A Study of Thin Films Based on Polylactide and Vanillic Acid-Crucial Properties Relevant to Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of Polylactide-Based Films
2.3. Methods of Analysis
2.3.1. Fourier Transform Infrared Analysis
2.3.2. SEM and AFM Study
2.3.3. DSC Analysis
2.3.4. Mechanical Properties
2.3.5. Assessment of Antioxidative Properties
2.3.6. Water Vapor Transmission Rate
2.3.7. Transparency and UV Barrier Properties
2.3.8. Blueberry Storage and Firmness
2.3.9. Statistics
3. Results and Discussion
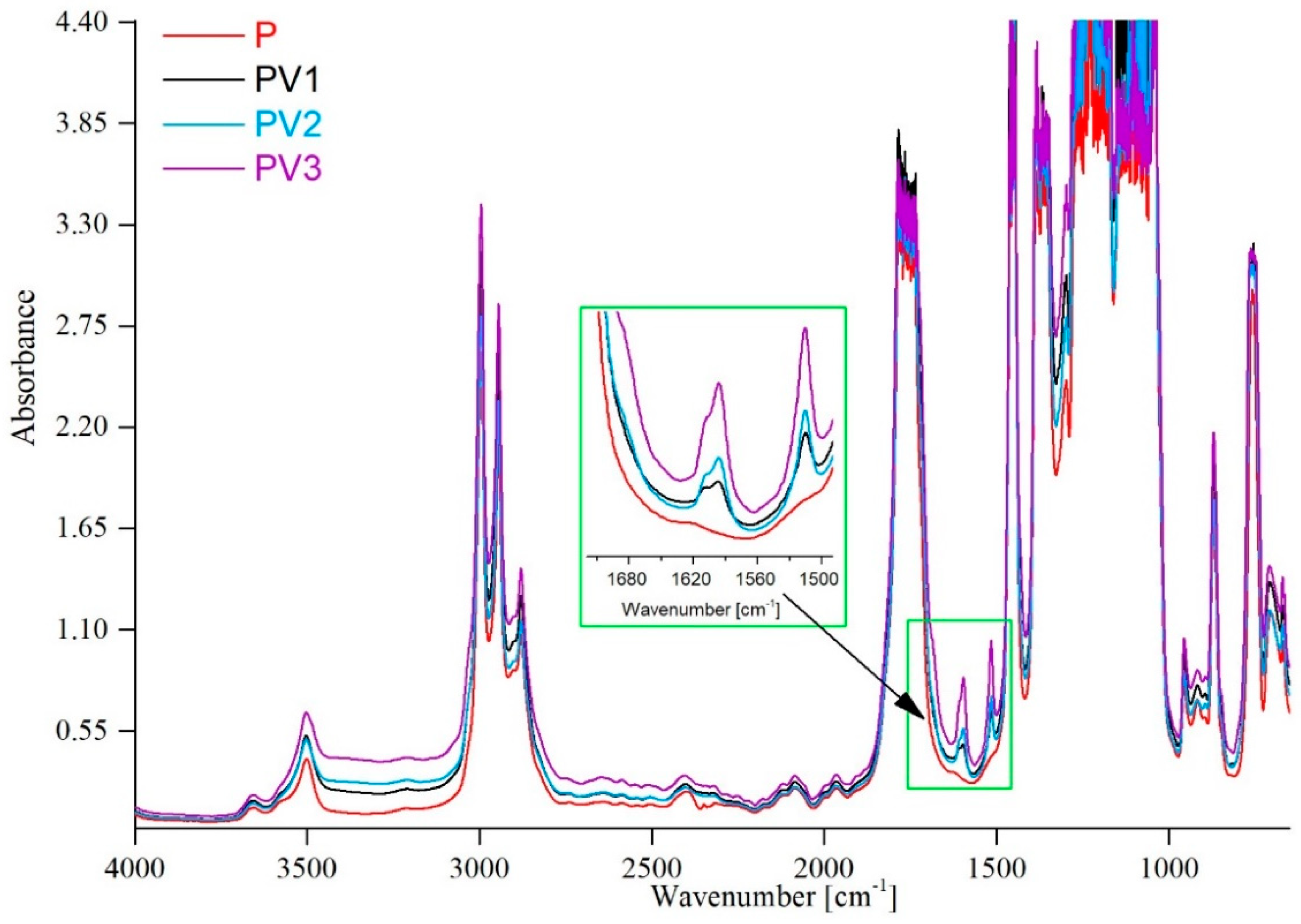
3.1. Structural Study by Means of FTIR Technique
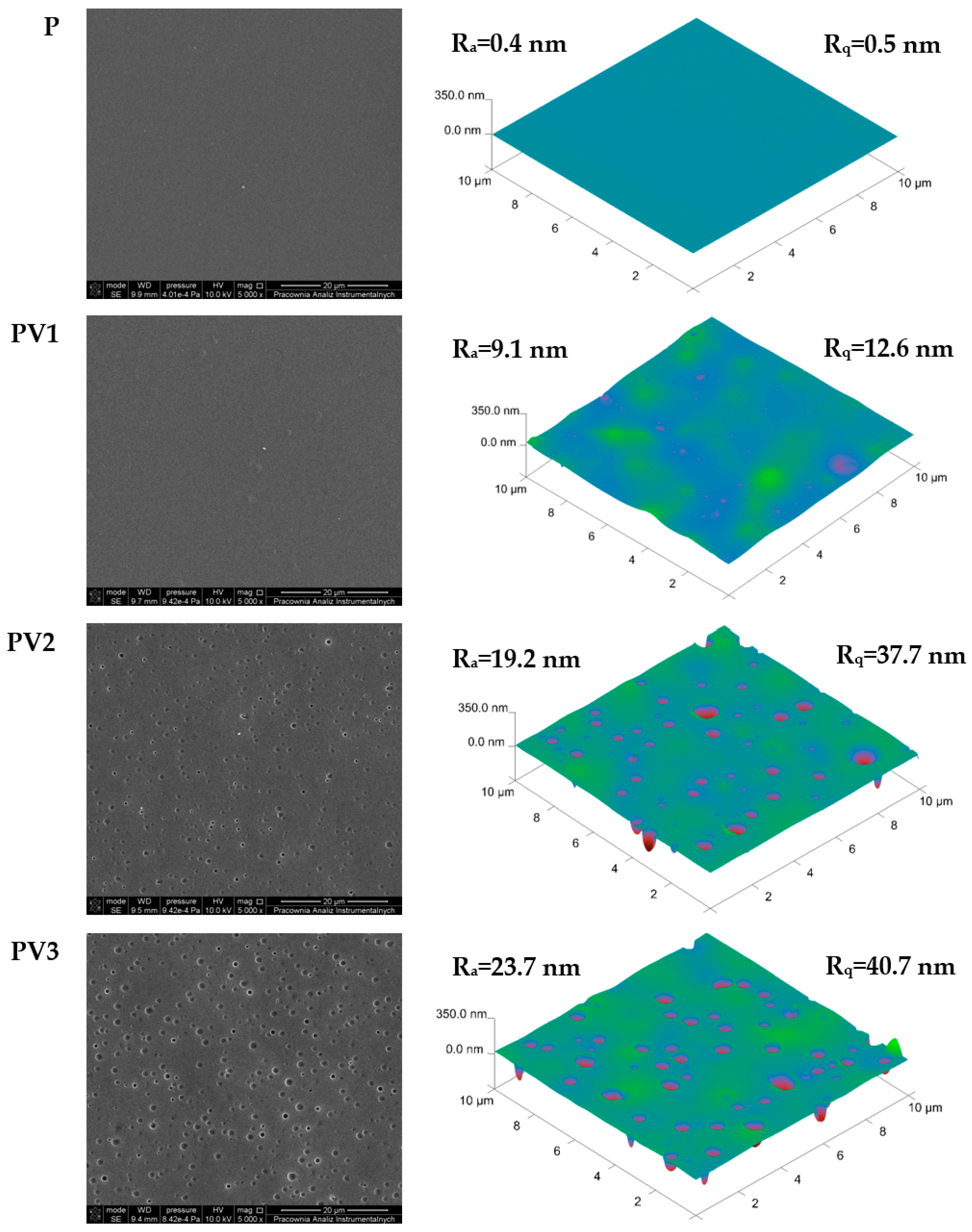
3.2. Morphology and Topography Evaluation
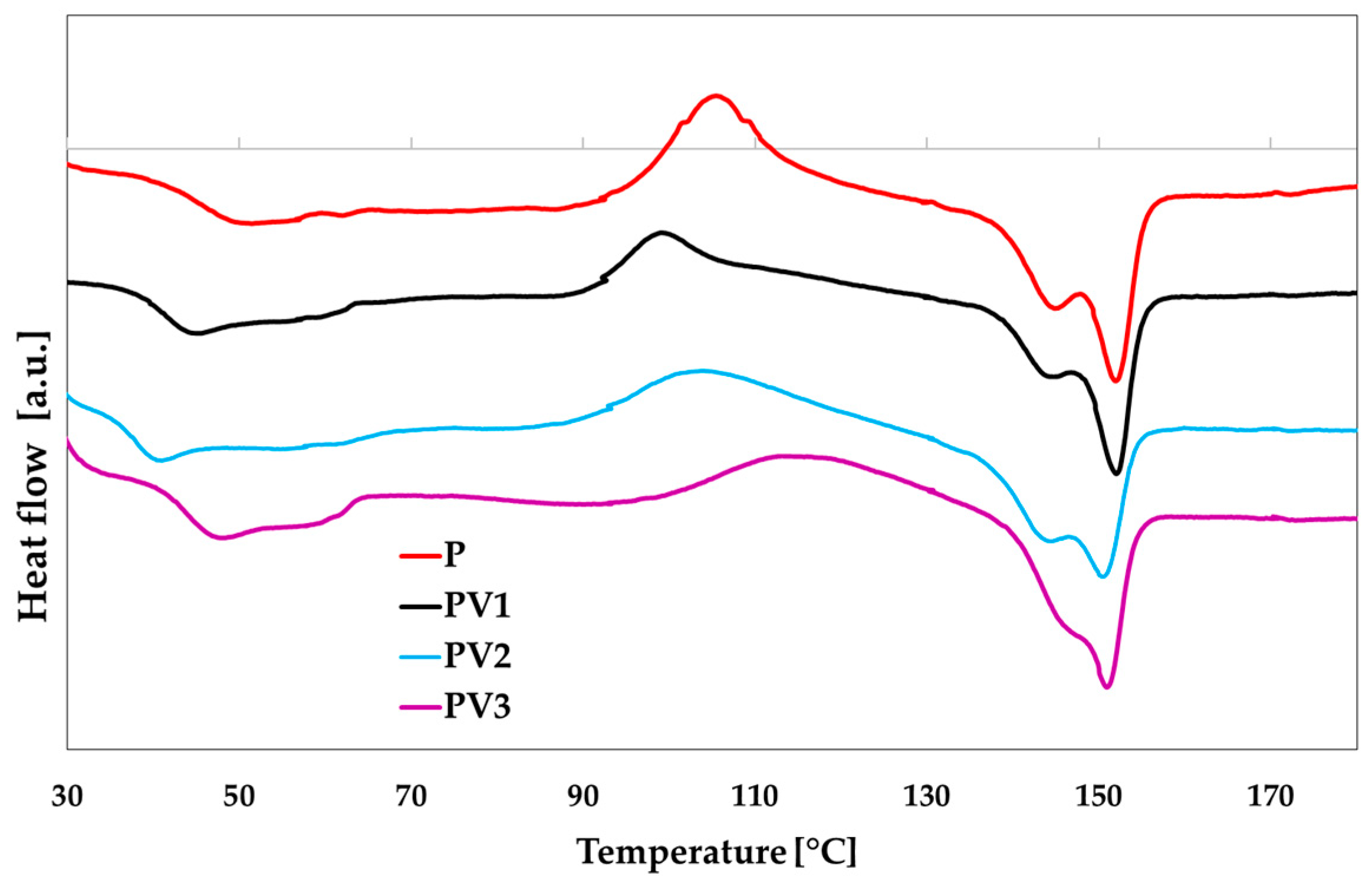
3.3. Determination of Thermal Properties of PLA-Based Materials
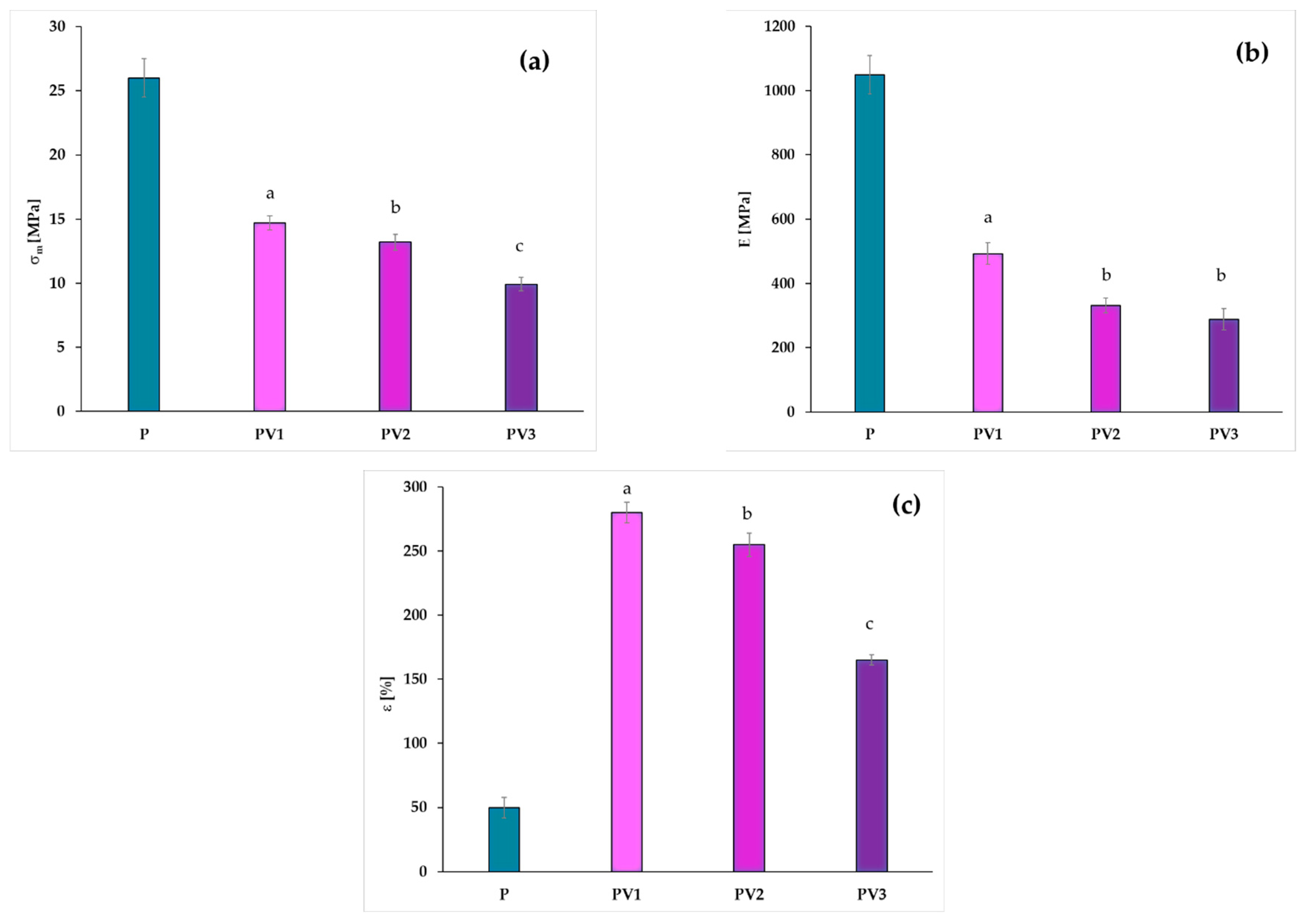
3.4. Changes in Mechanical Properties
3.5. Examination of WVTR
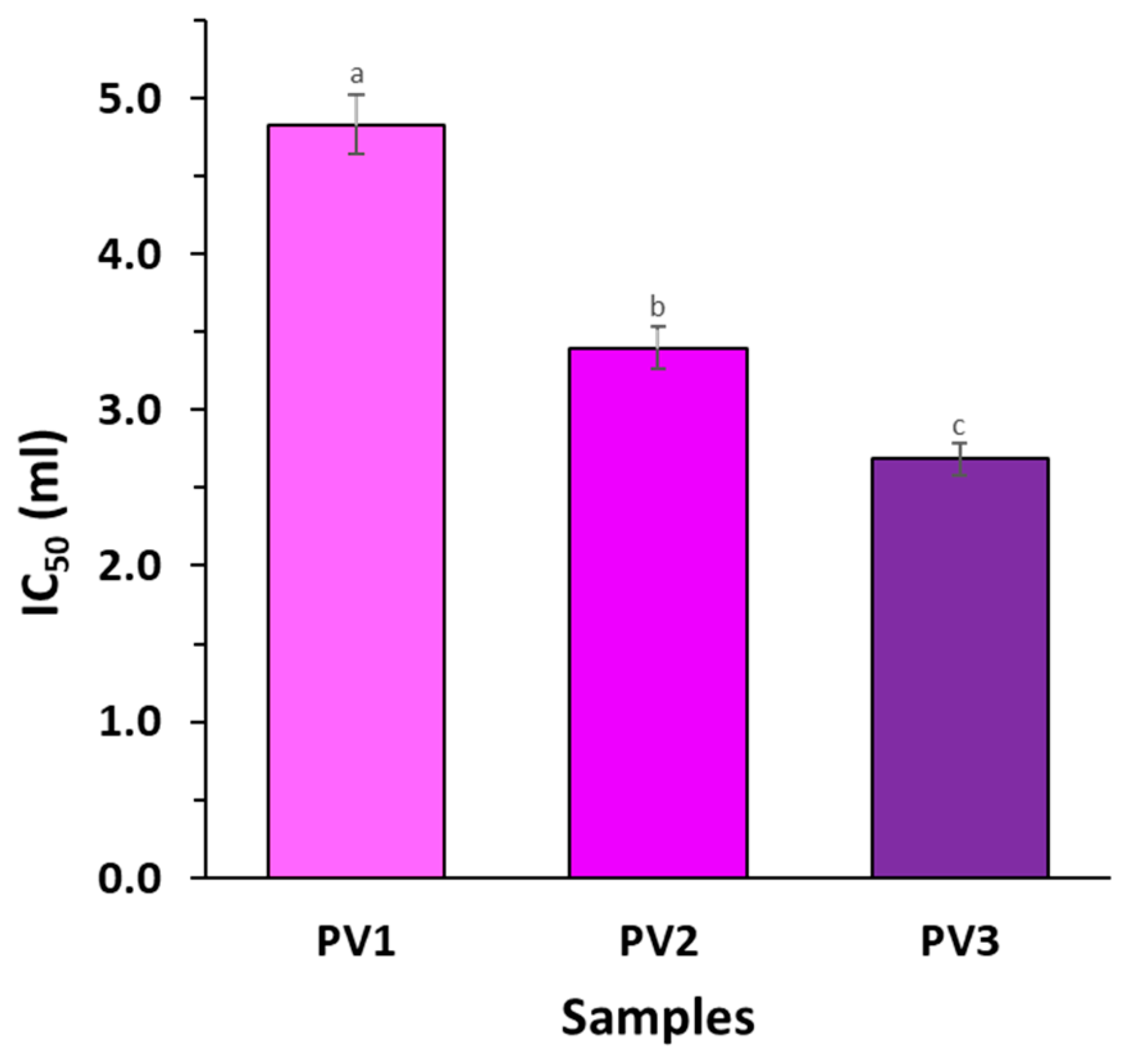
3.6. Differences in Antioxidative Properties
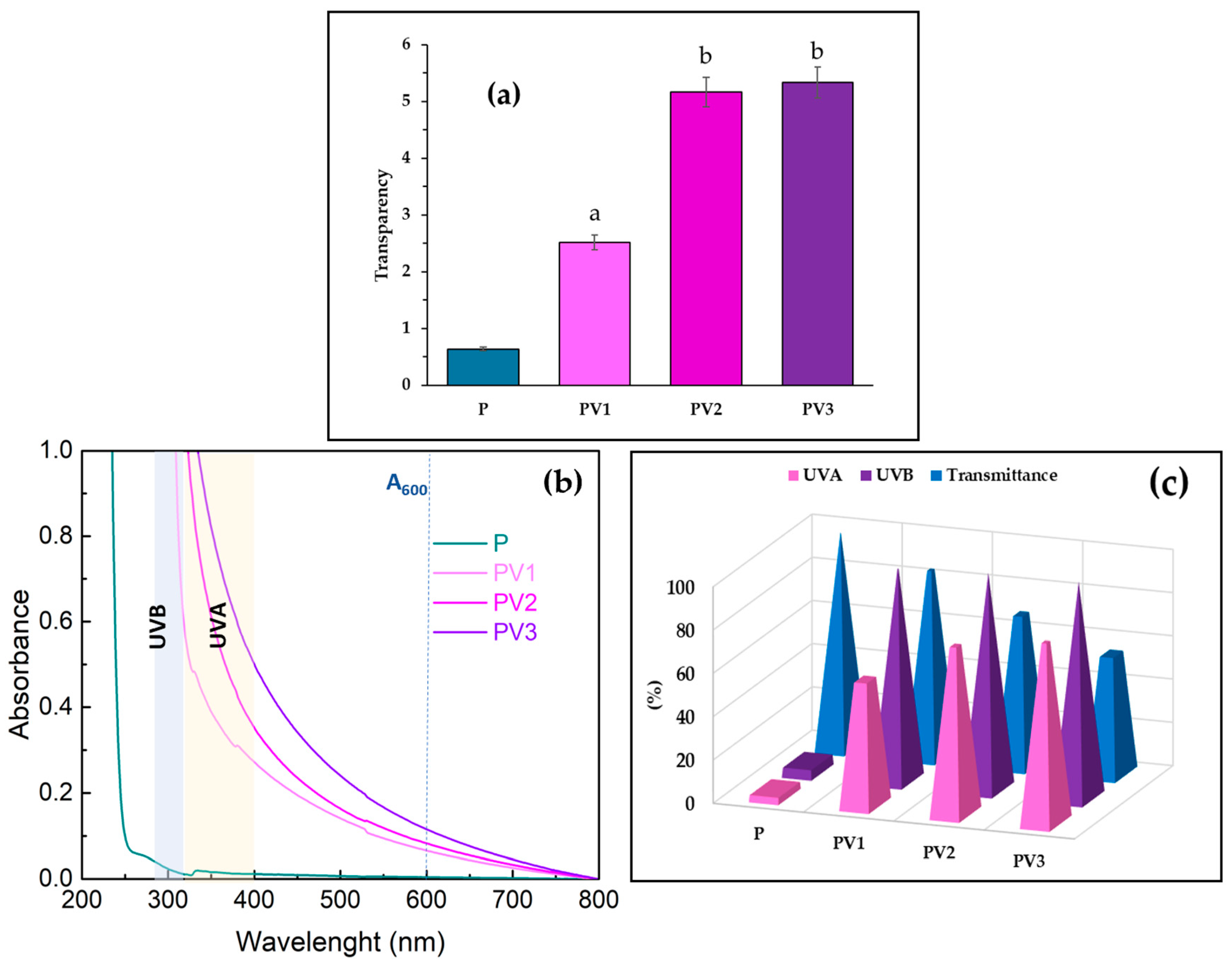
3.7. Evaluation of Transparency and UV Barrier
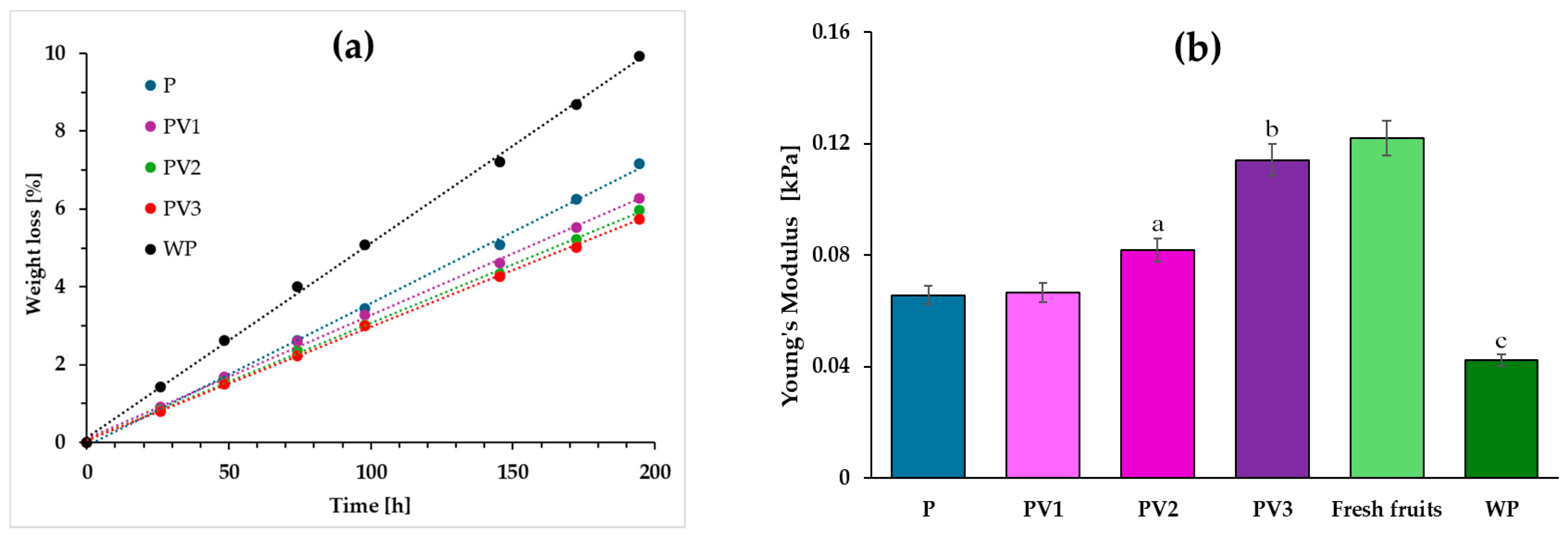
3.8. Storing Blueberries: Firmness and Weight Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, P.S.; Ribeiro, S.M.; Pontes, R.; Nunes, J. Production Methods and Applications of Bioactive Polylactic Acid: A Review. Environ. Chem. Lett. 2024, 22, 1831–1859. [Google Scholar] [CrossRef]
- Lombardi, A.; Fochetti, A.; Vignolini, P.; Campo, M.; Durazzo, A.; Lucarini, M.; Puglia, D.; Luzi, F.; Papalini, M.; Renzi, M.; et al. Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives. Antioxidants 2022, 11, 2074. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Extending the Shelf Life of Apples After Harvest Using Edible Coatings as Active Packaging—A Review. Appl. Sci. 2025, 15, 767. [Google Scholar] [CrossRef]
- Nahar, L.; Habibi, E.; Gavril, G.L.; Abdelfattah, G.M.M.; Wrona, M.; Nerín, C.; Guo, M.; Sarker, S.D. Towards Sustainable Food Packaging Using Natural Compounds: A Review of Current Research Update. Food Bioprod. Process 2025, 150, 260–274. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A. Application of High-Pressure Processing and Polylactide/Cinnamon Oil Packaging on Chicken Sample for Inactivation and Inhibition of Listeria Monocytogenes and Salmonella Typhimurium, and Post-Processing Film Properties. Food Control 2017, 78, 160–168. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-Mechanical, Structural Characterization and Antibacterial Performance of Solvent Casted Polylactide/Cinnamon Oil Composite Films. Food Control 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Efficacy of Antimicrobial Properties of Polylactide/Cinnamon Oil Film with and without High-Pressure Treatment against Listeria Monocytogenes and Salmonella Typhimurium Inoculated in Chicken Sample. Food Packag. Shelf Life 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Fadhel, Y.B.; Djidjelli, H.; Lacroix, M. Development of Antimicrobial Films Based on Poly(Lactic Acid) Incorporated with Thymus Vulgaris Essential Oil and Ethanolic Extract of Mediterranean Propolis. Int. J. Biol. Macromol. 2021, 185, 535–542. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/Graphene Oxide Nanosheets/Clove Essential Oil Composite Films for Potential Food Packaging Applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Vishwakarma, A.; Wrona, M.; Bertella, A.; Rudawska, A.; Gierszewska, M.; Schmidt, B. Comparative Study of Crucial Properties of Packaging Based on Polylactide and Selected Essential Oils. Foods 2025, 14, 204. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers 2023, 15, 4103. [Google Scholar] [CrossRef] [PubMed]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Wrona, M.; Richert, A.; Rudawska, A. Polylactide-Based Films Incorporated with Berberine—Physicochemical and Antibacterial Properties. Foods 2023, 12, 91. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef]
- Korra, S.; Madhurya, S.; Kumar, T.S.; Sainath, A.V.S.; Keshava Murthy, P.S.; Reddy, J.P. Extension of Shelf-Life of Mangoes Using PLA-Cardanol-Amine Functionalized Graphene Active Films. Int. J. Biol. Macromol. 2025, 297, 139849. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Properties of PLA Films with Cinnamic Acid: Effect of the Processing Method. Food Bioprod. Process 2022, 133, 25–33. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Multilayer Antimicrobial Films Based on Starch and PLA with Superficially Incorporated Ferulic or Cinnamic Acids for Active Food Packaging Purposes. Food Chem. Adv. 2023, 2, 100250. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative Properties of Vanillic Acid Esters in Multiple Antioxidant Assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U.; et al. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kołodziejczyk-Czepas, J.; Nowak, P. Nie Tylko Aromat—Wielokierunkowa Aktywność Biologiczna Kwasu Wanilinowego. Nauk. Przyr. 2017, 11, 169–184. [Google Scholar]
- Eelager, M.P.; Masti, S.P.; Chougale, R.B.; Hiremani, V.D.; Narasgoudar, S.S.; Dalbanjan, N.P.; Praveen Kumar, S.K. Evaluation of Mechanical, Antimicrobial, and Antioxidant Properties of Vanillic Acid Induced Chitosan/Poly (Vinyl Alcohol) Active Films to Prolong the Shelf Life of Green Chilli. Int. J. Biol. Macromol. 2023, 232, 123499. [Google Scholar] [CrossRef]
- Liu, W.; Xie, J.; Li, L.; Xue, B.; Li, X.; Gan, J.; Shao, Z.; Sun, T. Properties of Phenolic Acid-Chitosan Composite Films and Preservative Effect on Penaeus Vannamei. J. Mol. Struct. 2021, 1239, 130531. [Google Scholar] [CrossRef]
- Bakar, B.; Pekdemir, S.S.; Birhanlı, E.; Ulu, A.; Pekdemir, M.E.; Ateş, B. Unveiling the Effect of Molecular Weight of Vanillic Acid Grafted Chitosan Hydrogel Films on Physical, Antioxidant, and Antimicrobial Properties for Application in Food Packaging. Int. J. Biol. Macromol. 2024, 256, 128397. [Google Scholar] [CrossRef] [PubMed]
- González-Baró, A.C.; Parajón-Costa, B.S.; Franca, C.A.; Pis-Diez, R. Theoretical and Spectroscopic Study of Vanillic Acid. J. Mol. Struct. 2008, 889, 204–210. [Google Scholar] [CrossRef]
- Hong, M.M.; Oh, J.M.; Choy, J.H. Encapsulation of Flavor Molecules, 4-Hydroxy-3-Methoxy Benzoic Acid, into Layered Inorganic Nanoparticles for Controlled Release of Flavor. J. Nanosci. Nanotechnol. 2008, 8, 5018–5021. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Bouaziz, M. Polylactide Films with the Addition of Olive Leaf Extract— Physico-Chemical Characterization. Materials 2021, 14, 7623. [Google Scholar] [CrossRef]
- Lai, W.F. Design of Polymeric Films for Antioxidant Active Food Packaging. Int. J. Mol. Sci. 2022, 23, 12. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T. Design and Practical Considerations for Active Polymeric Films in Food Packaging. Int. J. Mol. Sci. 2022, 23, 6295. [Google Scholar] [CrossRef]
- Radhalakshmi, V.; Raman, M.; Joy, M.R. Development of Active Packaging Film Based on Poly (Lactic Acid) Incorporated with Piper Betel Leaf Ethanolic Extract and Its Application in the Shelf-Life Extension of Tuna Meat. Int. J. Biol. Macromol. 2023, 246, 125751. [Google Scholar] [CrossRef]
- Gao, C.; Chen, P.; Ma, Y.; Sun, L.; Yan, Y.; Ding, Y.; Sun, L. Multifunctional Polylactic Acid Biocomposite Film for Active Food Packaging with UV Resistance, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2023, 253, 126494. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Jacob, H.; Luciano, G.; Bini, T.B.; Almusallam, A. Polylactide/Poly(ε-Caprolactone)/Zinc Oxide/Clove Essential Oil Composite Antimicrobial Films for Scrambled Egg Packaging. Food Packag. Shelf Life 2019, 21, 100355. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(Lactic Acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Raj, A.; Yousfi, M.; Prashantha, K.; Samuel, C. Morphologies, Compatibilization and Properties of Immiscible PLA-Based Blends with Engineering Polymers: An Overview of Recent Works. Polymers 2024, 16, 1776. [Google Scholar] [CrossRef] [PubMed]
- Matumba, K.I.; Mokhena, T.C.; Ojijo, V.; Sadiku, E.R.; Ray, S.S. Morphological Characteristics, Properties, and Applications of Polylactide/Poly(ε-Caprolactone) Blends and Their Composites—A Review. Macromol. Mater. Eng. 2024, 309, 2400056. [Google Scholar] [CrossRef]
- Plamadiala, I.; Croitoru, C.; Pop, M.A.; Roata, I.C. Enhancing Polylactic Acid (PLA) Performance: A Review of Additives in Fused Deposition Modelling (FDM) Filaments. Polymers 2025, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, Y.; Wei, L.; Song, Y.; Guo, W.; Yu, L.; Gao, G.; Gao, J.; Huang, J.; Wang, Y.; et al. Enhancing the Compatibility and Performance of Poly (Lactic Acid) and Thermoplastic Polyolefin Elastomer Blends through a Dual Compatibilization Strategy. Int. J. Biol. Macromol. 2025, 303, 140513. [Google Scholar] [CrossRef]
- Ozawa, K.; Okuzono, T.; Doi, M. Diffusion Process during Drying to Cause the Skin Formation in Polymer Solutions. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2006, 45, 8817–8822. [Google Scholar] [CrossRef]
- Bassou, N.; Rharbi, Y. Role of Bénard-Marangoni Instabilities during Solvent Evaporation in Polymer Surface Corrugations. Langmuir 2009, 25, 624–632. [Google Scholar] [CrossRef]
- Tábi, T.; Sajó, I.E.; Szabó, F.; Luyt, A.S.; Kovács, J.G. Crystalline Structure of Annealed Polylactic Acid and Its Relation to Processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Li, J.; Xiao, P.; Li, H.; Zhang, Y.; Xue, F.; Luo, B.; Huang, S.; Shang, Y.; Wen, H.; De Claville Christiansen, J.; et al. Crystalline Structures and Crystallization Behaviors of Poly(l-Lactide) in Poly(l-Lactide)/Graphene Nanosheet Composites. Polym. Chem. 2015, 6, 3988–4002. [Google Scholar] [CrossRef]
- Zhuo, R.; Zhang, Y.; Li, G.; Shao, C.; Wang, Y.; Liu, C.; Li, Q.; Cao, W.; Shen, C. Structural Evolution of Poly(Lactic Acid) upon Uniaxial Stretching Investigated by in Situ Infrared Spectroscopy. Vib. Spectrosc. 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Giełdowska, M.; Puchalski, M.; Sztajnowski, S.; Krucińska, I. Evolution of the Molecular and Supramolecular Structures of PLA during the Thermally Supported Hydrolytic Degradation of Wet Spinning Fibers. Macromolecules 2022, 55, 10100–10112. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of Crystalline Forms (A′ and α) of Poly(Lactic Acid) on Its Mechanical, Thermo-Mechanical, Heat Deflection Temperature and Creep Properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Gao, P.; Alanazi, S.; Masato, D. Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers 2024, 16, 320. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Tashiro, K. Phase Transition Mechanism of Poly(l-Lactic Acid) among the α, δ, and β Forms on the Basis of the Reinvestigated Crystal Structure of the β Form. Macromolecules 2017, 50, 3285–3300. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of Active Phenolic Acids on Properties of PLA-PHBV Blend Films. Food Packag. Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Yu, M.; Du, Y.; Xu, P.; Yang, W.; Zhang, P.; Liu, T.; Lemstra, P.J.; Ma, P. Nucleation and Crystallization of Poly(L-Lactide) Assisted by Terminal Hydrogen-Bonding Segments. Polymers 2022, 254, 125031. [Google Scholar] [CrossRef]
- He, Y.; Dong, K.; Guo, Y.; Zhao, G.; Tang, S.; Wang, X.; Ma, B. Preparation and Properties of the Crosslinked Poly(L-Lactide) with High Crystallinity and High Gel Content. Polymers 2023, 287, 126441. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Effect of Ferulic and Cinnamic Acids on the Functional and Antimicrobial Properties in Thermo-Processed PLA Films. Food Packag. Shelf Life 2022, 33, 100882. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. The Role of a Deep Eutectic Solvent in Changes of Physicochemical and Antioxidative Properties of Chitosan-Based Films. Carbohydr. Polym. 2021, 255, 117527. [Google Scholar] [CrossRef]
- Zdulski, J.A.; Rutkowski, K.P.; Konopacka, D. Strategies to Extend the Shelf Life of Fresh and Minimally Processed Fruit and Vegetables with Edible Coatings and Modified Atmosphere Packaging. Appl. Sci. 2024, 14, 11074. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Bof, M.J.; Laurent, F.E.; Massolo, F.; Locaso, D.E.; Versino, F.; García, M.A. Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The Use of Pullulan Coatings with Propolis Extract to Extend the Shelf Life of Blueberry (Vaccinium corymbosum) Fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020. [Google Scholar] [CrossRef]
- Bugatti, V.; Cefola, M.; Montemurro, N.; Palumbo, M.; Quintieri, L.; Pace, B.; Gorrasi, G. Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries. Nanomaterials 2020, 10, 2513. [Google Scholar] [CrossRef]

| Sample | Photo | Thickness [mm] |
|---|---|---|
| P |  | 0.080 ± 0.003 |
| PV1 |  | 0.081 ± 0.002 |
| PV2 |  | 0.079 ± 0.003 |
| PV3 |  | 0.082 ± 0.002 |
| Sample | Tg (°C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc [%] |
|---|---|---|---|---|---|---|
| P | 44.69 | 105.43 | 5.80 | 144.93/152.01 | 6.04 | 5.83 |
| PV1 | 41.52 | 99.29 | 3.87 | 144.84/152.05 | 4.85 | 4.73 |
| PV2 | 40.77 | 104.04 | 3.30 | 144.47/150.55 | 4.49 | 4.47 |
| PV3 | 38.40 | 113.12 | 3.23 | 150.89 | 4.35 | 4.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olewnik-Kruszkowska, E.; Wrona, M.; Rudawska, A. A Study of Thin Films Based on Polylactide and Vanillic Acid-Crucial Properties Relevant to Packaging. Polymers 2025, 17, 882. https://doi.org/10.3390/polym17070882
Olewnik-Kruszkowska E, Wrona M, Rudawska A. A Study of Thin Films Based on Polylactide and Vanillic Acid-Crucial Properties Relevant to Packaging. Polymers. 2025; 17(7):882. https://doi.org/10.3390/polym17070882
Chicago/Turabian StyleOlewnik-Kruszkowska, Ewa, Magdalena Wrona, and Anna Rudawska. 2025. "A Study of Thin Films Based on Polylactide and Vanillic Acid-Crucial Properties Relevant to Packaging" Polymers 17, no. 7: 882. https://doi.org/10.3390/polym17070882
APA StyleOlewnik-Kruszkowska, E., Wrona, M., & Rudawska, A. (2025). A Study of Thin Films Based on Polylactide and Vanillic Acid-Crucial Properties Relevant to Packaging. Polymers, 17(7), 882. https://doi.org/10.3390/polym17070882








