, S.; Bardeen, C.J. Energy and charge transfer dynamics in fully decorated benzyl ether dendrimers and their disubstituted analogues. J. Phys. Chem. B 2006, 110, 24331–24339. [Google Scholar]
- Gu, T.; Whitesell, J.K.; Fox, M.A. Intramolecular charge transfer in 1:1 Cu(II)/pyrenylcyclam dendrimer complexes. J. Phys. Chem. B 2006, 110, 25149–25152. [Google Scholar]
- Li, Y.-Y.; Han, L.; Chen, J.; Zheng, S.; Zen, Y.; Li, Y.; Li, S.; Yang, G. Study on the extent of folding back conformation in poly(aryl ether) dendrimers by intramolecular electron transfer and exciplex formation. Macromolecules 2007, 40, 9384–9390. [Google Scholar]
- Denti, G.; Campagna, S.; Serroni, S.; Ciano, M.; Balzani, V. Decanuclear homo- and heterometallic polypyridine complexes: Syntheses, absorption spectra, luminescence, electrochemical oxidation, and intercomponent energy transfer. J. Am. Chem. Soc. 1992, 114, 2944–2950. [Google Scholar] [CrossRef]
- Gilat, S.L.; Adronov, A.; Fréchet, J.M.J. Light harvesting and energy transfer in novel convergently constructed dendrimers. Angew. Chem. Int. Ed. 1999, 38, 1422–1427. [Google Scholar] [CrossRef]
- Adronov, A.; Gilat, S.L.; Fréchet, J.M.J.; Ohta, K.; Neuwahl, F.V.R.; Fleming, G.R. Light harvesting and energy transfer in laser-dye-labeled poly(aryl ether) dendrimers. J. Am. Chem. Soc. 2000, 122, 1175–1185. [Google Scholar]
- Adronov, A.; Fréchet, J.M.J. Light-harvesting dendrimers. Chem. Commun. 2000. [Google Scholar]
- Chen, J.; Li, S.; Zhang, L.; Liu, B.; Han, Y.; Yang, G.; Li, Y. Light-harvesting and photoisomerization in benzophenone and norbornadiene-labeled poly(aryl ether) dendrimers via intramolecular triplet energy transfer. J. Am. Chem. Soc. 2005, 127, 2165–2171. [Google Scholar]
- Cicchi, S.; Fabbrizzi, P.; Ghini, G.; Brandi, A.; Foggi, P.; Marcelli, A.; Righini, R.; Botta, C. Pyrene-excimers-based antenna systems. Chem. Eur. J. 2009, 15, 754–764. [Google Scholar]
- Mourey, T.H.; Turner, S.R.; Rubinstein, M.; Fréchet, J.M.J.; Hawker, C.J.; Wooley, K.L. Unique behavior of dendritic macromolecules: Intrinsic viscosity of polyether dendrimers. Macromolecules 1992, 25, 2401–2406. [Google Scholar] [CrossRef]
- Gorman, C.B.; Hager, M.W.; Parkhurst, B.L.; Smith, J.C. Use of a paramagnetic core to affect longitudinal nuclear relaxation in dendrimers—A tool for probing dendrimer conformation. Macromolecules 1998, 31, 815–822. [Google Scholar]
- Wooley, K.L.; Klug, C.A.; Tasaki, K.; Schaefer, J. Shapes of dendrimers from rotational-echo double-resonance NMR. J. Am. Chem. Soc. 1997, 119, 53–58. [Google Scholar] [CrossRef]
- Malyarenko, D.I.; Vold, R.L.; Hoaston, G.L. Solid state deuteron NMR studies of polyamidoamine dendrimer salts. 2. Relaxation and molecular motion. Macromolecules 2000, 33, 7508–7520. [Google Scholar] [CrossRef]
- Chai, M.; Niu, Y.; Youngs, W.J.; Rinaldi, P.L. Structure and conformation of DAB dendrimers in solution via multidimensional NMR techniques. J. Am. Chem. Soc. 2001, 123, 4670–4678. [Google Scholar]
- Wind, M.; Saalwächter, K.; Wiesler, U.M.; Müllen, K.; Spiess, H.W. Solid-state NMR investigations of molecular dynamics in polyphenylene dendrimers: Evidence of dense-shell packing. Macromolecules 2002, 35, 10071–10086. [Google Scholar]
- Malveau, C.; Baille, W.E.; Zhu, X.X.; Ford, W.T. Molecular dynamics of hydrophilic poly(propylene imine) dendrimers in aqueous solutions by 1H BNR relaxation. J. Polym. Sci. B 2003, 41, 2969–2975. [Google Scholar] [CrossRef]
- Prosa, T.J.; Bauer, B.J.; Amis, E.J.; Tomalia, D.A.; Scherrenberg, R. A SAXS study of the internal structure of dendritic polymer systems. J. Polym. Sci. B 1997, 35, 2913–2924. [Google Scholar] [CrossRef]
- Topp, A.; Bauer, B.J.; Klimash, J.W.; Spindler, R.; Tomalia, D.A.; Amis, E.J. Probing the location of the terminal groups of dendrimers in dilute solution. Macromolecules 1999, 32, 7226–7231. [Google Scholar]
- Pötschke, D.; Ballauf, M.; Lindner, P.; Fischer, M.; Vögtle, F. Analysis of the structure of dendrimers in solution by small-angle neutron scattering including contrast variation. Macromolecules 1999, 32, 4079–4087. [Google Scholar] [CrossRef]
- Ballauff, M.; Likos, C.N. Dendrimers in solution: Insight from theory and simulation. Angew. Chem. Int. Ed. 2004, 43, 2998–3020. [Google Scholar] [CrossRef]
- Welch, P.; Muthukumar, M. Tuning the density profile of dendritic polyelectrolytes. Macromolecules 1998, 31, 5892–5897. [Google Scholar] [CrossRef]
- Lyulin, S.V.; Evers, L.J.; van der Schoot, P.; Darinskii, A.A.; Lyulin, A.V.; Michels, M.A.J. Effect of solvent quality and electrostatic interactions on size and structure of dendrimers. Brownian dynamics simulation and mean-field theory. Macromolecules 2004, 37, 3049–3063. [Google Scholar] [CrossRef]
- Bosman, A.W.; Bruining, M.J.; Kooijman, H.; Speck, A.; Janssen, R.A.J.; Meijer, E.W. Concerning the localization of end groups in dendrimers. J. Am. Chem. Soc. 1998, 120, 8547–8548. [Google Scholar]
- Meltzer, A.D.; Tirrell, D.A.; Jones, A.A.; Inglefield, P.T.; Hedstrand, D.M.; Tomalia, D.A. Chain dynamics in poly(amido amine) dendrimers. A study of 13C NMR relaxation parameters. Macromolecules 1992, 25, 4541–4548. [Google Scholar]
- Meltzer, A.D.; Tirrell, D.A.; Jones, A.A.; Inglefield, P.T. Chain dynamics in poly(amido amine) dendrimers. A study of 2H NMR relaxation parameters. Macromolecules 1992, 25, 4549–4552. [Google Scholar] [CrossRef]
- Rathgeber, S.; Monkenbusch, M.; Hedrick, J.L.; Trolsås, M.; Gast, A.P. Starlike dendrimers in solutions: Structural properties and internal dynamics. J. Chem. Phys. 2006. [Google Scholar]
- Stark, B.; Stühn, B.; Frey, H.; Lach, C.; Lorenz, K.; Frick, B. Segmental dynamics in dendrimers with perfluorinated end groups: A study using quasielastic neutron scattering. Macromolecules 1998, 31, 5415–5423. [Google Scholar] [CrossRef]
- Li, X.; Zamponi, M.; Hong, K.; Porcar, L.; Shew, C.-Y.; Jenkins, T.; Liu, E.; Smith, G.S.; Herwig, K.W.; Liu, Y.; Chen, W.-R. pH responsiveness of polyelectrolyte dendrimers: A dynamical perspective. Soft Matter 2011, 7, 618–622. [Google Scholar]
- Emran, S.K.; Newkome, G.R.; Weis, C.D.; Harmon, J.P. Molecular relaxations in ester-terminated, amine-based dendrimers. J. Polym. Sci. B 1999, 37, 2025–2038. [Google Scholar] [CrossRef]
- Mijović, J.; Ristić, S.; Kenny, J. Dynamics of six generations of PAMAM dendrimers as studied by dielectric relaxation spectroscopy. Macromolecules 2007, 40, 5212–5221. [Google Scholar] [CrossRef]
- Uppuluri, S.; Morrison, F.A.; Dvornic, P.R. Rheology of dendrimers. 2. Bulk polyamidoamine dendrimers under steady shear, creep, and dynamic oscillatory shear. Macromolecules 2000, 33, 2551–2560. [Google Scholar] [CrossRef]
- Aumanen, J.; Kesti, T.; Sundström, V.; Teobaldi, G.; Zerbetto, F.; Werner, N.; Richardt, G.; van Heyst, J.; Vögtle, F.; Korppi-Tommola, J. Internal dynamics and energy transfer in dansylated POPAM dendrimers and their eosin complexes. J. Phys. Chem. B 2010, 114, 1548–1558. [Google Scholar]
- Gorman, C.B.; Smith, J.C. Effect of repeat unit flexibility on dendrimer conformation as studied by atomistic molecular dynamics simulations. Polymer 2000, 41, 675–683. [Google Scholar] [CrossRef]
- Mazo, M.A.; Shamaev, M.Y.; Balabaev, N.K.; Darinskii, A.A.; Neelov, I.M. Conformational mobility of carbosilane dendrimer: Molecular dynamics simulation. Phys. Chem. Chem. Phys. 2004, 6, 1285–1289. [Google Scholar]
- Lyulin, S.V.; Darinskii, A.A.; Lyulin, A.V.; Michels, M.A.J. Computer simulation of the dynamics of neutral and charged dendrimers. Macromolecules 2004, 37, 4676–4685. [Google Scholar]
- Markelov, D.A.; Lyulin, S.V.; Gotlib, Y.Y.; Lyulin, A.V.; Matveev, V.V.; Lahderanta, E.; Darinski, A.A. Orientational mobility and relaxation spectra of dendrimers: Theory and computer simulation. J. Chem. Phys. 2009. [Google Scholar]
- Tuschl, T.; Gohlke, C.; Jovin, T.M.; Westhof, E.; Eckstein, F. A three-dimensional model for the hammerhead ribozyme based on fluorescence measurements. Science 1994, 266, 785–789. [Google Scholar]
- Cuniberti, C.; Perico, A. Intramolecular diffusion-controlled reactions and polymer dynamics. Prog. Polym. Sci. 1984, 10, 271–316. [Google Scholar] [CrossRef]
- Winnik, M.A. End-to-end cyclization of polymer chains. Acc. Chem. Res. 1985, 18, 73–79. [Google Scholar] [CrossRef]
- Duhamel, J. Polymer chain dynamics in solution probed with a fluorescence blob model. Acc. Chem. Res. 2006, 39, 953–960. [Google Scholar] [CrossRef]
- Winnik, F.M. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev. 1993, 93, 587–614. [Google Scholar]
- Tjipangandjara, K.F.; Somasundaran, P. Effects of charges in adsorbed polyacrylic acid conformation on alumina flocculation. Colloids Surf. 1991, 55, 245–255. [Google Scholar] [CrossRef]
- Cuniberti, C.; Perico, A. Intramolecular excimers and microbrownian motion of flexible polymer molecules in solution. Eur. Polym. J. 1977, 13, 369–374. [Google Scholar] [CrossRef]
- Winnik, M.A.; Redpath, T.; Richards, D.H. The dynamics of end-to-end cyclization in polystyrene probed by pyrene excimer formation. Macromolecules 1980, 13, 328–335. [Google Scholar] [CrossRef]
- Cheung, S.-T.; Winnik, M.A.; Redpath, A.E.C. cyclization dynamics of polymers, 5. The effect of solvent on end-to-end cyclization of poly(ethylene oxide) probed by intramolecular pyrene excimer formation. Macromol. Chem. Phys. 1982, 183, 1815–1824. [Google Scholar] [CrossRef]
- Svirskaya, P.; Danhelka, J.; Redpath, A.E.C.; Winnik, M.A. Cyclization dynamics of polymers: 7. Application of the pyrene excimer technique to the internal dynamics of poly(dimethylsiloxane) chains. Polymer 1983, 24, 319–322. [Google Scholar] [CrossRef]
- Redpath, A.E.C.; Winnik, M.A. Temperature dependence of excimer formation between pyrenes at the ends of a polymer in a good solvent. Cyclization dynamics of polymers. 9. J. Am. Chem. Soc. 1982, 104, 5604–5607. [Google Scholar]
- Winnik, M.A.; Redpath, A.E.C.; Paton, K.; Danhelka, J. Cyclization dynamics of polymers: 10 Synthesis, fractionation, and fluorescent spectroscopy of pyrene end-capped polystyrenes. Polymer 1984, 25, 91–99. [Google Scholar] [CrossRef]
- Boileau, S.; Méchin, F.; Martinho, J.M.G.; Winnik, M.A. End-to-end cyclization of a pyrene end-capped poly(bisphenol A-diethylene glycol carbonate). Macromolecules 1989, 22, 215–220. [Google Scholar]
- Ghiggino, K.P.; Snare, M.J.; Thistlethwaite, P.J. Cyclization dynamics in poly(ethylene oxide). chain length and temperature dependence. Eur. Polym. J. 1985, 21, 265–272. [Google Scholar] [CrossRef]
- Slomkowski, S.; Winnik, M.A. fluorescence quenching in pyrene/benzil end-labeled poly(tetramethylene oxide). Cyclization dynamics in polymers 19. Macromolecules 1986, 19, 500–501. [Google Scholar] [CrossRef]
- Xu, H.; Martinho, J.M.G.; Winnik, M.A.; Beinert, G. Transient effects in diffusion-controlled polymer cyclization. Makromol. Chem. 1989, 190, 1333–1343. [Google Scholar] [CrossRef]
- Farinha, J.P.S.; Martinho, J.M.G.; Xu, H.; Winnik, M.A.; Quirk, R.P. Influence of intrachain hydrogen bonding on the cyclization of a polystyrene chain. J. Polym. Sci. Part B 1994, 32, 1635–1642. [Google Scholar] [CrossRef]
- Martinho, J.M.G.; Reis e Sousa, A.T.; Winnik, M.A. Effect of temperature and solvent on the cyclization of a polystyrene chain. Macromolecules 1993, 26, 4484–4488. [Google Scholar] [CrossRef]
- Duhamel, J.; Khayakin, Y.; Hu, Y.Z.; Winnik, M.A.; Boileau, S.; Méchin, F. End-to-end cyclization of a pyrene end-capped poly(bisphenol AF-diethylene glycol carbonate) in solution. Eur. Polym. J. 1994, 30, 129–134. [Google Scholar] [CrossRef]
- Lee, S.; Winnik, M.A. Cyclization rates for two points in the interior of a polymer chain. Macromolecules 1997, 30, 2633–2641. [Google Scholar] [CrossRef]
- Lee, S.; Duhamel, J. Monitoring the hydrophobic interactions of internally pyrene-labeled poly(ethylene oxide)s in water by fluorescence spectroscopy. Macromolecules 1998, 31, 9193–9200. [Google Scholar] [CrossRef]
- Reis e Sousa, A.T.; Castanheira, E.M.S.; Fedorov, A.; Martinho, J.M.G. Polystyrene cyclization using pyrene excimer formation. Effect of geminate pairs in good solvents. J. Phys. Chem. A 1998, 102, 6406–6411. [Google Scholar]
- Picarra, S.; Gomes, P.T.; Martinho, J.M.G. Fluorescence study of the coil-globule transition of a poly(ε-caprolactone) chain labeled with pyrenes at both ends. Macromolecules 2000, 33, 3947–3950. [Google Scholar] [CrossRef]
- Farinha, J.P.S.; Picarra, S.; Miesel, K.; Martinho, J.M.G. Fluorescence study of the coil-globule transition of a PEO chain in toluene. J. Phys. Chem. B 2001, 105, 10536–10545. [Google Scholar]
- Kim, S.D.; Torkelson, J.M. Nanoscale confinement and temperature effects on associative polymers in thin films: Fluorescence study of a telechelic, pyrene-labeled poly(dimethylsiloxane). Macromolecules 2002, 35, 5943–5952. [Google Scholar]
- Gardinier, W.E.; Bright, F.V. Temperature-dependent tail-tail dynamics of pyrene-labeled poly(dimethylsiloxane) oligomers dissolved in ethyl acetate. J. Phys. Chem. B 2005, 109, 14824–14829. [Google Scholar] [CrossRef]
- Ingratta, M.; Hollinger, J.; Duhamel, J. A case for using randomly labeled polymers to study long-range polymer chain dynamics by fluorescence. J. Am. Chem. Soc. 2008, 130, 9420–9428. [Google Scholar]
- Costa, T.; Seixas de Melo, J.; Burrows, H.D. Fluorescence behavior of a pyrene-end-capped poly(ethylene oxide) in organic solvents and in dioxane-water mixtures. J. Phys. Chem. B 2009, 113, 618–626. [Google Scholar]
- Chen, S.; Duhamel, J.; Winnik, M.A. Probing end-to-end cyclization beyond willemski and fixmann. J. Phys. Chem. B 2011, 115, 3289–3302. [Google Scholar]
- Winnik, M.A.; Egan, L.S.; Tencer, M.; Croucher, M.D. Luminescence studies on sterically stabilized polymer colloid particles: Pyrene excimer formation. Polymer 1987, 28, 1553–1560. [Google Scholar] [CrossRef]
- Wang, F.W.; Lowry, R.E. Excimer fluorescence technique for study of polymer-segment mobility: Applications to pyrene-labelled poly(methyl methacrylate) and poly(methyl acrylate) in solution. Polymer 1985, 26, 1046–1052. [Google Scholar] [CrossRef]
- Mathew, A.; Siu, H.; Duhamel, J. A blob model to study chain folding by fluorescence. Macromolecules 1999, 32, 7100–7108. [Google Scholar] [CrossRef]
- Kanagalingam, S.; Ngan, C.F.; Duhamel, J. Effect of solvent quality on the level of association and encounter kinetics of hydrophobic pendants covalently attached onto a water-soluble polymer. Macromolecules 2002, 35, 8560–8570. [Google Scholar] [CrossRef]
- Kanagalingam, S.; Spartalis, J.; Cao, T.-C.; Duhamel, J. Scaling relations related to the kinetics of excimer formation between pyrene groups attached onto poly(N, N-dimethylacrylamide)s. Macromolecules 2002, 35, 8571–8577. [Google Scholar] [CrossRef]
- Irondi, K.; Zhang, M.; Duhamel, J. Study of the semidilute solutions of poly(N,N-dimethylacrylamide) by fluorescence and its implications to the kinetics of coil-to-globule transitions. J. Phys. Chem. B 2006, 110, 2628–2637. [Google Scholar]
- Ingratta, M.; Duhamel, J. Correlating pyrene excimer formation with polymer chain dynamics in solution. Possibilities and limitations. Macromolecules 2007, 40, 6647–6657. [Google Scholar] [CrossRef]
- Seixas de Melo, J.; Costa, T.; Francisco, A.; Macanita, A.L.; Gago, S.; Goncalves, I.S. Dynamics of short as compared with long poly(acrylic acid) chains hydrophobically modified with pyrene, as followed by fluorescence techniques. Phys. Chem. Chem. Phys. 2007, 9, 1370–1385. [Google Scholar]
- Picarra, S.; Relogio, P.; Afonso, C.A.M.; Martinho, J.M.G.; Farinha, J.P.S. Coil-globule transition of poly(dimethylacrylamide): Fluorescence and light scattering study. Macromolecules 2003, 36, 8119–8129. [Google Scholar] [CrossRef]
- Picarra, S.; Duhamel, J.; Fedorov, A.; Martinho, J.M.G. Coil-globule transition of pyrene-labeled polystyrene in cyclohexane: Determination of polymer chain radii by fluorescence. J. Phys. Chem. B 2004, 108, 12009–12015. [Google Scholar]
- Teertstra, S.J.; Lin, W.Y.; Gauthier, M.; Ingratta, M.; Duhamel, J. Comparison of the long range polymer chain dynamics of polystyrene and cis-polyisoprene using polymers randomly labeled with pyrene. Polymer 2009, 50, 5456–5466. [Google Scholar]
- Ingratta, M.; Duhamel, J. Effect of viscosity on long-range polymer chain dynamics in solution studied with a fluorescence blob model. Macromolecules 2009, 42, 1244–1251. [Google Scholar] [CrossRef]
- Ingratta, M.; Duhamel, J. Effect of time on the rate of long range polymer segmental intramolecular encounters. J. Phys. Chem. B 2009, 113, 2284–2292. [Google Scholar] [CrossRef]
- Ingratta, M.; Mathew, M.; Duhamel, J. How switching the substituent of a pyrene derivative from a methyl to a butyl affects the fluorescence response of polystyrene randomly labeled with pyrene. Can. J. Chem. 2010, 88, 217–227. [Google Scholar]
- Yip, J.; Duhamel, J.; Qiu, X.; Winnik, F.M. Fluorescence studies of a series of monodisperse telechelic α,ω-dipyrenyl poly(N-isopropylacrylamide)s in ethanol and in water. Can. J. Chem. 2011, 89, 163–172. [Google Scholar] [CrossRef]
- Yip, J.; Duhamel, J.; Qiu, X.P.; Winnik, F.M. Long-range polymer chain dynamics of pyrene-labelled poly(N-isopropylacrylamide)s studied by fluorescence. Macromolecules 2011, 44, 5363–5372. [Google Scholar]
- Modrakowski, C.; Camacho Flores, S.; Beinhoff, M.; Schlüter, A.D. Synthesis of pyrene containing building blocks for dendrimer synthesis. Synthesis 2001, 14, 2143–2155. [Google Scholar]
- Figueira-Duarte, T.M.; Simon, S.C.; Wagner, M.; Druzhinin, S.I.; Zachariasse, K.A.; Müllen, K. Polypyrene dendrimers. Angew. Chem. Int. Ed. 2008, 47, 10175–10178. [Google Scholar]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Stewart, G.M.; Fox, M.A. Fluorophore-labeled dendrons as light harvesting antennae. J. Am. Chem. Soc. 1996, 118, 4354–4360. [Google Scholar] [CrossRef]
- Giovanella, U.; Mróz, W.; Foggi, P.; Fabbrizzi, P.; Cicchi, S.; Botta, C. Multi-colour electroluminescence of dendronic antennae containing pyrenes as light harvesters. ChemPhysChem 2010, 11, 683–688. [Google Scholar] [CrossRef]
- Thomas, R.R.J.; Thompson, A.L.; Sivakumar, A.V.; Bardeen, C.J.; Thayumanavan, S. Energy and electron transfer in bifunctional non-conjugated dendrimers. J. Am. Chem. Soc. 2005, 127, 373–383. [Google Scholar]
- Vanjinathan, M.; Lin, H.-C.; Nasar, A.S. Synthesis, characterization and photophysical properties of DCM-based light-harvesting dendrimers. Macromol. Chem. Phys. 2011, 212, 849–859. [Google Scholar] [CrossRef]
- Gu, T.; Whitesell, J.K.; Fox, M.A. Intramolecular charge transfer in 1:1 Cu(II)/pyrenylcyclam dendrimer complexes. J. Phys. Chem. B 2006, 110, 25149–25152. [Google Scholar]
- Morales-Espinoza, E.G.; Lijanova, I.V.; Morales-Saavedra, O.G.; Torres-Zuñiga, V.; Hernandez-Ortega, S.; Martines-Garcia, M. Synthesis of porphyrin-dendrimers with a pyrene in the periphery and their cubic nonlinear optical properties. Molecules 2011, 16, 6950–6968. [Google Scholar]
- Yip, J.; Duhamel, J.; Bahun, G.J.; Adronov, A. A study of the branch ends of a series of pyrene-labeled dendrimers based on pyrene excimer formation. J. Phys. Chem. B 2010, 114, 10254–10265. [Google Scholar]
- Baker, L.A.; Crooks, R.M. Photophysical properties of pyrene-functionalized poly(propylene imine) dendrimers. Macromolecules 2000, 33, 9034–9039. [Google Scholar] [CrossRef]
- Wang, B.-B.; Zhang, X.; Jia, X.-R.; Li, Z.-C.; Ji, Y.; Yang, L.; Wei, Y. Fluorescence and aggregation behaviour of poly(amidoamine) dendrimers peripherally modified with aromatic fluorophores: The effect of dendritic architecture. J. Am. Chem. Soc. 2004, 126, 15180–15194. [Google Scholar]
- Wang, B.-B.; Zhang, X.; Yang, L.; Jia, X.-R.; Ji, Y.; Li, W.-S.; Wei, Y. Poly(amisoamine) dendrimers bearing electron-donating fluorophores: Fluorescence and electrochemical properties. Polym. Bull. 2006, 56, 63–74. [Google Scholar] [CrossRef]
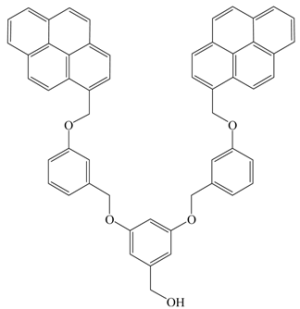
- Brauge, L.; Caminade, A.-M.; Majoral, J.-P.; Slomkowski, S.; Wolszczak, M. Segmental mobility in phosphorus-containing dendrimers. studies by fluorescent spectroscopy. Macromolecules 2001, 34, 5599–5606. [Google Scholar]
- Brauge, L.; Vériot, G.; Franc, G.; Deloncle, R.; Caminade, A.-M.; Majoral, J.-P. Synthesis of phosphorus dendrimers bearing chromophoric end groups: Toward organic blue light-emitting diodes. Tetrahedron 2006, 62, 11891–11899. [Google Scholar]
- Caminade, A.-M.; Hameau, A.; Turrin, C.-O.; Ianchuk, M.; Delavaux-Nicot, B.; Majoral, J.-P. fluorescent phosphorus dendrimers and their role in supramolecular interactions. Phosphorus Sulfur 2011, 186, 860–868. [Google Scholar] [CrossRef]
- Lekha, P.K.; Prasad, E. Tunable emission of static excimer in a pyrene-modified polyamidoamine dendrimer aggregate through positive solvatochromism. Chem. Eur. J. 2011, 17, 8609–8617. [Google Scholar] [CrossRef]
- Wilken, R.; Adams, J. End-group dynamics of fluorescently labelled dendrimers. Macromol. Rapid. Commun. 1997, 18, 659–665. [Google Scholar] [CrossRef]
- Sharma, A.; Schulman, S.G. Introduction to Fluorescence Spectroscopy; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Valeur, B. Molecular Fluorescence. Principles and Applications; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Springer: New York, NY, USA, 2006. [Google Scholar]
- Karstens, T.; Kobs, K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 1980, 84, 1871–1872. [Google Scholar] [CrossRef]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: New York, NY, USA, 1970; p. 301. [Google Scholar]
- Nakajima, A. Fluorescence spectra of pyrene in chlorinated aromatic solvents. J. Lumin. 1976, 11, 429–432. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Lianos, P.; Georghiou, S. Solute-solvent interaction and its effect on the vibronic and vibrational structure of pyrene spectra. Photochem. Photobiol. 1979, 30, 355–362. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py scale of solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorescence and empirical correlations with ET and Y values. Photochem. Photobiol. 1982, 35, 17–21. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py scale of solvent polarities. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
- Jones, A.; Dickson, T.J.; Wilson, B.E.; Duhamel, J. Fluorescence properties of poly(ethylene terephthalate-co-2,6-naphthalene dicarboxylate) with naphthalene contents ranging from 0.01 to 100 mole%. Macromolecules 1999, 32, 2956–2961. [Google Scholar]
- Genest, D.; Wahl, P. Time-Resolved Fluorescence Spectroscopy in Biochemistry and Biology; Cundall, R., Ed.; Springer: Berlin, Germany, 1980; pp. 523–539. [Google Scholar]
- Strickler, S.J.; Berg, R.A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Stevens, B.; Ban, M.I. Spectrophotometric determination of enthalpies and entropies of photoassociation for dissolved aromatic hydrocarbons. Trans. Far. Soc. 1964, 60, 1515–1523. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Duveneck, G.; Busse, R. Intramolecular excimer formation with 1,3-Di(1-pyrenyl)propane. Decay parameters and influence of viscosity. J. Am. Chem. Soc. 1984, 106, 1045–1051. [Google Scholar]
- Siu, H.; Duhamel, J. Comparison of the association level of a hydrophobically modified associative polymer obtained from an analysis based on two different models. J. Phys. Chem. B 2005, 109, 1770–1780. [Google Scholar]
- Zachariasse, K.; Kühnle, W. Intramolecular excimers with α,ω-diarylalkanes. Z. Phys. Chem. Neue Fol. 1976, 101, 267–276. [Google Scholar] [CrossRef]
- Kanaya, T.; Goshiki, K.; Yamamoto, M.; Nishijima, Y. Intramolecular end-to-end excimer formation of bis((1-pyrenylmethoxy)carbonyl)alkanes. A study of end-to-end collisional frequency on a chain molecule. J. Am. Chem. Soc. 1982, 104, 3580–3587. [Google Scholar]
- Eaton, D.F.; Smart, B.E. Are fluorocarbon chains “stiffer” than hydrocarbon chains? dynamics of end-to-end cyclization in a C8F16 segment monitored by fluorescence. J. Am. Chem. Soc. 1990, 112, 2821–2823. [Google Scholar] [CrossRef]
- Reynders, P.; Kühnle, W.; Zachariasse, K.A. Ground-state dimers in excimer-forming bichromophoric molecules. 1. Bis(pyrenylcarboxy)alkanes. J. Am. Chem. Soc. 1990, 112, 3929–3939. [Google Scholar]
- Snare, M.J.; Thistlethwaite, P.J.; Ghiggino, K.P. Kinetic studies of intramolecular excimer formation in dipyrenylalkanes. J. Am. Chem. Soc. 1983, 105, 3328–3332. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Duveneck, G.; Busse, R. Intramolecular excimer formation with 1,3-Di(1-pyrenyl)propane. Decay parameters and influence of viscosity. J. Am. Chem. Soc. 1984, 106, 1045–1051. [Google Scholar] [CrossRef]
- Reynders, P.; Kühnle, W.; Zachariasse, K.A. Ground-state dimers in excimer-forming bichromophoric molecules. NMR and single-photon-counting data. 2. Racemic and meso dipyrenylpentanes and dipyrenylalkanes. J. Phys. Chem. 1990, 94, 4073–4082. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Maçanita, A.L.; Kühnle, W. chain length dependence of intramolecular excimer formation with 1, n-bis(1-pyrenylcarboxy)alkanes for n = 1 – 16, 22, and 32. J. Phys. Chem. B 1999, 103, 9356–9365. [Google Scholar]
- Pandey, S.; Kane, M.A.; Baker, G.A.; Bright, F.V.; Fürstner, A.; Seidel, G.; Leitner, W. The photophysics of 6-(1-pyrenyl)hexyl-1,11(1-pyrenyl)undecanoate dissolved in organic liquids and supercritical carbon dioxide: Impact on olefin metathesis. J. Phys. Chem. B 2002, 106, 1820–1832. [Google Scholar]
- Maçanita, A.L.; Zachariasse, K.A. Viscosity dependence of intramolecular excimer formation with 1,5-bis(1-pyrenylcarboxy)pentane in alkane solvents as a function of temperature. J. Phys. Chem. A 2011, 115, 3183–3195. [Google Scholar]
- Eaton, W.A.; Muñoz, V.; Hagen, S.J.; Jas, G.S.; Lapidus, L.J.; Henry, E.R.; Hofrichter, J. Fast kinetics and mechanisms in protein folding. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 327–359. [Google Scholar] [CrossRef]
- Hagen, S.J.; Hofrichter, J.; Szabo, A.; Eaton, W.A. Diffusion-limited contact formation in unfolded cytochrome C: Estimating the maximum rate of protein folding. Proc. Natl. Acad. Sci. 1996, 93, 11615–11617. [Google Scholar]
- McGimpsey, W.G.; Chen, L.; Carraway, R.; Samaniego, W.N. Singlet-singlet and triplet-triplet energy transfer in bichromophoric peptides. J. Phys. Chem. A 1999, 103, 6082–6090. [Google Scholar] [CrossRef]
- Möglich, A.; Krieger, F.; Kiefhaber, T. Molecular basis for the effect of urea and guanidinium chloride on the dynamics of unfolded polypeptide chains. J. Mol. Biol. 2005, 345, 153–162. [Google Scholar] [CrossRef]
- Fierz, B.; Satzger, H.; Root, C.; Gich, P.; Zinth, W.; Kiefhaber, T. Loop formation in unfolded polypeptide chains on the picoseconds to microseconds time scale. Proc. Natl. Acad. Sci. USA 2007, 104, 2163–2168. [Google Scholar]
- Möglich, A.; Joder, K.; Kiefhaber, T. End-to-end distance distributions and intrachain diffusion constants in unfolded polypeptide chains indicate intramolecular hydrogen bond formation. Proc. Natl. Acad. Sci. USA 2006, 103, 12394–12399. [Google Scholar]
- Hudgins, R.R.; Huang, F.; Gramlich, G.; Nau, W.M. A fluorescence-based method for direct measurement of submicrosecond intramolecular contact formation in biopolymers: An exploratory study with polypeptides. J. Am. Chem. Soc. 2002, 124, 556–564. [Google Scholar]
- Huang, F.; Hudgins, R.R.; Nau, W.M. Primary and secondary structure dependence of peptide flexibility assessed by fluorescence-based measurement of end-to-end collision rates. J. Am. Chem. Soc. 2004, 126, 16665–16675. [Google Scholar]
- Roccatano, D.; Sahoo, H.; Zacharias, M.; Nau, W.M. Temperature dependence of looping rates in a short peptide. J. Phys. Chem. B 2007, 111, 2639–2646. [Google Scholar]
- Bieri, O.; Wirz, J.; Hellrung, B.; Schutkowski, M.; Drewello, M.; Kiefhaber, T. The speed limit for protein folding measured by triplet–triplet energy transfer. Proc. Natl. Acad. Sci. USA 1999, 96, 9597–9601. [Google Scholar]
- Krieger, F.; Fierz, B.; Bieri, O.; Drewello, M.; Kiefhaber, T. Dynamics of unfolded polypeptide chains as model for the earliest steps in protein folding. J. Mol. Biol. 2003, 332, 265–274. [Google Scholar] [CrossRef]
- Lapidus, L.J.; Eaton, W.A.; Hofrichter, J. Measuring the rate of intramolecular contact formation in polypeptides. Proc. Natl. Acad. Sci. USA 2000, 97, 7220–7225. [Google Scholar]
- Neuweiler, H.; Löllmann, M.; Doose, S.; Sauer, M. Dynamics of unfolded polypeptide chains in crowded environment studied by fluorescence correlation spectroscopy. J. Mol. Biol. 2007, 365, 856–869. [Google Scholar] [CrossRef]
- Wilemski, G.; Fixman, M. Diffusion-controlled intrachain reactions of polymers. I theory. J. Chem. Phys. 1974, 60, 866–877. [Google Scholar] [CrossRef]
- Wilemski, G.; Fixman, M. Diffusion-controlled intrachain reactions of polymers. II results for a pair of terminal reactive groups. J. Chem. Phys. 1974, 60, 878–890. [Google Scholar] [CrossRef]
- Chen, S.; Duhamel, J.; Bahun, G.; Adronov, A. Effect of fluorescent impurities in the study of pyrene-labeled macromolecules by fluorescence. J. Phys. Chem. B 2011, 115, 9921–9929. [Google Scholar]
- Keyes-Baig, C.; Duhamel, J.; Wettig, S. Characterization of the behavior of a pyrene substituted gemini surfactant in water by fluorescence. Langmuir 2011, 27, 3361–3371. [Google Scholar]
- Andriessen, R.; Boens, N.; Ameloot, M.; De Schryver, F.C. Non a priori analysis of fluorescence decay surfaces of excited-state processes. 2. Intermolecular excimer formation of pyrene. J. Phys. Chem. 1991, 95, 2047–2058. [Google Scholar]
- Andriessen, R.; Ameloot, M.; Boens, N.; de Schryver, F.C. Non a priori analysis of fluorescence decay surfaces of excited-state processes. 3. Intermolecular excimer formation of pyrene quenched by iodomethane. J. Phys. Chem. 1992, 96, 314–326. [Google Scholar]
- Boens, N.; Ameloot, M. Compartmental modeling and identifiability analysis in photophysics: Review. Int. J. Quantum Chem. 2006, 106, 300–315. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duhamel, J. Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation. Polymers 2012, 4, 211-239. https://doi.org/10.3390/polym4010211
Duhamel J. Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation. Polymers. 2012; 4(1):211-239. https://doi.org/10.3390/polym4010211
Chicago/Turabian StyleDuhamel, Jean. 2012. "Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation" Polymers 4, no. 1: 211-239. https://doi.org/10.3390/polym4010211
APA StyleDuhamel, J. (2012). Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation. Polymers, 4(1), 211-239. https://doi.org/10.3390/polym4010211