Microfluidization Preparation of Hybrid Graphene for Enhanced Wear Resistance of Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
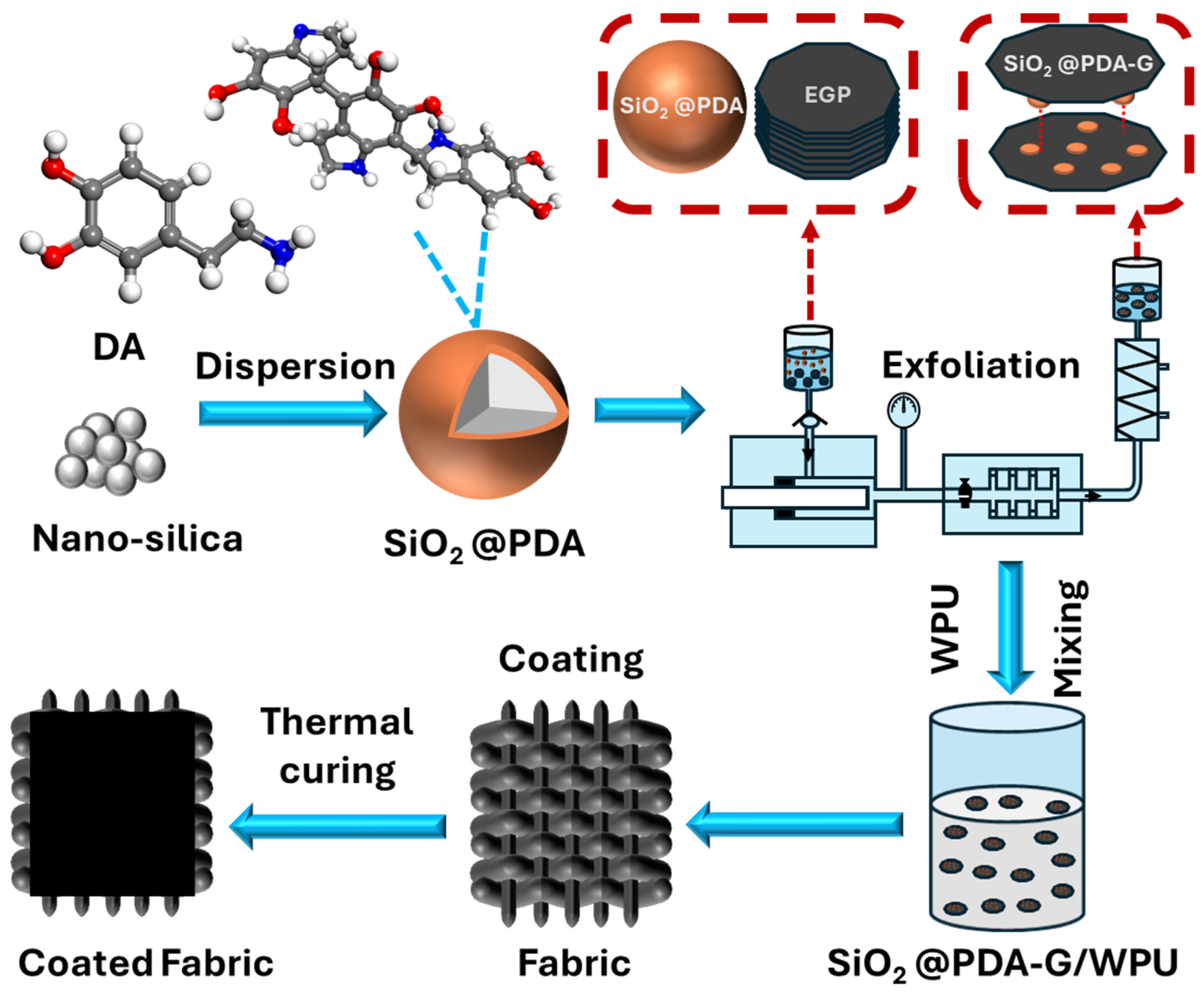
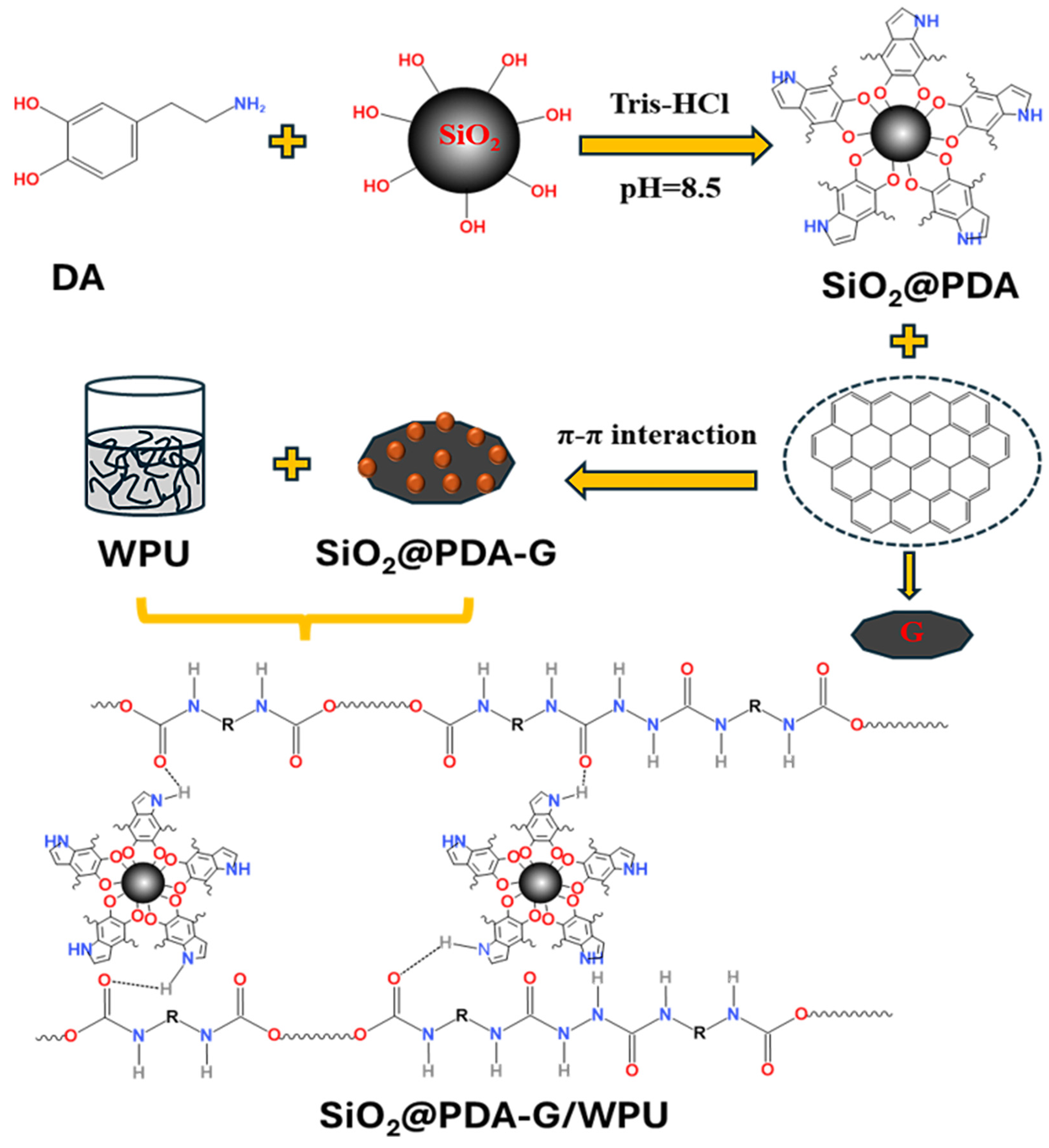
2.2. Preparation of Hybrid Graphene
2.3. Preparation of Hybrid Graphene/Polyurethane Composite Coating
2.4. Characterization
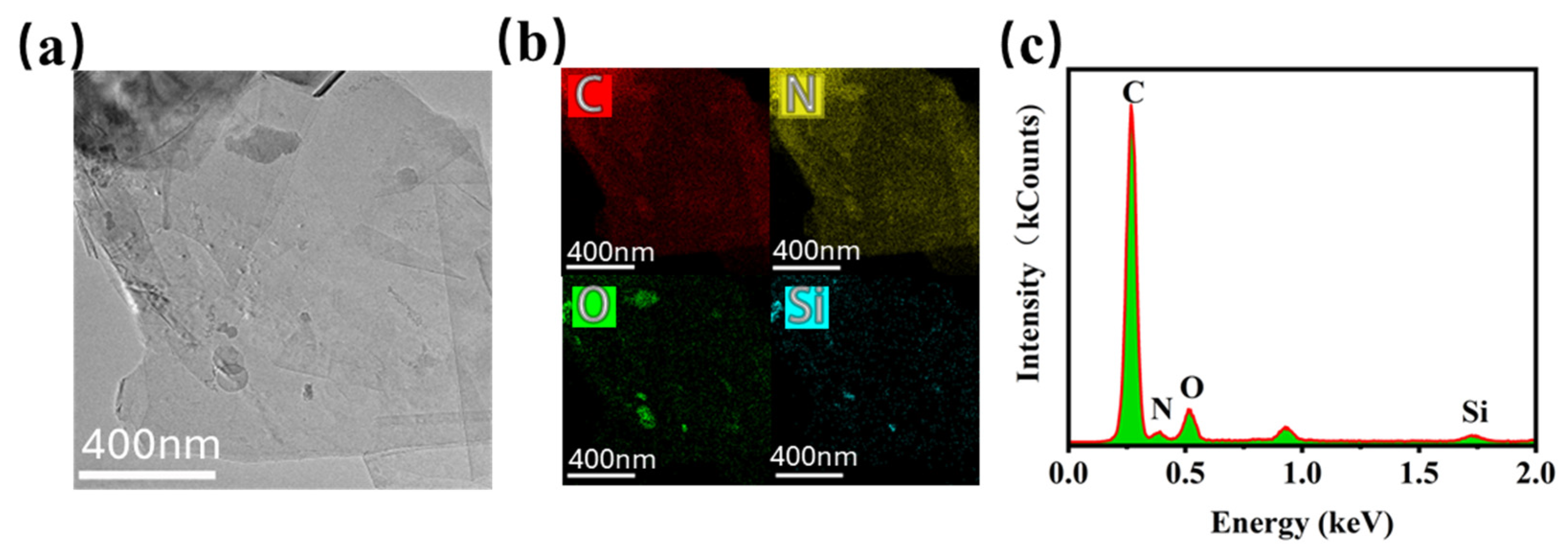
2.4.1. Characterization of Hybrids
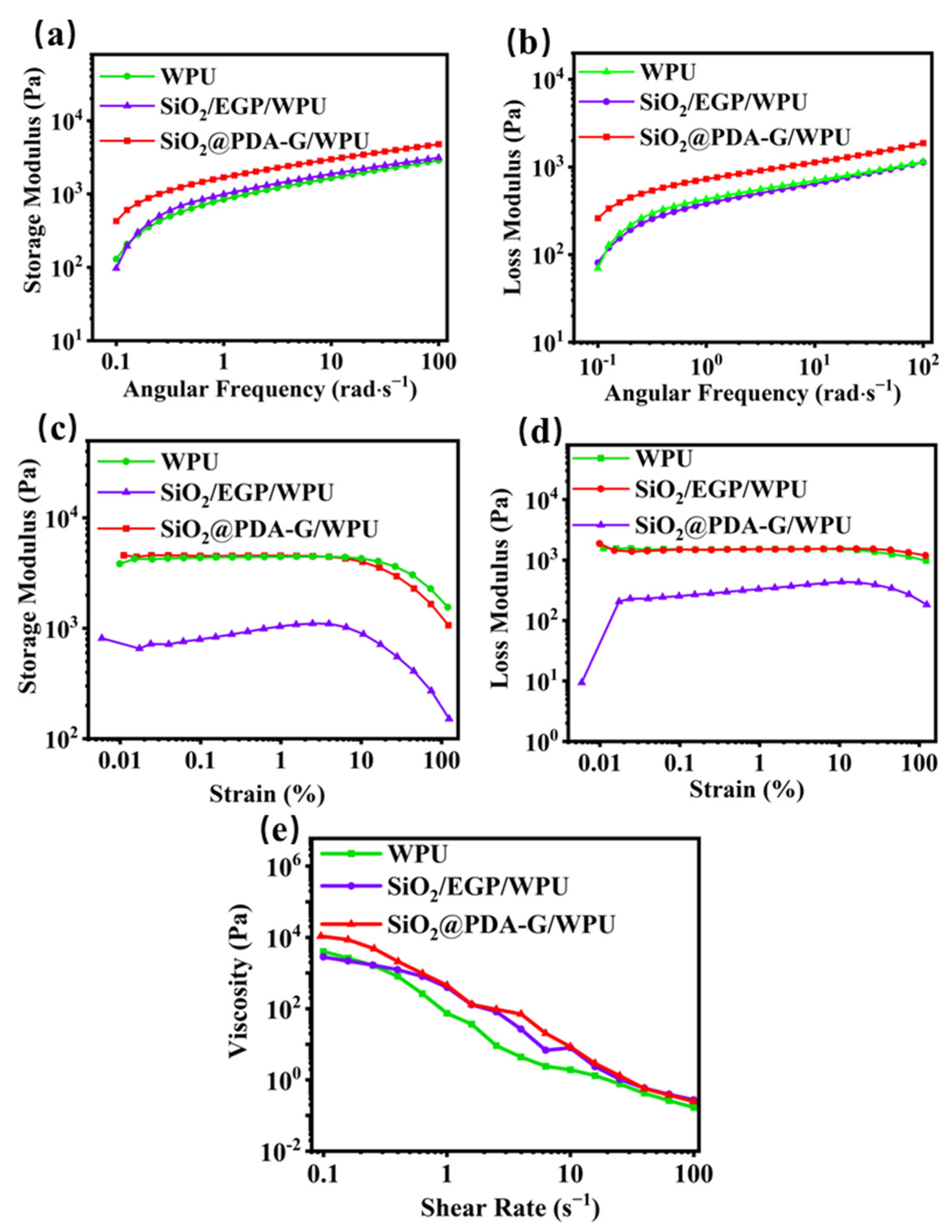
2.4.2. Rheological Testing
2.4.3. Characterization of Coating
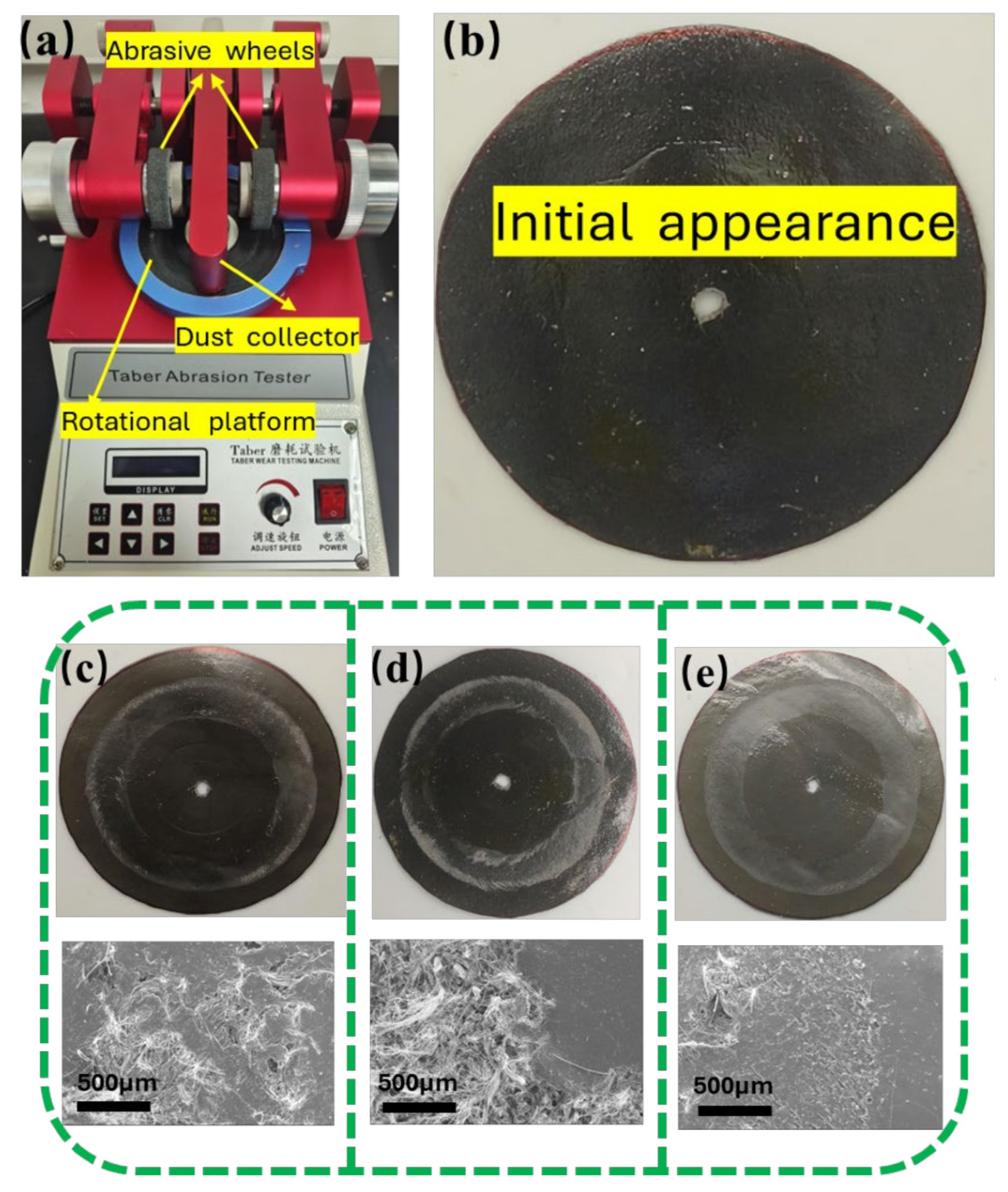
2.4.4. Wear Resistance Test of the Coating
2.4.5. Tensile Property Testing
3. Results and Discussion
3.1. Preparation and Characterization of Hybrid Graphene
3.2. Rheological Property
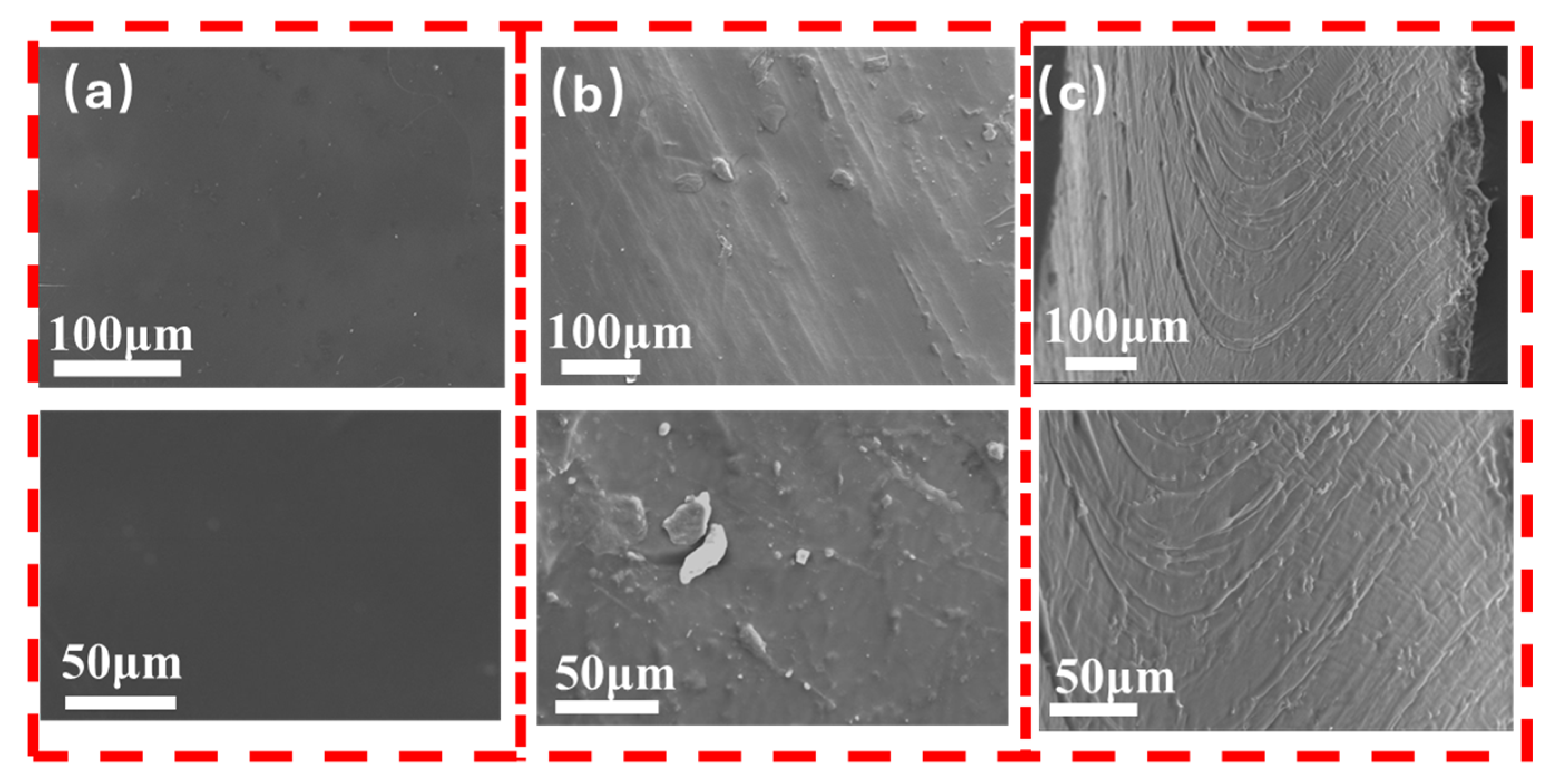
3.3. The Structure of Composite Coating
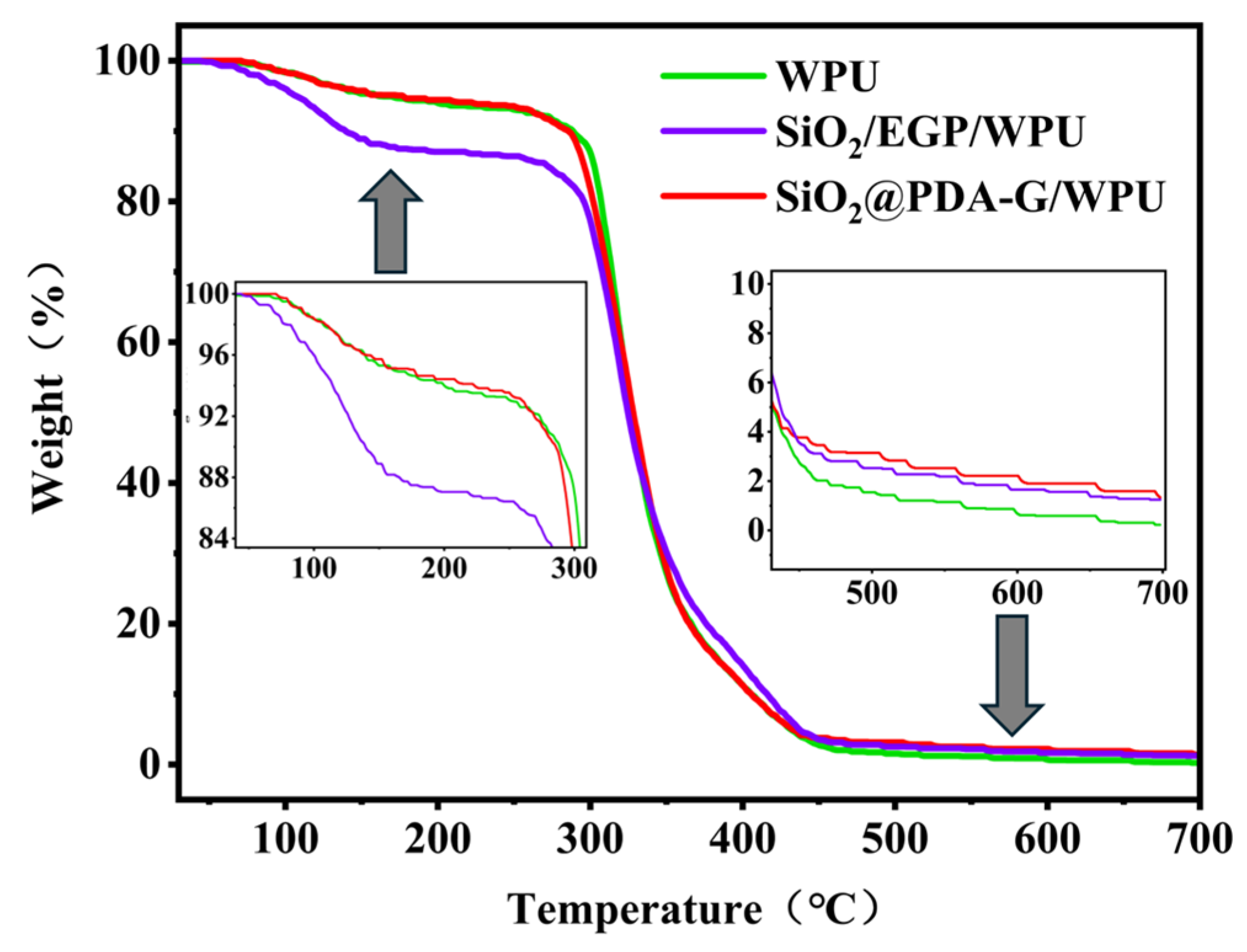
3.4. Wear Resistance Performance
3.5. Tensile Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGP | Expanded graphite powder |
| WPU | Waterborne polyurethane |
| PDA | Polydopamine |
| G | Graphene |
References
- Elsayed, H.; Attia, R.; Mohamed, O.; Haroun, A.; El-Sayed, N. Preparation of Polyurethane Silicon Oxide Nanomaterials as a Binder in Leather Finishing. Fibers Polym. 2018, 19, 832–842. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, K.-J. Study on Properties of Waterborne Polyurethane-Epoxy Hybrid Resin for Leather Garment Coationgs. J. Korean Appl. Sci. Technol. 2010, 27, 325–336. [Google Scholar]
- Lee, Y.J.; La, Y.; Jeon, O.S.; Lee, H.J.; Shin, M.K.; Yang, K.-H.; You, Y.J.; Park, S.Y. Effects of boron nitride nanotube content on waterborne polyurethane–acrylate composite coating materials. RSC Adv. 2021, 11, 12748–12756. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, W. Effect of Coating Process on Properties of Two-Component Waterborne Polyurethane Coatings for Wood. Coatings 2022, 12, 1857. [Google Scholar] [CrossRef]
- Karna, N.; Joshi, G.M.; Mhaske, S.T. Structure-property relationship of silane-modified polyurethane: A review. Prog. Org. Coat. 2023, 176, 107377. [Google Scholar] [CrossRef]
- Jian, H.; Wang, H.; Wen, M.; Shi, J.; Park, H.-J. High-performance flame-retardant, waterproof, and abrasion-resistant waterborne polyurethane-based transparent coatings for wood protection. Ind. Crops Prod. 2024, 222, 119804. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Z.; Wang, Y.; Guo, M.; Ban, T.; Zhang, Y.; Zhu, X. Abrasion resistant waterborne polyurethane coatings based on dual crosslinked structure. Prog. Org. Coat. 2024, 190, 108336. [Google Scholar] [CrossRef]
- Xu, D.; Liang, G.; Qi, Y.; Gong, R.; Zhang, X.; Zhang, Y.; Liu, B.; Kong, L.; Dong, X.; Li, Y. Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers 2022, 14, 5456. [Google Scholar] [CrossRef]
- Xu, H.; Liu, D.; Liang, L.; Tian, Z.; Shen, P. Nitrogen-Doped Graphene Sheets as Efficient Nanofillers at Ultra-Low Content for Reinforcing Mechanical and Wear-Resistant Properties of Acrylic Polyurethane Coatings. Crystals 2022, 12, 1820. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Yang, F.; Wu, X.; Ding, G. A novel waterborne polyurethane coating modified by highly dispersed nano-boron carbide particles. J. Appl. Polym. Sci. 2020, 138, 50214. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Li, F.; Ma, Y.; Chen, L.; Li, H.; Zhou, H.; Chen, J. In-situ polymerization of polyurethane/aniline oligomer functionalized graphene oxide composite coatings with enhanced mechanical, tribological and corrosion protection properties. Chem. Eng. J. 2021, 425, 130006. [Google Scholar] [CrossRef]
- Liu, Y.; Shin, D.-G.; Xu, S.; Kim, C.-L.; Kim, D.-E. Understanding of the lubrication mechanism of reduced graphene oxide coating via dual in-situ monitoring of the chemical and topographic structural evolution. Carbon 2021, 173, 941–952. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Wan, Q.; Wang, H.; Li, S.; Wang, J. Efficient liquid-phase exfoliation of few-layer graphene in aqueous 1, 1, 3, 3-tetramethylurea solution. J. Colloid Interface Sci. 2018, 526, 167–173. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Yusuf, M.; Kumar, M.; Khan, M.A.; Sillanpää, M.; Arafat, H. A review on exfoliation, characterization, environmental and energy applications of graphene and graphene-based composites. Adv. Colloid Interface Sci. 2019, 273, 102036. [Google Scholar] [CrossRef]
- Hu, Y.; Song, S.; Lopez-Valdivieso, A. Effects of oxidation on the defect of reduced graphene oxides in graphene preparation. J. Colloid Interface Sci. 2015, 450, 68–73. [Google Scholar] [CrossRef]
- Zhuo, Q.; Zhang, Y.; Du, Q.; Yan, C. Facile reduction of graphene oxide at room temperature by ammonia borane via salting out effect. J. Colloid Interface Sci. 2015, 457, 243–247. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.; Shi, Z.; Liu, C.-C.; Zhang, L.; Xie, G.; Cheng, M.; Wang, D.; Yang, R.; Shi, D.; et al. Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat. Mater. 2013, 12, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Park, H.J.; Konuma, M.; Lee, D.S.; Smet, J.H.; Starke, U. Long-Range Ordered Single-Crystal Graphene on High-Quality Heteroepitaxial Ni Thin Films Grown on MgO(111). Nano Lett. 2010, 11, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, G. Direct CVD Graphene Growth on Semiconductors and Dielectrics for Transfer-Free Device Fabrication. Adv. Mater. 2016, 28, 4956–4975. [Google Scholar] [CrossRef] [PubMed]
- Kwieciñski, W.; Sotthewes, K.; Poelsema, B.; Zandvliet, H.J.W.; Bampoulis, P. Chemical vapor deposition growth of bilayer graphene in between molybdenum disulfide sheets. J. Colloid Interface Sci. 2017, 505, 776–782. [Google Scholar] [CrossRef]
- Sagar, R.U.R.; Namvari, M.; Navale, S.T.; Stadler, F.J. Synthesis of scalable and tunable slightly oxidized graphene via chemical vapor deposition. J. Colloid Interface Sci. 2017, 490, 844–849. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Qiu, J.; Zhang, K.; Shao, J.; Yan, L. Efficient preparation of high-quality graphene via anodic and cathodic simultaneous electrochemical exfoliation under the assistance of microwave. J. Colloid Interface Sci. 2022, 608, 1422–1431. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Zhang, L.; Guo, H.; Weng, Z.; Wang, J.; Wu, L.; Zheng, L. Graphene prepared by microfluidization process using induced parallel orientation strategy to enhance anti-corrosion of photocurable epoxy coatings. Prog. Org. Coat. 2023, 181, 107603. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Zhang, L.; Peng, S.; Weng, Z.; Wang, J.; Wu, L.; Zheng, L. Mechanical exfoliation assisted with carbon nanospheres to prepare a few-layer graphene for flexible strain sensor. Appl. Surf. Sci. 2023, 611, 155649. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, J.; Chen, J.; Yan, H.; Zhang, Y.; Huang, J.; Zhao, G.; Zhang, Q.; Liu, Y. Polydopamine/polyethyleneimine co-crosslinked graphene oxide for the enhanced tribological performance of epoxy resin coatings. J. Mater. Sci. Technol. 2023, 136, 13–20. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Methods for Tensile Properties of Plastic. ASTM: West Conshohocken, PA, USA, 2014.
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Unravelling the polydopamine mystery: Is the end in sight? Polym. Chem. 2019, 10, 5771–5777. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Wan, H.; Chen, L.; Zhou, H.; Chen, J. Chemical modification of graphene oxide to reinforce the corrosion protection performance of UV-curable polyurethane acrylate coating. Prog. Org. Coat. 2020, 141, 105547. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F.; Rizk, R.A.; Ahmed, M.M. Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceram. Int. 2012, 38, 177–188. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.; et al. Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte. ACS Nano 2018, 12, 3140–3148. [Google Scholar] [CrossRef]
- Ren, J.; Li, Q.; Yan, L.; Jia, L.; Huang, X.; Zhao, L.; Ran, Q.; Fu, M. Enhanced thermal conductivity of epoxy composites by introducing graphene@boron nitride nanosheets hybrid nanoparticles. Mater. Des. 2020, 191, 108663. [Google Scholar] [CrossRef]
- Sam, R.T.; Umakoshi, T.; Verma, P. Probing stacking configurations in a few layered MoS2 by low frequency Raman spectroscopy. Sci. Rep. 2020, 10, 21227. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Ye, X.; Tian, K.; Huang, W.; He, J.; Guo, Y.; Tian, X. Synergistic enhancement of anisotropic thermal transport flexible polymer composites filled with multi-layer graphene (mG) and mussel-inspiring modified hexagonal boron nitride (h-BN). Compos. Part A Appl. Sci. Manuf. 2018, 111, 12–22. [Google Scholar] [CrossRef]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and Morphology of Pristine Graphene/Polyacrylamide Gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, A.; Chuang, R.; Xu, L.; Han, C.; Li, H.; Xia, N. Preparation and Characterization of Egg White Protein-Based Composite Edible Coating Containing Thymol Nanoemulsion. Foods 2024, 13, 3809. [Google Scholar] [CrossRef]
- Lin, S.; Zhong, Y.; Zhao, X.; Sawada, T.; Li, X.; Lei, W.; Wang, M.; Serizawa, T.; Zhu, H. Synthetic Multifunctional Graphene Composites with Reshaping and Self-Healing Features via a Facile Biomineralization-Inspired Process. Adv. Mater. 2018, 30, 1803004. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, Z.; Dong, L.; Yang, Z.; Cheng, L.; Zhou, X.; Xing, Y.; Wen, J.; Chen, M. In-situ modulation of interactions between polyaniline and graphene oxide films to develop waterborne epoxy anticorrosion coatings. Prog. Org. Coat. 2019, 133, 106–116. [Google Scholar] [CrossRef]
- Jing, L.-C.; Wang, T.; Cao, W.-W.; Wen, J.-G.; Zhao, H.; Ning, Y.-J.; Yuan, X.-T.; Tian, Y.; Teng, L.-H.; Geng, H.-Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
- Son, S.Y.; Lee, G.; Wang, H.; Samson, S.; Wei, Q.; Zhu, Y.; You, W. Integrating charge mobility, stability and stretchability within conjugated polymer films for stretchable multifunctional sensors. Nat. Commun. 2022, 13, 2739. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Carpick, R.W.; Gumbsch, P.; Liu, X.Z.; Ding, X.; Sun, J.; Li, J. The evolving quality of frictional contact with graphene. Nature 2016, 539, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, X.; Zhang, C.; Chen, X.; Lin, S.; Sun, H.; Lin, C.-T.; Zhu, H.; Luo, J. Self-Assembled Graphene Film as Low Friction Solid Lubricant in Macroscale Contact. ACS Appl. Mater. Interfaces 2017, 9, 21554–21562. [Google Scholar] [CrossRef]
- Dou, X.; Koltonow, A.R.; He, X.; Jang, H.D.; Wang, Q.; Chung, Y.-W.; Huang, J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. USA 2016, 113, 1528–1533. [Google Scholar] [CrossRef]
- Tripathi, K.; Gyawali, G.; Lee, S.W. Graphene Coating via Chemical Vapor Deposition for Improving Friction and Wear of Gray Cast Iron at Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32336–32351. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Wang, Q. Enhancement of tribological properties of polymer composites reinforced by functionalized graphene. Compos. Part B Eng. 2017, 120, 83–91. [Google Scholar] [CrossRef]
- Mungse, H.P.; Khatri, O.P. Chemically Functionalized Reduced Graphene Oxide as a Novel Material for Reduction of Friction and Wear. J. Phys. Chem. C 2014, 118, 14394–14402. [Google Scholar] [CrossRef]
- Huang, T.; Xin, Y.; Li, T.; Nutt, S.; Su, C.; Chen, H.; Liu, P.; Lai, Z. Modified Graphene/Polyimide Nanocomposites: Reinforcing and Tribological Effects. ACS Appl. Mater. Interfaces 2013, 5, 4878–4891. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Wang, J.; Wu, L.; Zhang, X. Mechanically robust nanocomposites from screen-printable polymer/graphene nanosheet pastes. Nanoscale 2019, 11, 2343–2354. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, J.; Curtis, T.E.; Luo, S.; Huang, D.; Feng, Z.; Morales-Ferreiro, J.O.; Sapkota, P.; Lei, F.; Zhang, J.; et al. Exfoliated Graphene Leads to Exceptional Mechanical Properties of Polymer Composite Films. ACS Nano 2019, 13, 1097–1106. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, N.; Kuzhandaivel, D.; Chen, Y.; Wu, L.; Zheng, L. Microfluidization Preparation of Hybrid Graphene for Enhanced Wear Resistance of Coatings. Polymers 2025, 17, 824. https://doi.org/10.3390/polym17060824
Chen Q, Wang N, Kuzhandaivel D, Chen Y, Wu L, Zheng L. Microfluidization Preparation of Hybrid Graphene for Enhanced Wear Resistance of Coatings. Polymers. 2025; 17(6):824. https://doi.org/10.3390/polym17060824
Chicago/Turabian StyleChen, Qi, Na Wang, Dhandapani Kuzhandaivel, Yingxian Chen, Lixin Wu, and Longhui Zheng. 2025. "Microfluidization Preparation of Hybrid Graphene for Enhanced Wear Resistance of Coatings" Polymers 17, no. 6: 824. https://doi.org/10.3390/polym17060824
APA StyleChen, Q., Wang, N., Kuzhandaivel, D., Chen, Y., Wu, L., & Zheng, L. (2025). Microfluidization Preparation of Hybrid Graphene for Enhanced Wear Resistance of Coatings. Polymers, 17(6), 824. https://doi.org/10.3390/polym17060824






