Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals
Abstract
:1. Introduction
| Biological Analogues | In vitro Solutions | In vivo Solutions |
|---|---|---|
| Connective tissues in mammals [2] Dermal scutes of fish [3] Exoskeleton of insects and crustaceans [2,3] Membranes of animal eggs [2,4] Plant cell walls [2] | Collagen [4] Cellulose [4,19,11] Chitin [4] DNA [12,13] Viral suspensions [16] Actin [17] Flagella of Salmonella typhimurium [17] Mucin [18] | DNA [3,13] Chromosomes of dinoflagellate and bacteria [14] Collagen in egg shell and glands of dogfish [5] Oothecal gland protein of S. tenuidentata [6] Spider silk [7] Sickle cell hemoglobin [8] Synovial fluid [9] |
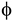 ), i.e.,
), i.e., 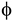 Leff/Deff, exceed a critical value (usually close to 4) at which excluded volume is minimized [31]. For semi-flexible chain BLCPs, like cellulose fibrils, Leff is the persistence length of the polymer chain and Deff is effective diameter that takes into account deviations from uncharged rigid-rods (due to dispersion forces, hydration interactions, and surface charges) [1]. The persistence length of cellulose chains obtained from various cellulose-solvent systems in vitro has been reported in the range of 11–25 nm [32]. It is erroneous to arrive at a conclusion that there is not enough material to form a LC phase during development of helicoidal plywoods in plant cell walls solely based on this calculation for the following reasons: (a) These calculations use absolute diameter instead of Deff of the CMFs, due to the lack of in vivo experiments in plant cell wall; (b) it has been frequently observed that the isotropic-nematic transition predicted by the Onsager theory may be an order-of-magnitude larger than that observed experimentally, perhaps due to other factors such as molecular interactions. Theory and simulations based on well established liquid crystal theory can be used as a tool to resolve these uncertainties arising due to the lack of in vivo experimental evidence and/or data. This is evident from the recent review [33] that emphasizes the Landau-de Gennes theory that yields testable and verifiable predictions of thermodynamical, textural, and rheological phenomena observed in BLCPs. For an extensive review of mesoscopic models employed to quantitatively describe the biological liquid crystalline phases and processes, refer to [33].
Leff/Deff, exceed a critical value (usually close to 4) at which excluded volume is minimized [31]. For semi-flexible chain BLCPs, like cellulose fibrils, Leff is the persistence length of the polymer chain and Deff is effective diameter that takes into account deviations from uncharged rigid-rods (due to dispersion forces, hydration interactions, and surface charges) [1]. The persistence length of cellulose chains obtained from various cellulose-solvent systems in vitro has been reported in the range of 11–25 nm [32]. It is erroneous to arrive at a conclusion that there is not enough material to form a LC phase during development of helicoidal plywoods in plant cell walls solely based on this calculation for the following reasons: (a) These calculations use absolute diameter instead of Deff of the CMFs, due to the lack of in vivo experiments in plant cell wall; (b) it has been frequently observed that the isotropic-nematic transition predicted by the Onsager theory may be an order-of-magnitude larger than that observed experimentally, perhaps due to other factors such as molecular interactions. Theory and simulations based on well established liquid crystal theory can be used as a tool to resolve these uncertainties arising due to the lack of in vivo experimental evidence and/or data. This is evident from the recent review [33] that emphasizes the Landau-de Gennes theory that yields testable and verifiable predictions of thermodynamical, textural, and rheological phenomena observed in BLCPs. For an extensive review of mesoscopic models employed to quantitatively describe the biological liquid crystalline phases and processes, refer to [33].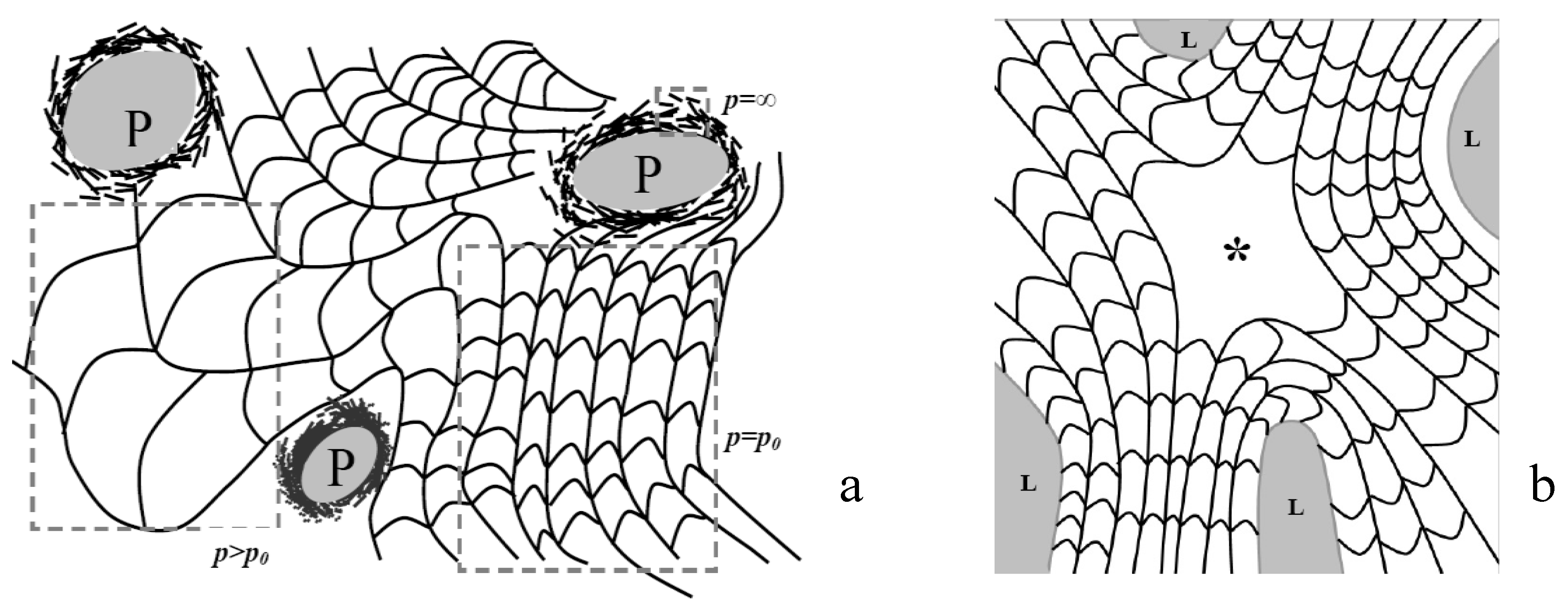
2. Theory and Governing Equations
2.1. Description of Long-Range Orientational Order
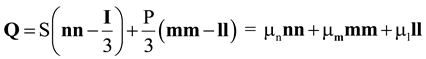
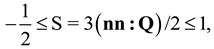
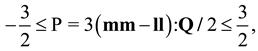
2.2. Landau- de Gennes Theory for Liquid Crystalline Materials
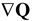 ). In the absence of an external field, the total free energy density of the mesophase (f) can be given in the following dimensionless form [15,33,34,46]:
). In the absence of an external field, the total free energy density of the mesophase (f) can be given in the following dimensionless form [15,33,34,46]:
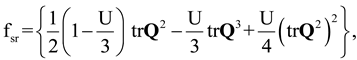
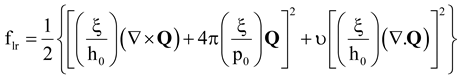
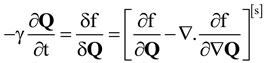
2.3. Characterization Methods: Lubensky- de Gennes Coarse Grained Elastic Theory and Chiral Order Parameter
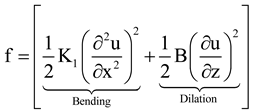
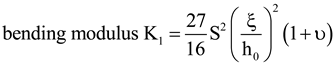
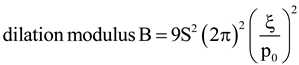
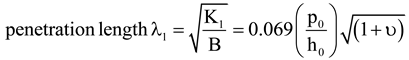
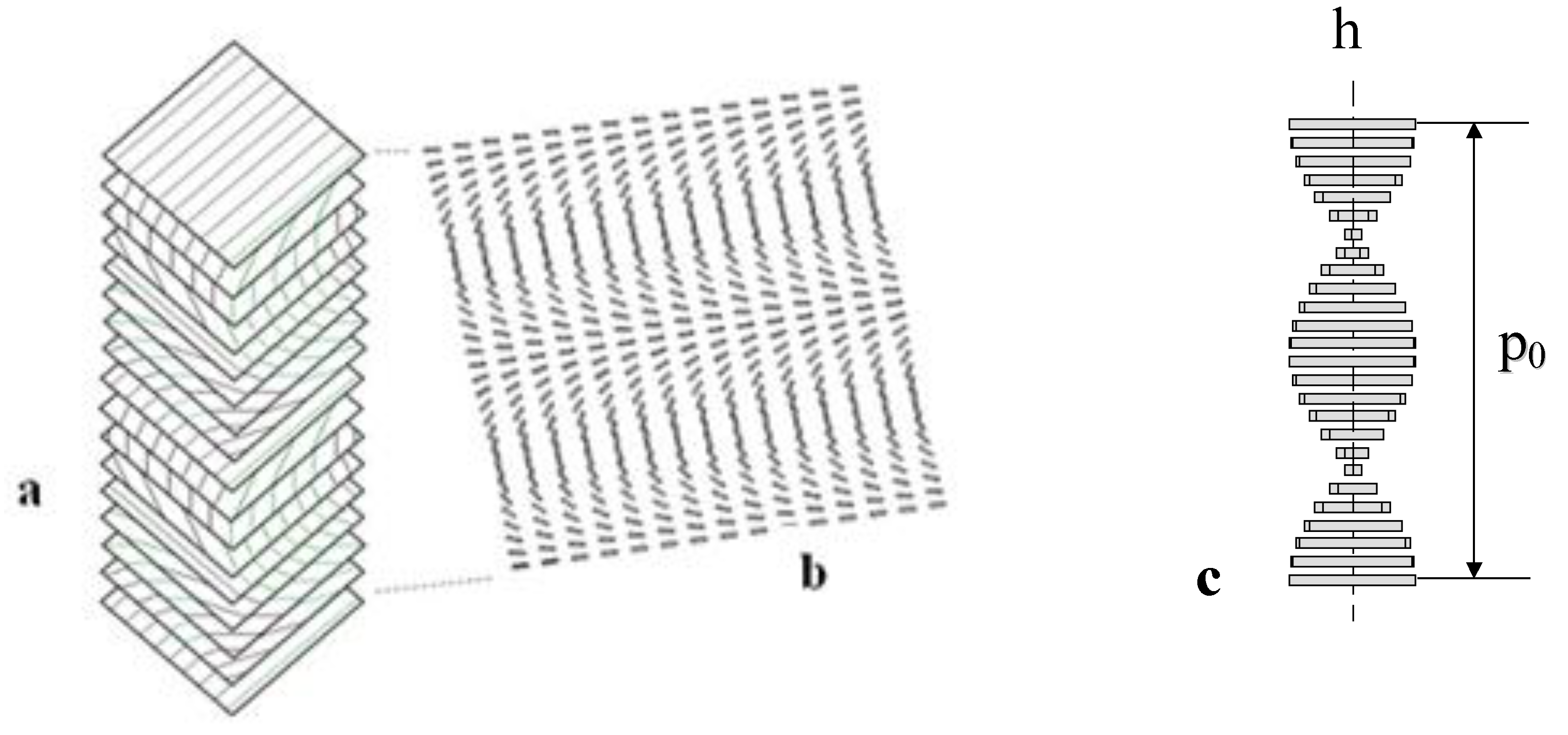
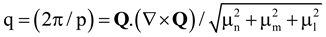 , where p is the modified pitch due to layer dilation. Hence by tracking q(x), dislocation defects associated with q changes are identified.
, where p is the modified pitch due to layer dilation. Hence by tracking q(x), dislocation defects associated with q changes are identified. 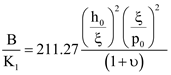
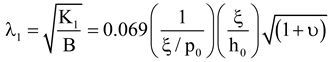
3. Computational Modeling
3.1. Computational Domain
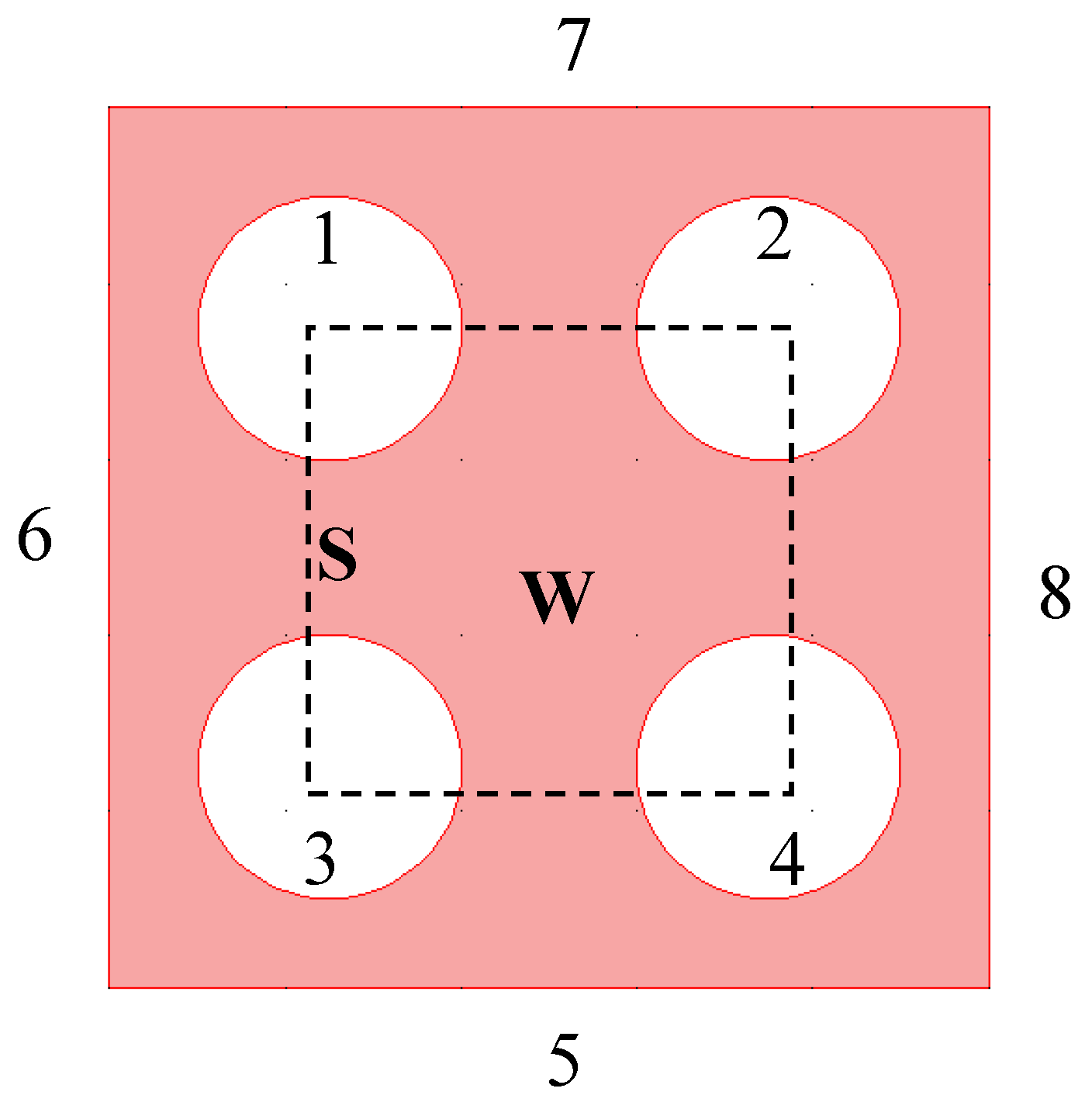
3.2. Boundary and Initial Conditions
3.3. Elastic Constants for BLCPs
- (a)
- internal/external length scale ratio (ξ/h0)
- (b)
- internal/pitch length scale ratio (ξ/p0)
- (c)
- elastic anisotropy (υ).
3.4. Classification of Defects in Chiral Nematic LCs
4. Results and Discussion
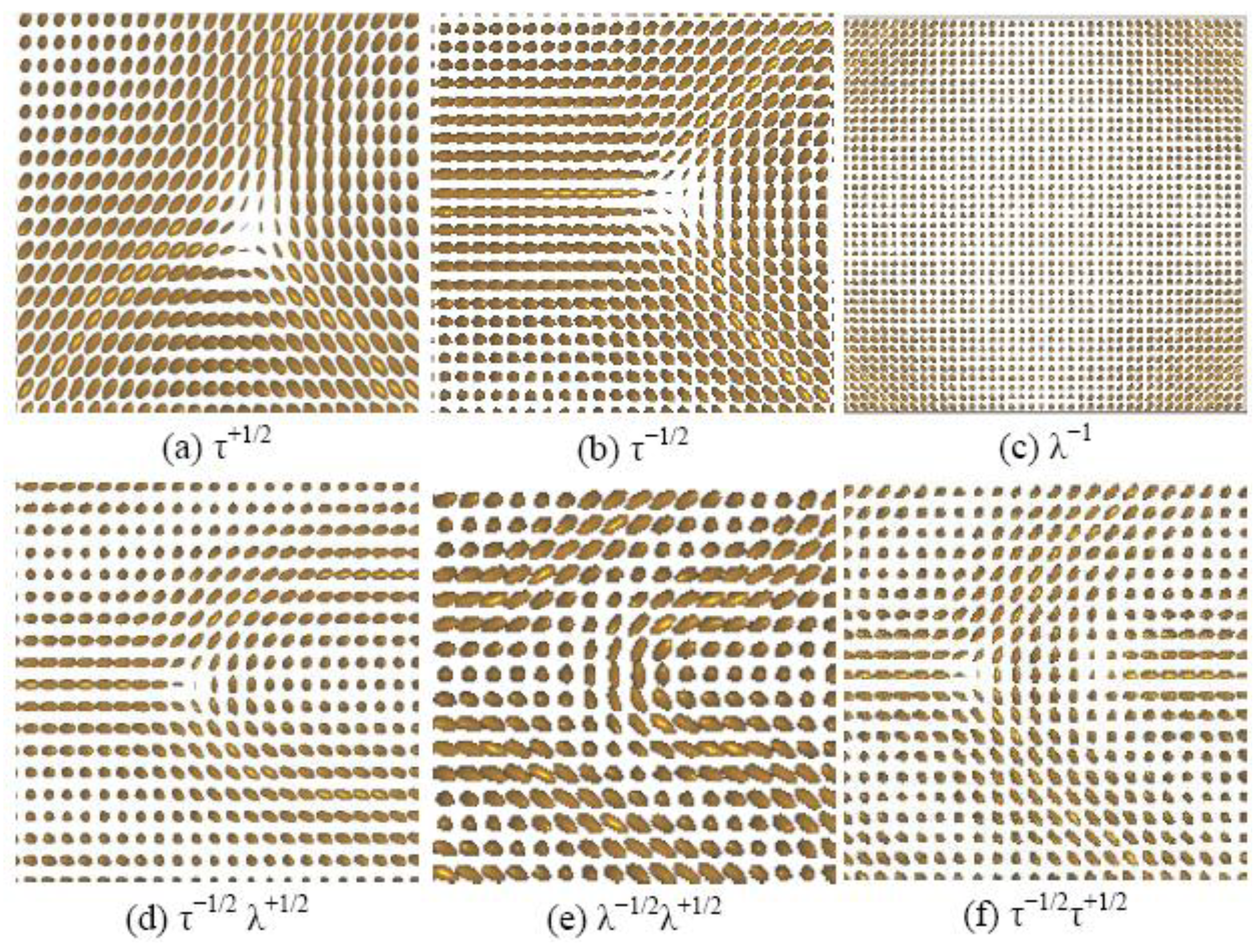
4.1. Defect Textures and Charge Balance
| Type of defect | υ = 1 | υ = 5 | υ = 21 |
|---|---|---|---|
| τ+1/2 | 5 | Absent | Absent |
| τ−1/2 | 7 | Absent | Absent |
| λ−1 | Absent | 1 | 1 |
| τ−1/2λ+1/2 | 8 | 4 | Absent |
| λ−1/2λ+1/2 | Absent | 8 | Absent |
| τ−1/2τ+1/2 | 5 | Absent | Absent |
| Total number of defects | 25 | 13 | 1 |
| Net Charge | −1 | −1 | −1 |
4.2. Effect of Confinement on the Type of Dislocations Nucleated
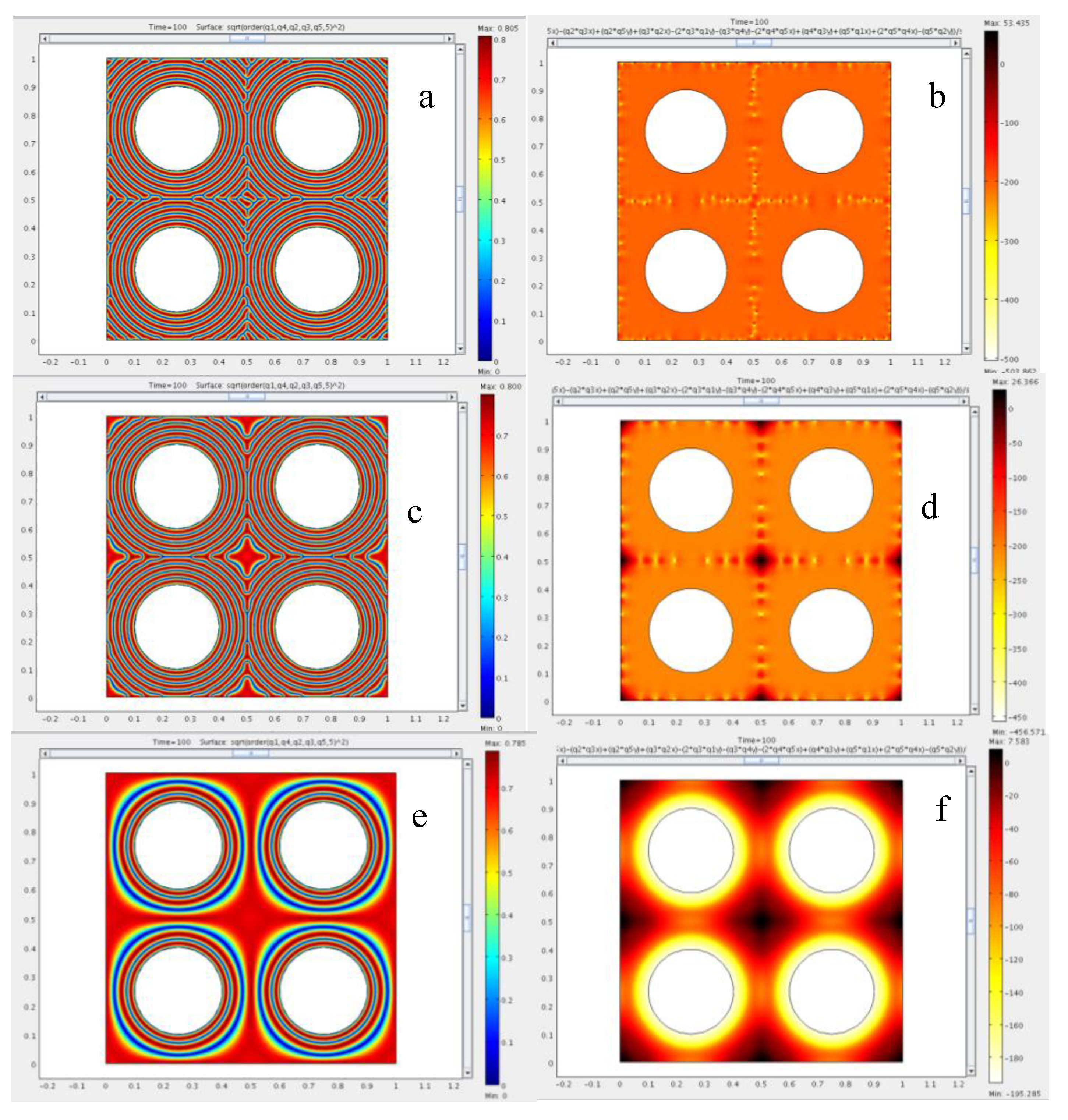
4.3. The Role of Bending and Dilation Energies on Defect Texture
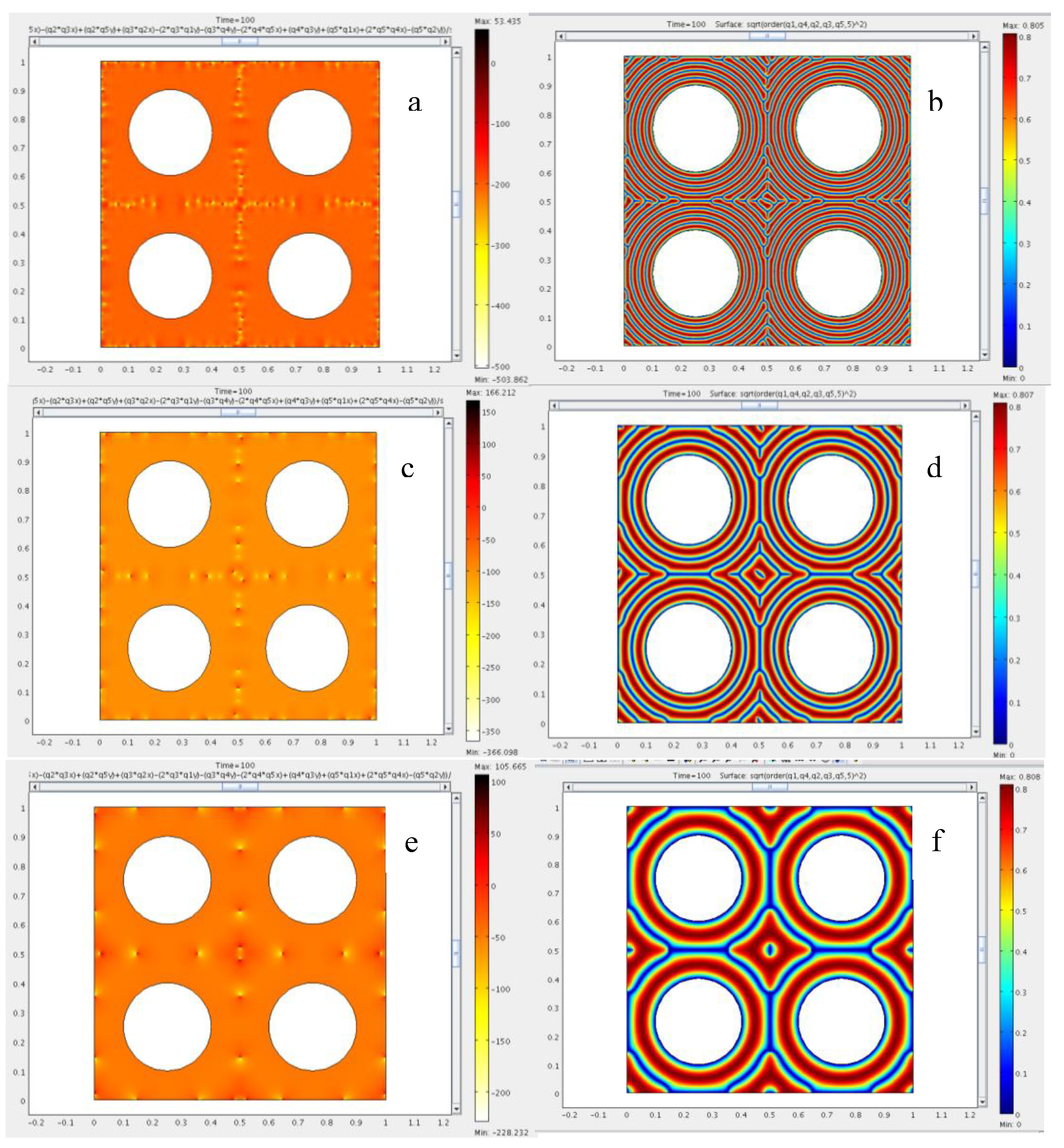
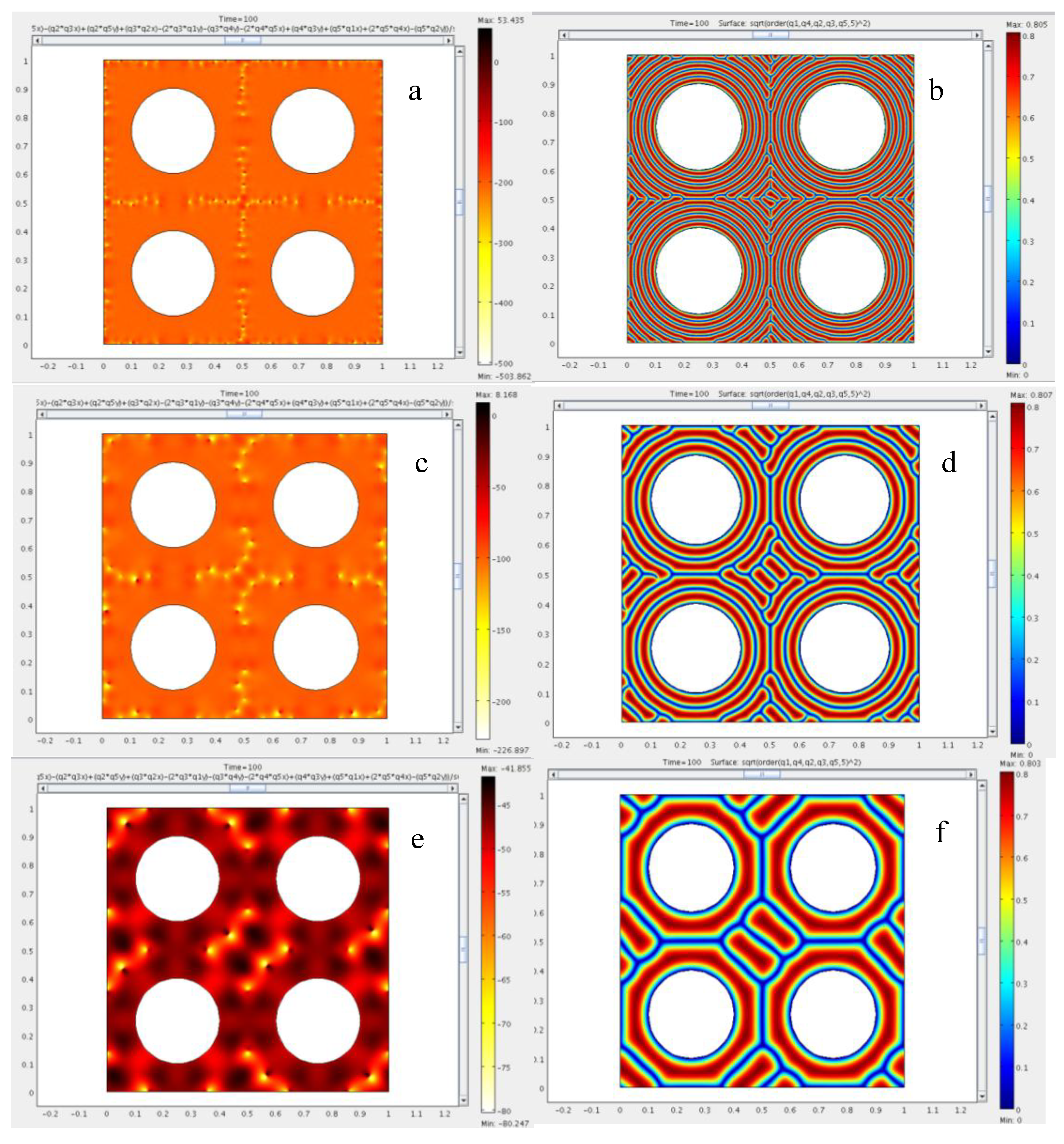
4.4. Saddle Defect in a Plant-Based Helicoidal Plywood
5. Conclusions
Acknowledgements
References
- Donald, A.; Windle, A.; Hanna, S. Liquid Crystalline Polymers; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Neville, A. Biology of Fibrous Composites: Development beyond the Cell Membrane; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Guille, M. Twisted liquid crystalline supramolecular arrangements in morphogenesis. Int. Rev. Cytology 1996, 166, 59–101. [Google Scholar]
- Knight, D.; Feng, D. Some observations on the collagen fibrils of the egg capsule of the dogfish, Scyliorhinus canicula. Tissue Cell 1994, 26, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.; Luke, B. A biological system producing a self-assembling cholesteric protein liquid crystal. J. Cell Sci. 1971, 8, 93. [Google Scholar]
- Vollrath, F.; Knight, D. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Fraden, S. Phase behavior of mixtures of rods (tobacco mosaic virus) and spheres (polyethylene oxide, bovine serum albumin). Biophys. J. 1998, 74, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Kupchinov, B.; Ermakov, S.; Rodnenkov, V.; Bobrysheva, S.; Beloenko, E.; Kestelman, V. Role of liquid crystals in the lubrication of living joints. Smart Mater. Struct. 1993, 2, 7–12. [Google Scholar] [CrossRef]
- Revol, J.; Bradford, H.; Giasson, J.; Marchessault, R.; Gray, D. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.; Godbout, L.; Dong, X.; Gray, D.; Chanzy, H.; Maret, G. Chiral nematic suspensions of cellulose crystallites; phase separation and magnetic field orientation. Liq. Cryst. 1994, 16, 127–134. [Google Scholar] [CrossRef]
- Livolant, F.; Bouligand, Y. Liquid crystalline phases given by helical biological polymers (DNA, PBLG and xanthan). Columnar textures. J. de Phys. 1986, 47, 1813–1827. [Google Scholar] [CrossRef]
- Livolant, F. Ordered phases of DNA in vivo and in vitro. Phys. A Statist. Theor. Phys. 1991, 176, 117–137. [Google Scholar]
- Livolant, F.; Bouligand, Y. New observations on the twisted arrangement of Dinoflagellate chromosomes. Chromosoma 1978, 68, 21–44. [Google Scholar] [CrossRef]
- de Gennes, P.; Prost, J. The Physics of Liquid Crystals; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Clore, G.; Starich, M.; Gronenborn, A. Measurement of residual dipolar couplings of macromolecules aligned in the nematic phase of a colloidal suspension of rod-shaped viruses. J. Amer. Chem. Soc. 1998, 120, 10571–10572. [Google Scholar] [CrossRef]
- Barry, E.; Hensel, Z.; Dogic, Z.; Shribak, M.; Oldenbourg, R. Entropy-driven formation of a chiral liquid-crystalline phase of helical filaments. Phys. Rev. Lett. 2006, 96, 18305–18305. [Google Scholar] [CrossRef]
- Davies, J.; Viney, C. Water-mucin phases: Conditions for mucus liquid crystallinity1. Thermochim. Acta 1998, 315, 39–49. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshöft, M.; Zühlke, S.; Spiteller, M. An endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 2008, 71, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Im, J.; Kim, J. Surface-enhanced raman spectroscopic studies of oriented monolayers on electrode surfaces. Anal. Sci. Technol. 1995, 8, 699–706. [Google Scholar]
- Kusari, S.; Zühlke, S.; Kosuth, J.; Cellárová, E.; Spiteller, M. Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J. Nat. Prod. 2009, 72, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P.; Arnold, N.; Spiteller, M.; Steglich, W. Lilacinone, a red aminobenzoquinone pigment from Lactarius lilacinus. J. Nat. Prod. 2003, 66, 1402–1403. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Verma, V.; Amna, T.; Qazi, G.; Spiteller, M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 2005, 68, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, R.; Spiteller, P.; Mycenaaurin, A. An antibacterial polyene pigment from the fruiting bodies of mycena aurantiomarginata. J. Nat. Prod. 2010, 73, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M. Cellulose nanocrystals reinforced electrospun Poly (lactic acid) fibers as potential scaffold for bone tissure engineering. M.S. Thesis, North Carolina State University, Raleigh, NC, USA, 2010. [Google Scholar]
- Nassif, N.; Gobeaux, F.; Seto, J.; Belamie, E.; Davidson, P.; Panine, P.; Mosser, G.; Fratzl, P.; Guille, M. Self-assembled collagen-apatite matrix with bone-like hierarchy. Chem. Mater. 2010, 22, 3307–3309. [Google Scholar] [CrossRef]
- Donaldson, L. Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci. Technol. 2007, 41, 443–460. [Google Scholar] [CrossRef]
- Belamie, E.; Mosser, G.; Gobeaux, F.; Giraud-Guille, M. Possible transient liquid crystal phase during the laying out of connective tissues: α-Chitin and collagen as models. J. Phys. Condens. Matter 2006, 18, S115–S129. [Google Scholar] [CrossRef]
- Vian, B.; Roland, J.; Reis, D. Primary cell wall texture and its relation to surface expansion. Int. J. Plant Sci. 1993, 154, 1–9. [Google Scholar] [CrossRef]
- Cowin, S. Do liquid crystal-like flow processes occur in the supramolecular assembly of biological tissues? J. Non-Newtonian Fluid Mech. 2004, 119, 155–162. [Google Scholar] [CrossRef]
- Onsager, L. The effects of shape on the interaction of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Kroon-Batenburg, L.; Kruiskamp, P.; Vliegenthart, J.; Kroon, J. Estimation of the persistence length of polymers by MD simulations on small fragments in solution. Application to cellulose. J. Phys. Chem. B 1997, 101, 8454–8459. [Google Scholar] [CrossRef]
- Rey, A. Liquid crystal models of biological materials and processes. Soft Matter 2010, 6, 3402–3429. [Google Scholar]
- De Luca, G.; Rey, A. Chiral front propagation in liquid-crystalline materials: Formation of the planar monodomain twisted plywood architecture of biological fibrous composites. Phys. Rev. E 2004, 69, 11706. [Google Scholar] [CrossRef]
- De Luca, G.; Rey, A. Monodomain and polydomain chiral liquid crystals and their biological analogues. Eur. Phys. J. E 2003, 12, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Murugesan, Y. Mechanical model for fiber-laden membranes. Continuum Mech. Thermodyn. 2010. [Google Scholar] [CrossRef]
- Murugesan, Y.; Rey, A. Thermodynamic model of structure and shape in rigid polymer-laden membranes. Macromol. Theory Simul. 2010, 19, 113–126. [Google Scholar]
- Murugesan, Y.; Rey, A. Structure and rheology of fiber-laden membranes via integration of nematodynamics and membranodynamics. J. Non-Newtonian Fluid Mech. 2010, 165, 32–44. [Google Scholar] [CrossRef]
- Roland, J.; Reis, D.; Vian, B.; Satiat-Jeunemaitre, B.; Mosiniak, M. Morphogenesis of plant cell walls at the supramolecular level: Internal geometry and versatility of helicoidal expression. Protoplasma 1987, 140, 75–91. [Google Scholar] [CrossRef]
- Reis, D.; Roland, J.; Mosiniak, M.; Darzens, D.; Vian, B. The sustained and warped helicoidal pattern of a xylan-cellulose composite: the stony endocarp model. Protoplasma 1992, 166, 21–34. [Google Scholar]
- Smalyukh, I.; Lavrentovich, O. Defects, surface anchoring, and three-dimensional director fields in the lamellar structure of cholesteric liquid crystals as studied by fluorescence confocal polarizing microscopy. In Topology in Condensed Matter; Monastyrskiĭ, M.I., Ed.; Springer-Verlag: Berlin, Germany, 2006; pp. 205–250. [Google Scholar]
- Gupta, G.; Rey, A. Texture rules for concentrated filled nematics. Phys. Rev. Lett. 2005, 95, 127802. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; White, J. Disclination structures in the carbonaceous mesophase. In Advances in Liquid Crystals 5; Brown, H., Ed.; Academic Press: New York, NY, USA, 1982; Volume 5, pp. 157–213. [Google Scholar]
- Abukhdeir, N.; Rey, A. Defect kinetics and dynamics of pattern coarsening in a two-dimensional smectic-A system. New J. Phys. 2008, 10, 063025. [Google Scholar] [CrossRef]
- Abukhdeir, N.; Rey, A. Edge dislocation core structure in lamellar smectic-A liquid crystals. Soft Matter 2010, 6, 1117–1120. [Google Scholar] [CrossRef]
- Wright, D.; Mermin, N. Crystalline liquids: the blue phases. Rev. Mod. Phys. 1989, 61, 385–432. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S. The Theory of Polymer Dynamics; Oxford University Press: New York, NY, USA, 1988. [Google Scholar]
- Barmpoutis, A.; Vemuri, B.; Shepherd, T.; Forder, J. Tensor splines for interpolation and approximation of DT-MRI with applications to segmentation of isolated rat hippocampi. IEEE Trans. Med. Imaging 2007, 26, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. Capillary models for liquid crystal fibers, membranes, films, and drops. Soft Matter 2007, 3, 1349–1368. [Google Scholar]
- Rey, A. Thermodynamics of soft anisotropic interfaces. J. Chem. Phys. 2004, 120, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. Viscoelastic theory for nematic interfaces. Phys. Rev. E 2000, 61, 1540–1549. [Google Scholar]
- Meyer, R.; Lonberg, F.; Taratuta, V.; Fraden, S.; Lee, S.; Hurd, A. Measurements of the anisotropic viscous and elastic properties of lyotropic polymer nematics. Faraday Discuss. Chem. Soc. 1985, 79, 125–132. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murugesan, Y.K.; Rey, A.D. Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals. Polymers 2010, 2, 766-785. https://doi.org/10.3390/polym2040766
Murugesan YK, Rey AD. Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals. Polymers. 2010; 2(4):766-785. https://doi.org/10.3390/polym2040766
Chicago/Turabian StyleMurugesan, Yogesh K., and Alejandro D. Rey. 2010. "Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals" Polymers 2, no. 4: 766-785. https://doi.org/10.3390/polym2040766
APA StyleMurugesan, Y. K., & Rey, A. D. (2010). Modeling Textural Processes during Self-Assembly of Plant-Based Chiral-Nematic Liquid Crystals. Polymers, 2(4), 766-785. https://doi.org/10.3390/polym2040766





