Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation
Abstract
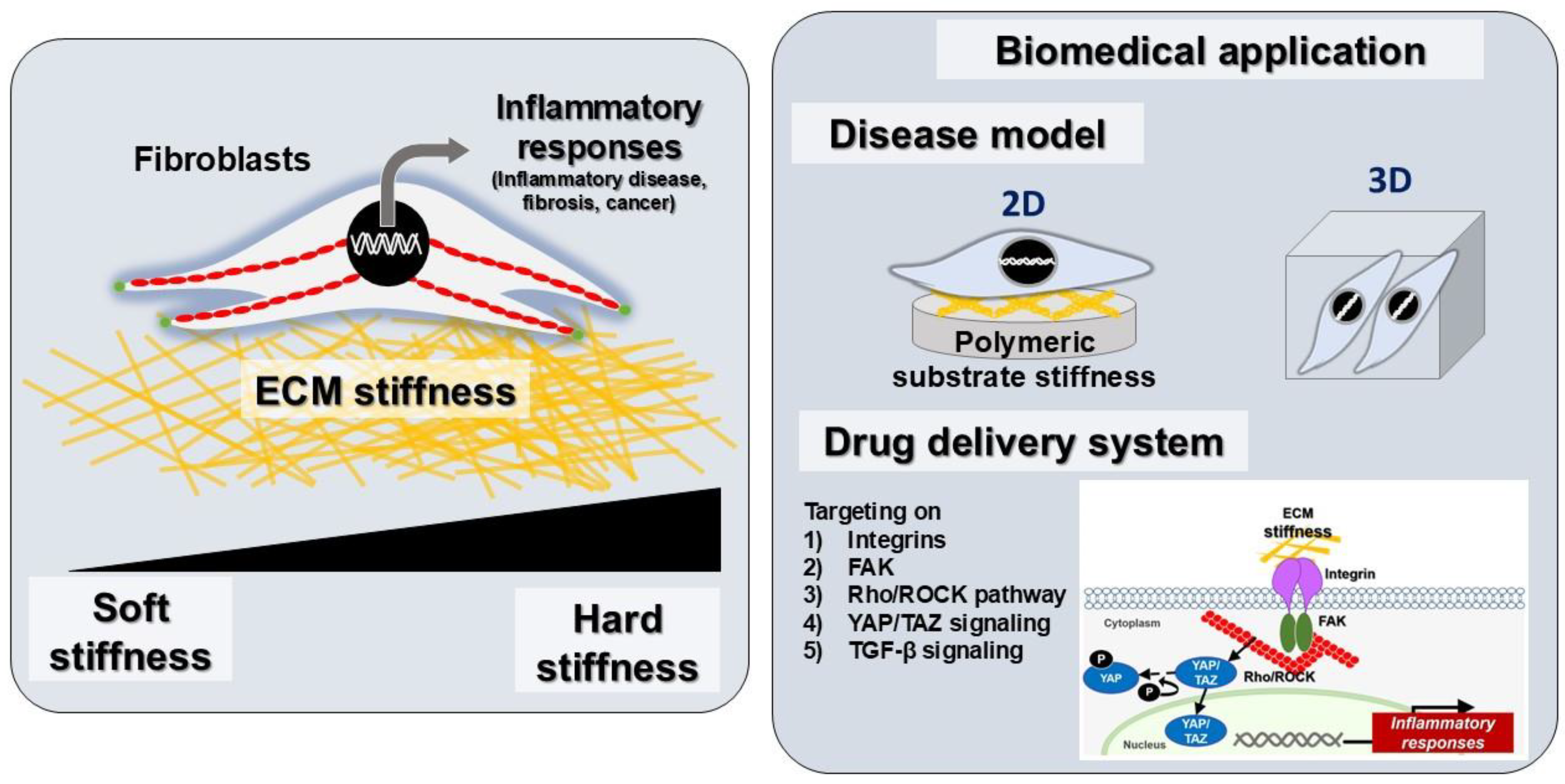
1. Introduction
2. Biomaterials for Mimicking Extracellular Matrix Stiffness in Tissue
2.1. Polydimethylsiloxane (PDMS)
2.2. Hydrogel
3. Surface Functionalization of Substrate Stiffness Materials
3.1. Type I Collagen
3.2. Polydopamine
3.3. Fibronectin
3.4. Gelatin
4. Cellular Mechanotransduction Pathway Mediated by ECM Stiffness
4.1. Integrin-Mediated Adhesion Complexes and Cytoskeletal Remodeling
4.2. Yes-Associated Protein (YAP) and Mechanotransduction
4.3. ECM Stiffness-Dependent Regulation of Transcription Factors and Signaling Pathways in Inflammatory Responses
5. Fibroblasts as Key Regulators of Inflammation and Tissue Remodeling: From Homeostasis to Disease
6. The Role of ECM Stiffness in Fibroblast Activation and Inflammatory Diseases
7. ECM Stiffness and Inflammatory Conditions Across Various Tissues
7.1. Cardiac Fibrosis
7.2. Pulmonary Fibrosis
7.3. Skin Fibrosis
7.4. Intestinal Fibrosis
7.5. Skeletal Muscle Fibrosis
7.6. Gingival and Periodontal Disease
| Tissue Mimetic | Cell Type | Substrate Material | Coating | Results | Refs. |
|---|---|---|---|---|---|
| Heart fibrosis | Cardiac fibroblasts | poly(ethylene glycol) (PEG) hydrogels | CRGDS peptide | Substrate stiffness regulates fibrotic gene and inflammatory response via NF-κB pathway | [130] |
| Heart fibrosis | Cardiac fibroblasts | polyacrylamide gels | Type I Collagen | Substrate stiffness influences the releasing of IL-6, IL-11, sIL-6R | [131] |
| Lung fibrosis | Lung fibroblasts | polyacrylamide gels | Type I Collagen | Substrate stiffness controls fibrotic marker and RANKL expression | [18] |
| Intestinal fibrosis | Colonic myofibroblasts | polyacrylamide gels | Type I Collagen | Substrate stiffness modulates profibrotic response of colonic myofibroblasts | [145] |
| Gingival tissue | Gingival fibroblasts | PDMS | Type I Collagen | Substrate stiffness controls pro-inflammatory cytokines and ECM production | [35] |
| Skeleton muscle fibrosis | Muscle stem cell | PDMS | Fibronectin | Substrate stiffness regulates TNF-related apoptosis-inducing ligand (TRAIL) pathway | [140] |
8. Targeting Fibroblast-Mediated Inflammation for Biomedical Applications
8.1. Disease Modeling Applications
- (1)
- Two-dimensional and three-dimensional tumor models
- (2)
- In vitro model for fibrosis tissue
8.2. Therapeutic Strategy for Fibroblast Inflammation in Mechanotransduction Mechanisms
- (1)
- Targeting integrins
- (2)
- Targeting the FAK pathway
- (3)
- Targeting the Rho/ROCK pathway
- (4)
- Targeting YAP/TAZ signaling
- (5)
- Targeting TGF-β signaling
8.3. ECM-Modifying Strategies:
- (1)
- Enzymatic ECM degradation
- (2)
- Biomaterials with tunable stiffness
- (3)
- Small molecules and increased ECM stiffness
- (4)
- Hydrogels in therapeutic applications
8.4. Diagnostic and Prognostic Tools for ECM Stiffness-Related Diseases
- (1)
- Magnetic resonance elastography (MRE)
- (2)
- Ultrasound elastography
- (3)
- Atomic force microscopy (AFM)
9. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Wipff, P.-J.; Rifkin, D.B.; Meister, J.-J.; Hinz, B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration?-Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef]
- Kim, H.S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.-W. Advanced materials technologies to unravel mechanobiological phenomena. Trends Biotechnol. 2024, 42, 179–196. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Choi, Y.; Seong, H. Nanoscale surface coatings and topographies for neural interfaces. Acta Biomater. 2024, 175, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef] [PubMed]
- Nattasit, P.; Niibe, K.; Yamada, M.; Ohori-Morita, Y.; Limraksasin, P.; Tiskratok, W.; Yamamoto, M.; Egusa, H. Stiffness-Tunable Hydrogel-Sandwich Culture Modulates the YAP-Mediated Mechanoresponse in Induced-Pluripotent Stem Cell Embryoid Bodies and Augments Cardiomyocyte Differentiation. Macromol. Biosci. 2023, 23, e2300021. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef]
- Blokland, K.E.C.; Nizamoglu, M.; Habibie, H.; Borghuis, T.; Schuliga, M.; Melgert, B.N.; Knight, D.A.; Brandsma, C.A.; Pouwels, S.D.; Burgess, J.K. Substrate stiffness engineered to replicate disease conditions influence senescence and fibrotic responses in primary lung fibroblasts. Front. Pharmacol. 2022, 13, 989169. [Google Scholar] [CrossRef]
- Meli, V.S.; Rowley, A.T.; Veerasubramanian, P.K.; Heedy, S.E.; Liu, W.F.; Wang, S.W. Modulation of Stiffness-Dependent Macrophage Inflammatory Responses by Collagen Deposition. ACS Biomater. Sci. Eng. 2024, 10, 2212–2223. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Tirella, A.; Mattei, G.; La Marca, M.; Ahluwalia, A.; Tirelli, N. Functionalized Enzyme-Responsive Biomaterials to Model Tissue Stiffening in vitro. Front. Bioeng. Biotechnol. 2020, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, H.E.; Erden, A.; Kuru Oz, D.; Unal, S.; Erden, I. Magnetic resonance elastography: Basic principles, technique, and clinical applications in the liver. Diagn. Interv. Radiol. 2018, 24, 328–335. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.C.C.; Brokken-Zijp, J.; Laven, J.; Huinink, H.P.; Reuvers, N.J.W.; Van, M.P.; de With, G. Influence of cross-linker concentration on the cross-linking of PDMS and the network structures formed. Polymer 2009, 50, 3955–3966. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, B.; Ziemer, K.S.; Barabino, G.A.; Carrier, R.L. Chemical and physical modifications to poly(dimethylsiloxane) surfaces affect adhesion of Caco-2 cells. J. Biomed. Mater. Res. A 2010, 93, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Lima, R.A. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids 2025, 10, 41. [Google Scholar] [CrossRef]
- Tiskratok, W.; Yamada, M.; Watanabe, J.; Kartikasari, N.; Kimura, T.; Egusa, H. Substrate stiffness controls proinflammatory responses in human gingival fibroblasts. Sci. Rep. 2023, 13, 1358. [Google Scholar] [CrossRef]
- Verma, B.K.; Chatterjee, A.; Kondaiah, P.; Gundiah, N. Substrate Stiffness Modulates TGF-beta Activation and ECM-Associated Gene Expression in Fibroblasts. Bioengineering 2023, 10, 998. [Google Scholar] [CrossRef]
- Sip, C.G.; Folch, A. Stable chemical bonding of porous membranes and poly(dimethylsiloxane) devices for long-term cell culture. Biomicrofluidics 2014, 8, 036504. [Google Scholar] [CrossRef]
- Seethapathy, S.; Gorecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta 2012, 750, 48–62. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, e2304506. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Chen, Q. Influences of heating temperature on mechanical properties of polydimethylsiloxane. Sens. Actuators A Phys. 2009, 151, 42–45. [Google Scholar] [CrossRef]
- Mayer, M.; Rabindranath, R.; Börner, J.; Hörner, E.; Bentz, A.; Salgado, J.; Han, H.; Böse, H.; Probst, J.; Shamonin, M.; et al. Ultra-Soft PDMS-Based Magnetoactive Elastomers as Dynamic Cell Culture Substrata. PLoS ONE 2013, 8, e76196. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. Engl. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nikov, R.G.; Nikov, R.G.; Nedyalkov, N.N.; Stoyanchov, T.R.; Fukata, N.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; et al. Optical properties of polydimethylsiloxane (PDMS) during nanosecond laser processing. Appl. Surf. Sci. 2016, 374, 96–103. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- White, E.S. Lung extracellular matrix and fibroblast function. Ann. Am. Thorac. Soc. 2015, 12, S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012, 3, 792. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Regehr, K.J.; Domenech, M.; Koepsel, J.T.; Carver, K.C.; Ellison-Zelski, S.J.; Murphy, W.L.; Schuler, L.A.; Alarid, E.T.; Beebe, D.J. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab. Chip 2009, 9, 2132–2139. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Berthier, E.; Young, E.W.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab. Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Li, T.-Y.; Yang, H.-C.; Ding, M.-H.; Chen, L.-J.; Yu, S.-Y.; Meng, X.-S.; Jin, J.-J.; Sun, S.-Z.; Zhang, J.; et al. Design and Fabrication of Viscoelastic Hydrogels as Extracellular Matrix Mimicry for Cell Engineering. Chem. Bio Eng. 2024, 1, 916–933. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef]
- Yang, D.H.; Jung, S.; Kim, J.Y.; Lee, N.Y. Fabrication of a Cell-Friendly Poly(dimethylsiloxane) Culture Surface via Polydopamine Coating. Micromachines 2022, 13, 1122. [Google Scholar] [CrossRef]
- Jiao, J.; Sun, L.; Guo, Z.; Hou, S.; Holyst, R.; Lu, Y.; Feng, X. Antibacterial and anticancer PDMS surface for mammalian cell growth using the Chinese herb extract paeonol(4-methoxy-2-hydroxyacetophenone). Sci. Rep. 2016, 6, 38973. [Google Scholar] [CrossRef]
- Etezadi, F.; Le, M.N.T.; Shahsavarani, H.; Alipour, A.; Moazzezy, N.; Samani, S.; Amanzadeh, A.; Pahlavan, S.; Bonakdar, S.; Shokrgozar, M.A.; et al. Optimization of a PDMS-Based Cell Culture Substrate for High-Density Human-Induced Pluripotent Stem Cell Adhesion and Long-Term Differentiation into Cardiomyocytes under a Xeno-Free Condition. ACS Biomater. Sci. Eng. 2022, 8, 2040–2052. [Google Scholar] [CrossRef]
- Lee, M.; Chu, K.; Chakraborty, M.; Kotoulas, N.; Akbari, M.; Goh, C.; Clemente-Casares, X.; Winer, D.A.; Shrestha, A.; Tsai, S. PDMS hydrogel-coated tissue culture plates for studying the impact of substrate stiffness on dendritic cell function. STAR Protoc. 2022, 3, 101233. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Oh, J.M.; Kwon, K.W.; Huh, D. Polydopamine-Based Interfacial Engineering of Extracellular Matrix Hydrogels for the Construction and Long-Term Maintenance of Living Three-Dimensional Tissues. ACS Appl. Mater. Interfaces 2019, 11, 23919–23925. [Google Scholar] [CrossRef]
- Kasálková, N.S.; Juřicová, V.; Fajstavr, D.; Frýdlová, B.; Rimpelová, S.; Švorčík, V.; Slepička, P. Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance. Int. J. Mol. Sci. 2024, 25, 2779. [Google Scholar] [CrossRef]
- Stanton, A.E.; Tong, X.; Yang, F. Varying solvent type modulates collagen coating and stem cell mechanotransduction on hydrogel substrates. APL Bioeng. 2019, 3, 036108. [Google Scholar] [CrossRef]
- Harati, J.; Tao, X.; Shahsavarani, H.; Du, P.; Galluzzi, M.; Liu, K.; Zhang, Z.; Shaw, P.; Shokrgozar, M.A.; Pan, H.; et al. Polydopamine-Mediated Protein Adsorption Alters the Epigenetic Status and Differentiation of Primary Human Adipose-Derived Stem Cells (hASCs). Front. Bioeng. Biotechnol. 2022, 10, 934179. [Google Scholar] [CrossRef]
- Hernandez-Hatibi, S.; Guerrero, P.E.; Garcia-Aznar, J.M.; Garcia-Gareta, E. Polydopamine Interfacial Coating for Stable Tumor-on-a-Chip Models: Application for Pancreatic Ductal Adenocarcinoma. Biomacromolecules 2024, 25, 5169–5180. [Google Scholar] [CrossRef]
- Dabaghi, M.; Shahriari, S.; Saraei, N.; Da, K.; Chandiramohan, A.; Selvaganapathy, P.R.; Hirota, J.A. Surface Modification of PDMS-Based Microfluidic Devices with Collagen Using Polydopamine as a Spacer to Enhance Primary Human Bronchial Epithelial Cell Adhesion. Micromachines 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Hashimoto, Y.; Honda, Y.; Li, P.; Yao, Y.; Zhao, Z.; Matsumoto, N. Accelerated construction of an in vitro model of human periodontal ligament tissue: Vacuum plasma combined with fibronectin coating and a polydimethylsiloxane matrix. PeerJ 2019, 7, e7036. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.; Pang, S.W. Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms. Int. J. Mol. Sci. 2023, 24, 12409. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Yu, Z.T.; Kamei, K.; Takahashi, H.; Shu, C.J.; Wang, X.; He, G.W.; Silverman, R.; Radu, C.G.; Witte, O.N.; Lee, K.B.; et al. Integrated microfluidic devices for combinatorial cell-based assays. Biomed. Microdevices 2009, 11, 547–555. [Google Scholar] [CrossRef]
- Toworfe, G.K.; Composto, R.J.; Adams, C.S.; Shapiro, I.M.; Ducheyne, P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J. Biomed. Mater. Res. A 2004, 71, 449–461. [Google Scholar] [CrossRef]
- Joo, H.; Park, J.; Sutthiwanjampa, C.; Kim, H.; Bae, T.; Kim, W.; Choi, J.; Kim, M.; Kang, S.; Park, H. Surface Coating with Hyaluronic Acid-Gelatin-Crosslinked Hydrogel on Gelatin-Conjugated Poly(dimethylsiloxane) for Implantable Medical Device-Induced Fibrosis. Pharmaceutics 2021, 13, 269. [Google Scholar] [CrossRef]
- Kemkemer, R.; Zenghao, Z.; Linxiao, Y.; Athanasopulu, K.; Frey, K.; Cui, Z.; Su, H.; Luo, L. Surface modification of Polydimethylsiloxane by hydrogels for microfluidic applications. Curr. Dir. Biomed. Eng. 2019, 5, 93–96. [Google Scholar] [CrossRef]
- Lee, J.; Song, B.; Subbiah, R.; Chung, J.J.; Choi, U.H.; Park, K.; Kim, S.H.; Oh, S.J. Effect of chain flexibility on cell adhesion: Semi-flexible model-based analysis of cell adhesion to hydrogels. Sci. Rep. 2019, 9, 2463. [Google Scholar] [CrossRef]
- Fu, J.; Chuah, Y.J.; Liu, J.; Tan, S.Y.; Wang, D.A. Respective Effects of Gelatin-Coated Polydimethylsiloxane (PDMS) Substrates on Self-renewal and Cardiac Differentiation of Induced Pluripotent Stem Cells (iPSCs). ACS Biomater. Sci. Eng. 2018, 4, 4321–4330. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Ji, Y.R.; Hsu, Y.H.; Liao, F.C.; Kao, N.T.; Huang, A.P.; Young, T.H. Gelatin-Based Hydrogel for Three-Dimensional Neuron Culture Application. ACS Omega 2023, 8, 45288–45300. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, A.; Orr, A.W.; Tzima, E.; Schwartz, M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004, 279, 12001–12004. [Google Scholar] [CrossRef]
- Janota, C.S.; Calero-Cuenca, F.J.; Gomes, E.R. The role of the cell nucleus in mechanotransduction. Curr. Opin. Cell Biol. 2020, 63, 204–211. [Google Scholar] [CrossRef]
- Katoh, K.; Kano, Y.; Amano, M.; Onishi, H.; Kaibuchi, K.; Fujiwara, K. Rho-kinase–mediated contraction of isolated stress fibers. J. Cell Biol. 2001, 153, 569–584. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.-W.; Summers, L.J.; Dorn, G.W.; Wei, L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 2008, 44, 551–560. [Google Scholar] [CrossRef]
- Htwe, S.S.; Cha, B.H.; Yue, K.; Khademhosseini, A.; Knox, A.J.; Ghaemmaghami, A.M. Role of Rho-Associated Coiled-Coil Forming Kinase Isoforms in Regulation of Stiffness-Induced Myofibroblast Differentiation in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 772–783. [Google Scholar] [CrossRef]
- Guan, J.L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997, 29, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. Biomed. Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Tan, X.; Yan, Y.; Song, B.; Zhu, S.; Mei, Q.; Wu, K. Focal adhesion kinase: From biological functions to therapeutic strategies. Exp. Hematol. Oncol. 2023, 12, 83. [Google Scholar] [CrossRef]
- Wang, W.; Lollis, E.M.; Bordeleau, F.; Reinhart-King, C.A. Matrix stiffness regulates vascular integrity through focal adhesion kinase activity. FASEB J. 2019, 33, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.K.; Cheng, Y.; Liang Cheng, M.; Yu, L.; Mu, M.; Li, H.; Liu, Y.; Zhang, B.; Yao, Y.; Guo, H.; et al. Focal Adhesion Kinase Regulates Fibroblast Migration via Integrin beta-1 and Plays a Central Role in Fibrosis. Sci. Rep. 2016, 6, 19276. [Google Scholar] [CrossRef]
- Greenberg, R.S.; Bernstein, A.M.; Benezra, M.; Gelman, I.H.; Taliana, L.; Masur, S.K. FAK-dependent regulation of myofibroblast differentiation. FASEB J. 2006, 20, 1006–1008. [Google Scholar] [CrossRef]
- Kim, E.; Riehl, B.D.; Bouzid, T.; Yang, R.; Duan, B.; Donahue, H.J.; Lim, J.Y. YAP mechanotransduction under cyclic mechanical stretch loading for mesenchymal stem cell osteogenesis is regulated by ROCK. Front. Bioeng. Biotechnol. 2023, 11, 1306002. [Google Scholar] [CrossRef]
- Emon, B.; Joy, M.S.H.; Lalonde, L.; Ghrayeb, A.; Doha, U.; Ladehoff, L.; Brockstein, R.; Saengow, C.; Ewoldt, R.H.; Saif, M.T.A. Nuclear deformation regulates YAP dynamics in cancer associated fibroblasts. Acta Biomater. 2024, 173, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Yasuda, M.; Harada, I.; Mizutani, T.; Kawabata, K.; Haga, H. Substrate stiffness regulates temporary NF-kappaB activation via actomyosin contractions. Exp. Cell Res. 2013, 319, 2916–2927. [Google Scholar] [CrossRef]
- Marozzi, M.; Parnigoni, A.; Negri, A.; Viola, M.; Vigetti, D.; Passi, A.; Karousou, E.; Rizzi, F. Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 8102. [Google Scholar] [CrossRef]
- Sero, J.E.; Sailem, H.Z.; Ardy, R.C.; Almuttaqi, H.; Zhang, T.; Bakal, C. Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. Mol. Syst. Biol. 2015, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Kohan, M.; Muro, A.F.; White, E.S.; Berkman, N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010, 24, 4503–4512. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Swaminathan, V.; Garcia-Mata, R.; O'Brien, E.T.; Superfine, R.; Burridge, K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011, 13, 722–727. [Google Scholar] [CrossRef]
- Secker, G.A.; Shortt, A.J.; Sampson, E.; Schwarz, Q.P.; Schultz, G.S.; Daniels, J.T.T. GFbeta stimulated re-epithelialisation is regulated by CTGF and Ras/MEK/ERK signalling. Exp. Cell Res. 2008, 314, 131–142. [Google Scholar] [CrossRef]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Buechler, M.B.; Fu, W.; Turley, S.J. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 2021, 54, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef]
- Buckley, C.D.; Pilling, D.; Lord, J.M.; Akbar, A.N.; Scheel-Toellner, D.; Salmon, M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001, 22, 199–204. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.H. Biology of fibroblasts and myofibroblasts. Proc. Am. Thorac. Soc. 2008, 5, 334–337. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral. Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef]
- Alfonso Garcia, S.L.; Parada-Sanchez, M.T.; Arboleda Toro, D. The phenotype of gingival fibroblasts and their potential use in advanced therapies. Eur. J. Cell Biol. 2020, 99, 151123. [Google Scholar] [CrossRef]
- Wang, K.; Wen, D.; Xu, X.; Zhao, R.; Jiang, F.; Yuan, S.; Zhang, Y.; Gao, Y.; Li, Q. Extracellular matrix stiffness-The central cue for skin fibrosis. Front. Mol. Biosci. 2023, 10, 1132353. [Google Scholar] [CrossRef]
- You, E.; Ko, P.; Jeong, J.; Keum, S.; Kim, J.W.; Seo, Y.J.; Song, W.K.; Rhee, S. Dynein-mediated nuclear translocation of yes-associated protein through microtubule acetylation controls fibroblast activation. Cell Mol. Life Sci. 2020, 77, 4143–4161. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X. Transforming Growth Factor-beta Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Tiskratok, W.; Yamada, M.; Watanabe, J.; Pengyu, Q.; Kimura, T.; Egusa, H. Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness. Int. J. Mol. Sci. 2023, 24, 8959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, L.; Qiang, C.; Chen, C.; Shen, Z.; Ding, F.; Lv, L.; Zhu, T.; Lu, Y.; Cui, X. The role of matrix metalloproteinase 9 in fibrosis diseases and its molecular mechanisms. Biomed. Pharmacother. 2024, 171, 116116. [Google Scholar] [CrossRef]
- Guo, T.; He, C.; Venado, A.; Zhou, Y. Extracellular Matrix Stiffness in Lung Health and Disease. Compr. Physiol. 2022, 12, 3523–3558. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Niu, L.; Jia, Y.; Wu, M.; Liu, H.; Feng, Y.; Hu, Y.; Zhang, X.; Gao, D.; Xu, F.; Huang, G. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT(1) R. J. Cell Physiol. 2020, 235, 8345–8357. [Google Scholar] [CrossRef]
- Han, Y.; Shao, Z.; Zhang, Y.; Zhao, H.; Sun, Z.; Yang, C.; Tang, H.; Han, Y.; Gao, C. 3D matrix stiffness modulation unveils cardiac fibroblast phenotypic switching. Sci. Rep. 2024, 14, 17015. [Google Scholar] [CrossRef]
- Felisbino, M.B.; Rubino, M.; Travers, J.G.; Schuetze, K.B.; Lemieux, M.E.; Anseth, K.S.; Aguado, B.A.; McKinsey, T.A. Substrate stiffness modulates cardiac fibroblast activation, senescence, and proinflammatory secretory phenotype. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H61–H73. [Google Scholar] [CrossRef]
- Galdyszynska, M.; Bobrowska, J.; Lekka, M.; Radwanska, P.; Piera, L.; Szymanski, J.; Drobnik, J. The stiffness-controlled release of interleukin-6 by cardiac fibroblasts is dependent on integrin alpha2beta1. J. Cell Mol. Med. 2020, 24, 13853–13862. [Google Scholar] [CrossRef]
- Ebrahimighaei, R.; Tarassova, N.; Bond, S.C.; McNeill, M.C.; Hathway, T.; Vohra, H.; Newby, A.C.; Bond, M. Extracellular matrix stiffness controls cardiac fibroblast proliferation via the nuclear factor-Y (NF-Y) transcription factor. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119640. [Google Scholar] [CrossRef]
- Deng, Z.; Fear, M.W.; Suk Choi, Y.; Wood, F.M.; Allahham, A.; Mutsaers, S.E.; Prele, C.M. The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2020, 126, 105802. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Ito, S.; Takahashi, K.; Furuya, K.; Kondo, M.; Sokabe, M.; Hasegawa, Y. Matrix stiffness regulates migration of human lung fibroblasts. Physiol. Rep. 2017, 5, e13281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, L.; Wang, A.; Liu, J.; Huang, X.; Zan, T. Positive feedback loops between fibroblasts and the mechanical environment contribute to dermal fibrosis. Matrix Biol. 2023, 121, 1–21. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Onfroy-Roy, L.; Hamel, D.; Foncy, J.; Malaquin, L.; Ferrand, A. Extracellular Matrix Mechanical Properties and Regulation of the Intestinal Stem Cells: When Mechanics Control Fate. Cells 2020, 9, 26299. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohns Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef]
- Alakhdar, A.A.; Sivakumar, S.; Kopchak, R.M.; Hunter, A.N.; Ambrosio, F.; Washburn, N.R. Age-Related ECM Stiffness Mediates TRAIL Activation in Muscle Stem Cell Differentiation. Adv. Biol. 2024, 8, 2400334. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478. [Google Scholar] [CrossRef]
- Johnson, L.A.; Rodansky, E.S.; Sauder, K.L.; Horowitz, J.C.; Mih, J.D.; Tschumperlin, D.J.; Higgins, P.D. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef]
- Hinz, B. Mechanical aspects of lung fibrosis: A spotlight on the myofibroblast. Proc. Am. Thorac. Soc. 2012, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lagares, D.; Santos, A.; Grasberger, P.E.; Liu, F.; Probst, C.K.; Rahimi, R.A.; Sakai, N.; Kuehl, T.; Ryan, J.; Bhola, P.; et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 2017, 9, eaal3765. [Google Scholar] [CrossRef]
- Chu, L.; Xie, D.; Xu, D. Epigenetic Regulation of Fibroblasts and Crosstalk between Cardiomyocytes and Non-Myocyte Cells in Cardiac Fibrosis. Biomolecules 2023, 13, 1382. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers (Basel) 2022, 14, 1049. [Google Scholar] [CrossRef]
- Jahin, I.; Phillips, T.; Marcotti, S.; Gorey, M.A.; Cox, S.; Parsons, M. Extracellular matrix stiffness activates mechanosensitive signals but limits breast cancer cell spheroid proliferation and invasion. Front. Cell Dev. Biol. 2023, 11, 1292775. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development -focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Jin, A.; Jiang, S.; Lei, L.; Liu, L. Biomaterial-assisted organoid technology for disease modeling and drug screening. Mater. Today Bio 2025, 30, 101438. [Google Scholar] [CrossRef]
- Sahan, A.Z.; Baday, M.; Patel, C.B. Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives. Gels 2022, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.; Bennet, T.; Rowe, E.M.; Anwer, M.; Wellington, C.L.; Cheung, K.C. Review of Design Considerations for Brain-on-a-Chip Models. Micromachines 2021, 12, 441. [Google Scholar] [CrossRef]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinkovic, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, L.; Wang, S.; Gong, J.; Selvaraj, J.N.; Ye, L.; Chen, H.; Zhang, Y.; Wang, G.; Song, W.; et al. Engineered model of heart tissue repair for exploring fibrotic processes and therapeutic interventions. Nat. Commun. 2024, 15, 7996. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Sui, A.; Zhong, Y.; Demetriades, A.M.; Shen, J.; Su, T.; Yao, Y.; Gao, Y.; Zhu, Y.; Shen, X.; Xie, B. ATN-161 as an Integrin α5β1 Antagonist Depresses Ocular Neovascularization by Promoting New Vascular Endothelial Cell Apoptosis. Med. Sci. Monit. 2018, 24, 5860–5873. [Google Scholar] [CrossRef]
- Bellani, S.; Molyneaux, P.L.; Maher, T.M.; Spagnolo, P. Potential of αvβ6 and αvβ1 integrin inhibition for treatment of idiopathic pulmonary fibrosis. Expert. Opin. Ther. Targets 2024, 28, 575–585. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Nishimichi, N. New Therapeutic Targets for Hepatic Fibrosis in the Integrin Family, α8β1 and α11β1, Induced Specifically on Activated Stellate Cells. Int. J. Mol. Sci. 2021, 22, 12794. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Wang, S.-Q.; Shang, H.-L.; Lv, H.-F.; Chen, B.-B.; Gao, S.-G.; Chen, X.-B. Roles and inhibitors of FAK in cancer: Current advances and future directions. Front. Pharmacol. 2024, 15, 1274209. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Zhen, Y.-Y.; Tsai, Y.-C.; Chuang, C.-H.; Hsiao, M.; Huang, M.-S.; Yang, C.-J. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.-Y.E.; Lim, S.-T.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Pozzato, C.; Outeiro-Pinho, G.; Galiè, M.; Ramadori, G.; Konstantinidou, G. ERK5 suppression overcomes FAK inhibitor resistance in mutant KRAS-driven non-small cell lung cancer. EMBO Mol. Med. 2024, 16, 2402–2426. [Google Scholar] [CrossRef]
- Baba, I.; Egi, Y.; Utsumi, H.; Kakimoto, T.; Suzuki, K. Inhibitory effects of fasudil on renal interstitial fibrosis induced by unilateral ureteral obstruction. Mol. Med. Rep. 2015, 12, 8010–8020. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase signaling in cancer progression and dissemination. Physiol. Rev. 2022, 102, 455–510. [Google Scholar] [CrossRef]
- Rao, J.; Ye, Z.; Tang, H.; Wang, C.; Peng, H.; Lai, W.; Li, Y.; Huang, W.; Lou, T. The RhoA/ROCK Pathway Ameliorates Adhesion and Inflammatory Infiltration Induced by AGEs in Glomerular Endothelial Cells. Sci. Rep. 2017, 7, 39727. [Google Scholar] [CrossRef]
- Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes. Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Battilana, G.; Cordenonsi, M.; Piccolo, S. YAP/TAZ as therapeutic targets in cancer. Curr. Opin. Pharmacol. 2016, 29, 26–33. [Google Scholar] [CrossRef]
- Meli, V.S.; Veerasubramanian, P.K.; Downing, T.L.; Wang, W.; Liu, W.F. Mechanosensation to inflammation: Roles for YAP/TAZ in innate immune cells. Sci. Signal. 2023, 16, eadc9656. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ju, S.; Lee, J.; Kim, H.R.; Sub, Y.; Park, D.J.; Park, S.; Kwon, D.; Kang, H.G.; Shin, J.E.; et al. Noncanonical role of Golgi-associated macrophage TAZ in chronic inflammation and tumorigenesis. Sci. Adv. 2025, 11, eadq2395. [Google Scholar] [CrossRef]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Devel Ther. 2015, 9, 4479–4499. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccin. Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Chan, M.K.-K.; Chan, E.L.-Y.; Ji, Z.Z.; Chan, A.S.-W.; Li, C.; Leung, K.-t.; To, K.-F.; Tang, P.M.-K. Transforming growth factor-β signaling: From tumor microenvironment to anticancer therapy. Explor. Target. Anti-Tumor Ther. 2023, 4, 316–343. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Bhati, A.; Fageeh, H.; Ibraheem, W.; Fageeh, H.; Chopra, H.; Panda, S. Role of hyaluronic acid in periodontal therapy (Review). Biomed. Rep. 2022, 17, 91. [Google Scholar] [CrossRef]
- Assi, M.M.; Grawish, M.E.; Elsabaa, H.M.; Helal, M.E.; Ezzat, S.K. Therapeutic potential of hyaluronic acid hydrogel combined with bone marrow stem cells-conditioned medium on arthritic rats’ TMJs. Sci. Rep. 2024, 14, 26828. [Google Scholar] [CrossRef]
- Talaat, W.M.; Haider, M.; Kawas, S.A.; Kandil, N.G.; Harding, D.R. Chitosan-Based Thermosensitive Hydrogel for Controlled Drug Delivery to the Temporomandibular Joint. J. Craniofac Surg. 2016, 27, 735–740. [Google Scholar] [CrossRef]
- Diez-Guardia, V.; Tian, Y.; Guo, Y.; Li, J.; Cui, S.; Dreiss, C.A.; Gentleman, E.; Wang, X. Controlled Release of Human Dental Pulp Stem Cell-Derived Exosomes from Hydrogels Attenuates Temporomandibular Joint Osteoarthritis. Adv. Healthc. Mater. 2024, e2402923. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sui, B.; Liu, X.; Sun, J. A bioinspired and high-strengthed hydrogel for regeneration of perforated temporomandibular joint disc: Construction and pleiotropic immunomodulatory effects. Bioact. Mater. 2023, 25, 701–715. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J. A mini-invasive injectable hydrogel for temporomandibular joint osteoarthritis: Its pleiotropic effects and multiple pathways in cartilage regeneration. Biomater. Adv. 2024, 169, 214162. [Google Scholar] [CrossRef]
- Bensamoun, S.F.; Wang, L.; Robert, L.; Charleux, F.; Latrive, J.-P.; Ho Ba Tho, M.-C. Measurement of liver stiffness with two imaging techniques: Magnetic resonance elastography and ultrasound elastometry. J. Magn. Reson. Imaging 2008, 28, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Qiu, B.S. The Advance of Magnetic Resonance Elastography in Tumor Diagnosis. Front. Oncol. 2021, 11, 722703. [Google Scholar] [CrossRef]
- Wang, K.; Ning, S.; Zhang, S.; Jiang, M.; Huang, Y.; Pei, H.; Li, M.; Tan, F. Extracellular matrix stiffness regulates colorectal cancer progression via HSF4. J. Exp. Clin. Cancer Res. 2025, 44, 30. [Google Scholar] [CrossRef]
- Oglat, A.A.; Abukhalil, T. Ultrasound Elastography: Methods, Clinical Applications, and Limitations: A Review Article. Appl. Sci. 2024, 14, 4308. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom Radiol 2018, 43, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Cloutier, G.; Szeverenyi, N.M.; Sirlin, C.B. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 1, Principles and Techniques. AJR Am. J. Roentgenol. 2015, 205, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Sidhu, P.S. Ultrasound-based liver elastography: Current results and future perspectives. Abdom Radiol 2020, 45, 3463–3472. [Google Scholar] [CrossRef]
- Cho, D.H.; Aguayo, S.; Cartagena-Rivera, A.X. Atomic force microscopy-mediated mechanobiological profiling of complex human tissues. Biomaterials 2023, 303, 122389. [Google Scholar] [CrossRef] [PubMed]




| Property | PDMS | Hydrogel | Refs. |
|---|---|---|---|
| Stiffness range | 5 kPa–10 MPa | 0.1–100 kPa (cell culture plate) | [26,41,42] |
| Tunability | Stiffness is adjusted by varying crosslinker ratio and curing conditions | Stiffness is tuned by adjusting polymer concentration and crosslinking density | [33,59] |
| Biocompatibility | Generally biocompatible but requires surface functionalization for cell adhesion | Naturally biocompatible and can incorporate bioactive molecules (e.g., peptides, growth factors) | [9,49,60] |
| Degradability | Non-degradable | Degradable or non-degradable depending on polymer composition and crosslinking | [30,61] |
| Hydration | Low (hydrophobic) | High (hydrophilic) | [47,62] |
| Applications | Microfluidics, cell mechanobiology, and static ECM stiffness models | Dynamic ECM stiffness models, tissue engineering, and drug delivery | [56,63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiskratok, W.; Chuinsiri, N.; Limraksasin, P.; Kyawsoewin, M.; Jitprasertwong, P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers 2025, 17, 822. https://doi.org/10.3390/polym17060822
Tiskratok W, Chuinsiri N, Limraksasin P, Kyawsoewin M, Jitprasertwong P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers. 2025; 17(6):822. https://doi.org/10.3390/polym17060822
Chicago/Turabian StyleTiskratok, Watcharaphol, Nontawat Chuinsiri, Phoonsuk Limraksasin, Maythwe Kyawsoewin, and Paiboon Jitprasertwong. 2025. "Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation" Polymers 17, no. 6: 822. https://doi.org/10.3390/polym17060822
APA StyleTiskratok, W., Chuinsiri, N., Limraksasin, P., Kyawsoewin, M., & Jitprasertwong, P. (2025). Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers, 17(6), 822. https://doi.org/10.3390/polym17060822






