Fluorescent Molecular Imprinted Sensor Based on Carbon Quantum Dot for Nitrofen Detection in Water Sample
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments
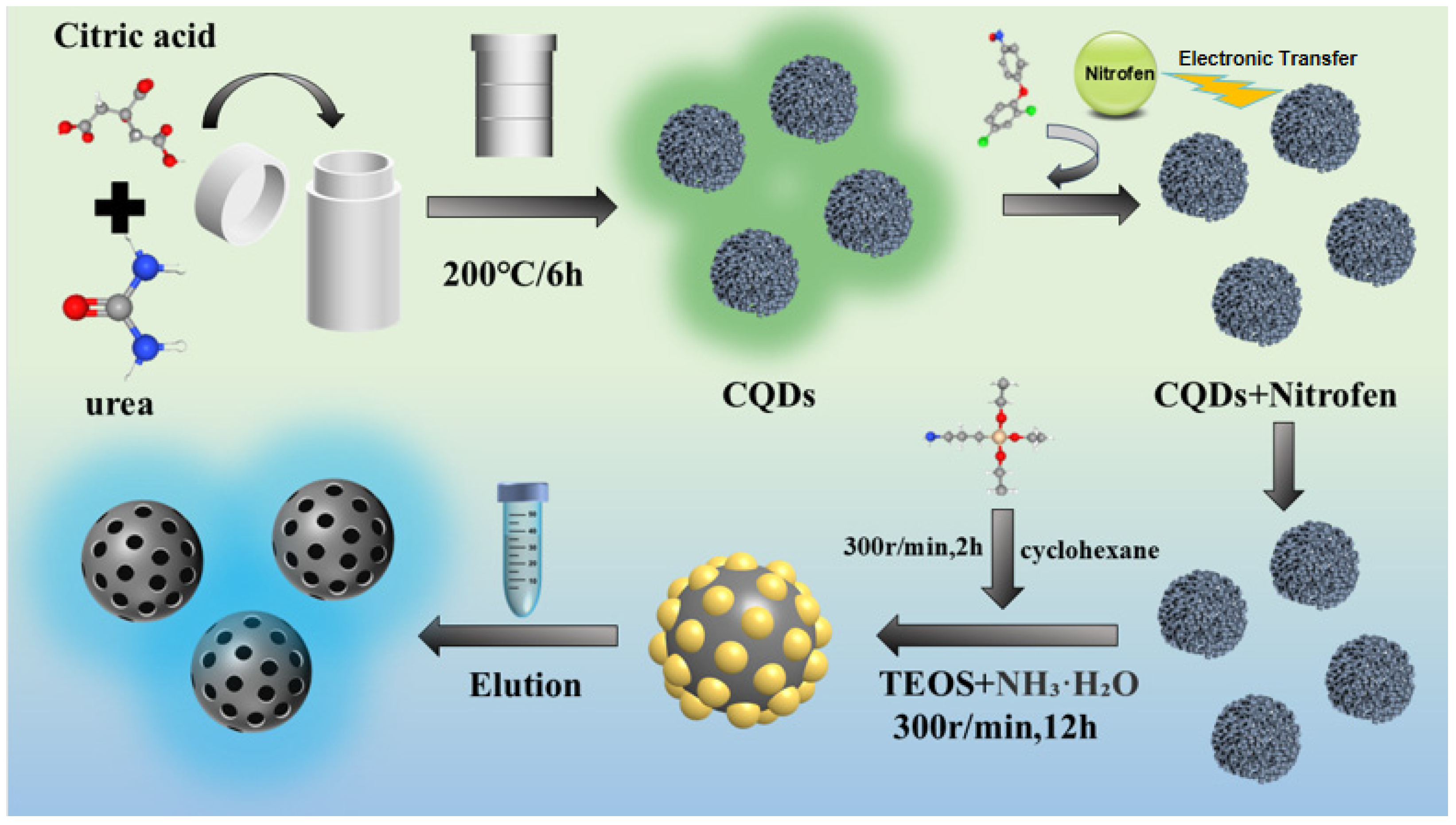
2.3. Synthesis of CQDs
2.4. Synthesis of CQDs@MIPs and NIPs
2.5. Fluorescence Determination for Nitrofen
2.6. Determination of Nitrofen in Water Samples
3. Results and Discussion
3.1. Synthetic System Optimization of CQDs and CQDs@MIPs
3.2. Characterization of CQDs and CQDs@MIPs
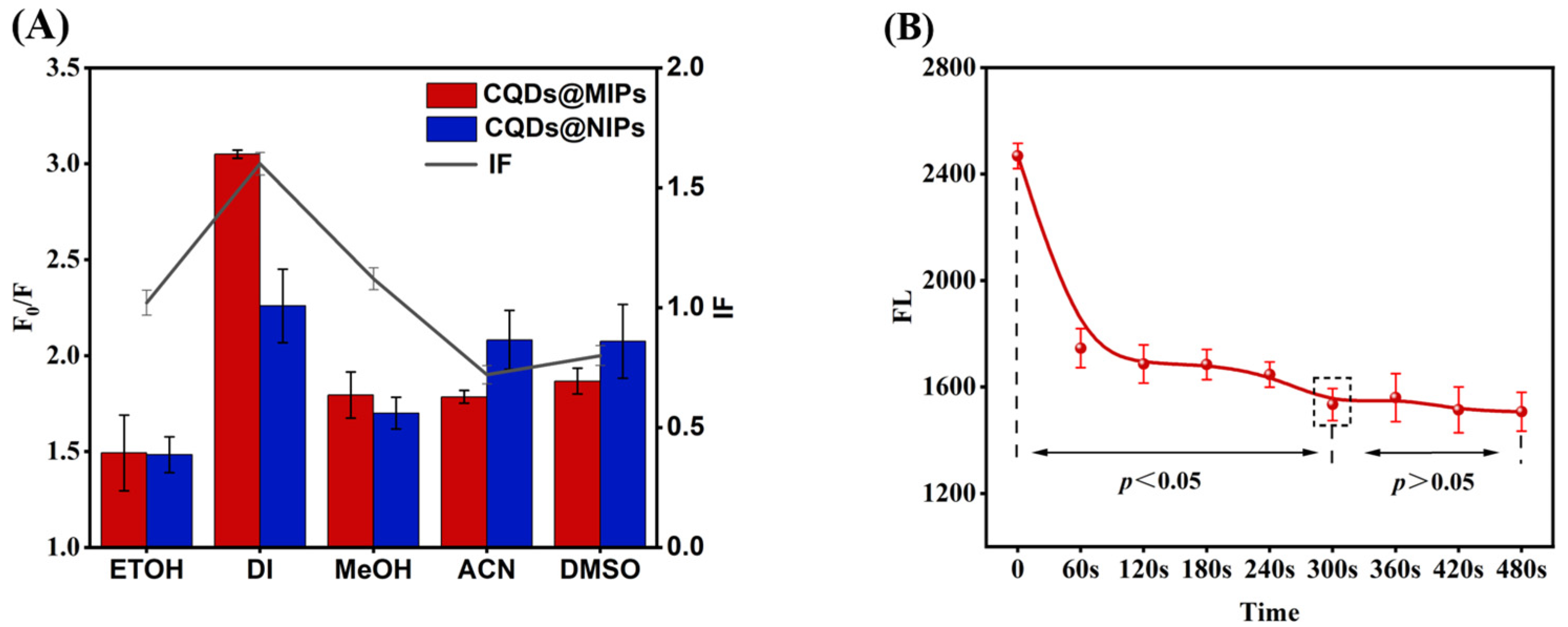
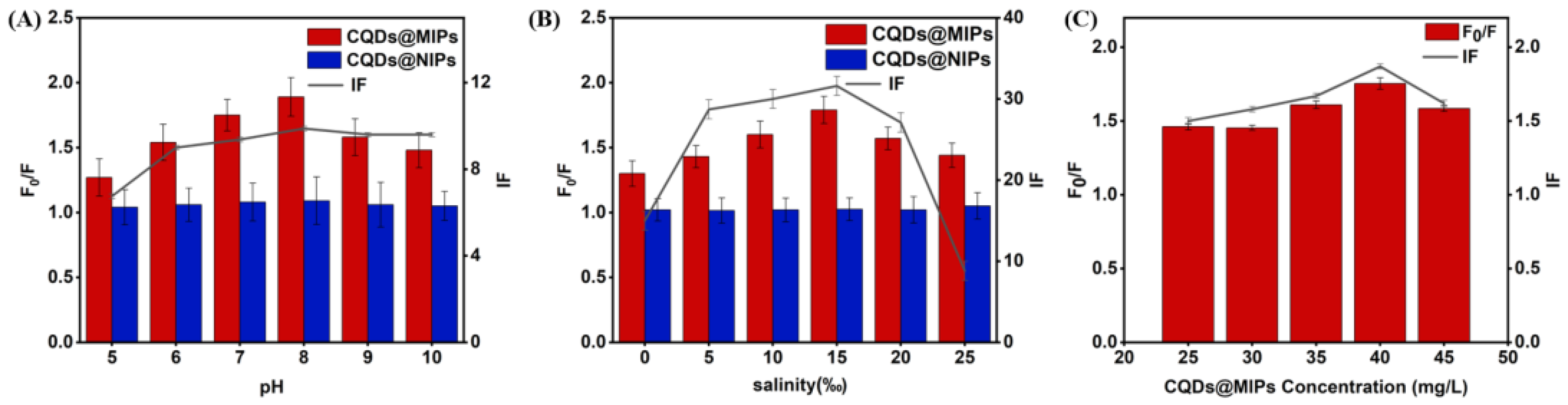
3.3. Optimization of CQDs@MIPs Detection Conditions
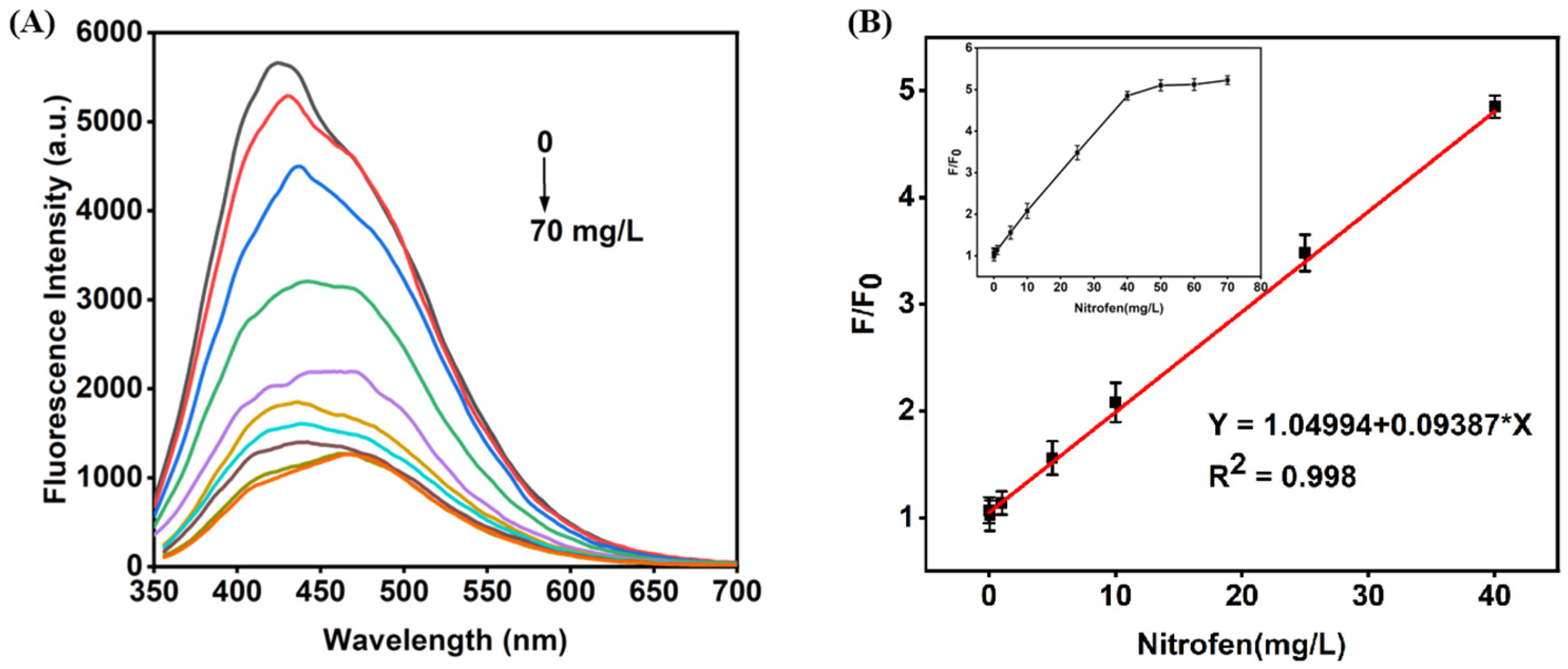
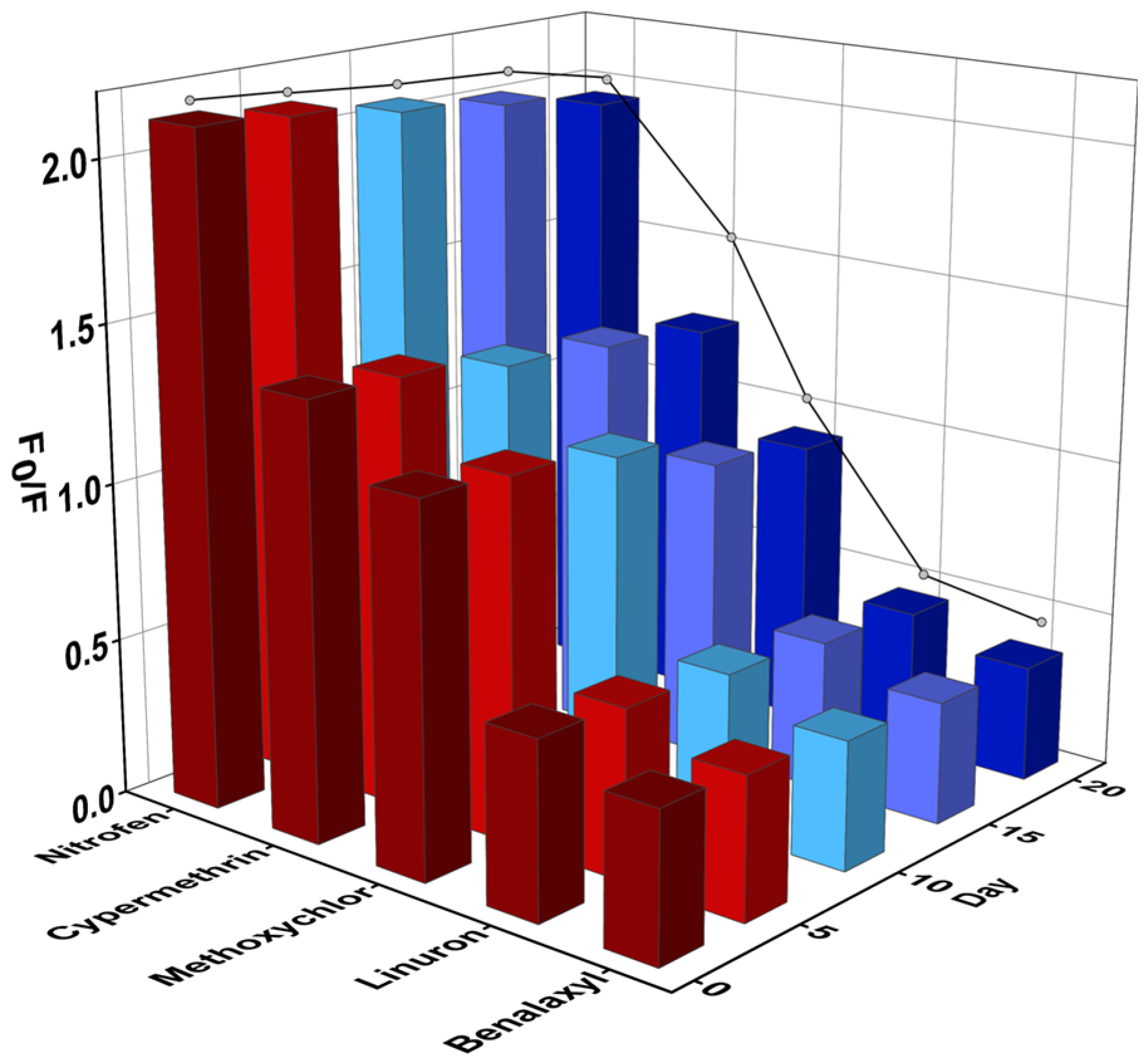
3.4. Performance Determination of CQDs@MIPs
3.5. Real Samples Analysis
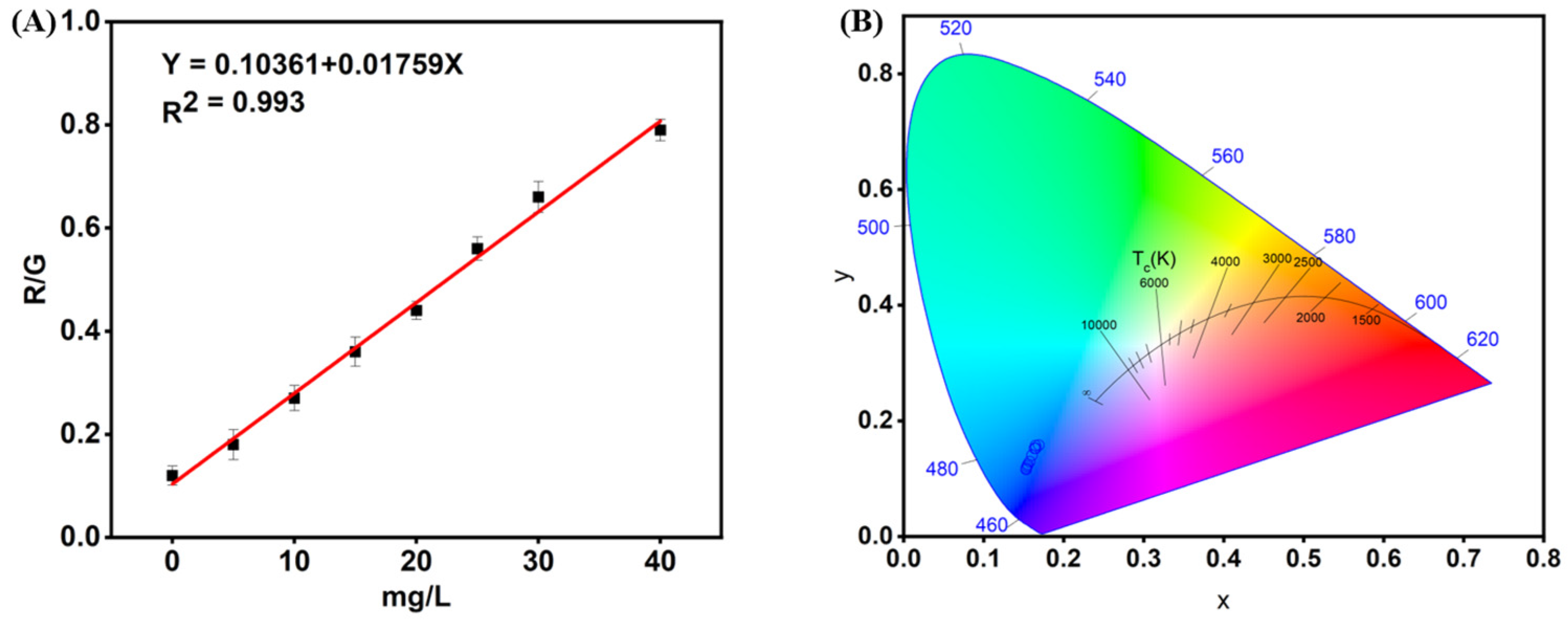
3.6. Performance Evaluation of Deep Learning in RGB Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sharkey, A.M.; Hartig, A.M.; Dang, A.J.; Chatterjee, A.; Williams, B.J.; Parker, K.M. Amine Volatilization from Herbicide Salts: Implications for Herbicide Formulations and Atmospheric Chemistry. Environ. Sci. Technol. 2022, 56, 13644–13653. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbo, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.E.; Hladik, M.L.; Kolpin, D.W. Occurrence of Dichloroacetamide Herbicide Safeners and Co-Applied Herbicides in Midwestern U.S. Streams. Environ. Sci. Technol. Lett. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- da Silva, A.P.; Poquioma Hernandez, H.V.; Comelli, C.L.; Guillen Portugal, M.A.; Moreira Delavy, F.; de Souza, T.L.; de Oliveira, E.C.; de Oliveira-Ribeiro, C.A.; Silva de Assis, H.C.; de Castilhos Ghisi, N. Meta-analytical review of antioxidant mechanisms responses in animals exposed to herbicide 2,4-D herbicide. Sci. Total Environ. 2024, 924, 171680. [Google Scholar] [CrossRef]
- Duque, T.S.; Pinheiro, R.A.; Souza, I.M.; Silva, G.G.; Soares, M.A.; Barbosa Dos Santos, J. Herbicides and bio-inputs: Compatibility and challenges for sustainable agriculture. Chemosphere 2024, 369, 143878. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, X.; Zhang, Z.; Han, C.; Mao, Z.; Gao, C.; Huang, F. Pillar[6]arene/paraquat molecular recognition in water: High binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 2012, 134, 19489–19497. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, L.H.; Wang, Y.; Wang, H.; Xu, Z.L.; Tian, Y.X.; Sun, Y.M.; Yang, J.Y.; Shen, Y.D. Ultrasensitive and rapid colorimetric detection of paraquat via a high specific VHH nanobody. Biosens. Bioelectron. 2022, 205, 114089. [Google Scholar] [CrossRef]
- Yoshioka, N.; Asano, M.; Kuse, A.; Matsuoka, T.; Akiyama, Y.; Mitsuhashi, T.; Nagasaki, Y.; Ueno, Y. Rapid and sensitive quantification of paraquat and diquat in human serum by liquid chromatography/time-of-flight mass spectrometry using atmospheric pressure photoionization. Forensic Toxicol. 2012, 30, 135–141. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; Labuza, T.P. Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chem. 2014, 148, 42–46. [Google Scholar] [CrossRef]
- Li, N.; Ng, T.B.; Wong, J.H.; Qiao, J.X.; Zhang, Y.N.; Zhou, R.; Chen, R.R.; Liu, F. Separation and purification of the antioxidant compounds, caffeic acid phenethyl ester and caffeic acid from mushrooms by molecularly imprinted polymer. Food Chem. 2013, 139, 1161–1167. [Google Scholar] [CrossRef]
- Fan, D.; Jia, L.; Xiang, H.; Peng, M.; Li, H.; Shi, S. Synthesis and characterization of hollow porous molecular imprinted polymers for the selective extraction and determination of caffeic acid in fruit samples. Food Chem. 2017, 224, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Cytosensing of cancer cells using antibody-based molecular imprinting: A short-review. TrAC Trends Anal. Chem. 2018, 99, 129–134. [Google Scholar] [CrossRef]
- Zhu, L.; Mei, X.; Peng, Z.; Yang, J.; Li, Y. A paper-based microfluidic sensor array combining molecular imprinting technology and carbon quantum dots for the discrimination of nitrophenol isomers. J. Hazard. Mater. 2022, 435, 129012. [Google Scholar] [CrossRef]
- Wu, L.C.; Li, X.L.; Miao, H.H.; Xu, J.J.; Pan, G.Q. State of the art in development of molecularly imprinted sensors. View 2021, 3, 20200170. [Google Scholar] [CrossRef]
- Huo, P.; Lu, Z.; Liu, X.; Wu, D.; Liu, X.; Pan, J.; Gao, X.; Guo, W.; Li, H.; Yan, Y. Preparation photocatalyst of selected photodegradation antibiotics by molecular imprinting technology onto TiO2/fly-ash cenospheres. Chem. Eng. J. 2012, 189, 75–83. [Google Scholar] [CrossRef]
- Hassan, M.; Gomes, V.G.; Dehghani, A.; Ardekani, S.M. Engineering carbon quantum dots for photomediated theranostics. Nano Res. 2017, 11, 1–41. [Google Scholar] [CrossRef]
- Ma, Y.; Cen, Y.; Sohail, M.; Xu, G.; Wei, F.; Shi, M.; Xu, X.; Song, Y.; Ma, Y.; Hu, Q.A. Ratiometric fluorescence universal platform based on N, Cu codoped carbon dots to detect metabolites participating in H2O2-generation reactions. ACS Appl. Mater. Interfaces 2017, 9, 33011–33019. [Google Scholar] [CrossRef]
- Katzen, J.M.; Tserkezis, C.; Cai, Q.; Li, L.H.; Kim, J.M.; Lee, G.; Yi, G.R.; Hendren, W.R.; Santos, E.J.G.; Bowman, R.M. Strong Coupling of Carbon Quantum Dots in Plasmonic Nanocavities. ACS Appl. Mater. Interfaces 2020, 12, 19866–19873. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hu, Y.; Wang, G.; Dong, S.; Hao, J. Magnetic networks of carbon quantum dots and Ag particles. J. Colloid Interface Sci. 2019, 539, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. Engl. 2017, 56, 9910–9914. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E. Carbon quantum dots derived from polysaccharides: Chemistry and potential applications. Carbohydr. Polym. 2024, 324, 121503. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Richtera, L.; Xhaxhiu, K.; Hynek, D.; Heger, Z.; Zitka, O.; Adam, V. Carbon dots based FRET for the detection of DNA damage. Sens. Bioelectron. 2017, 92, 133–139. [Google Scholar] [CrossRef]
- Lu, S.; Li, G.; Lv, Z.; Qiu, N.; Kong, W.; Gong, P.; Chen, G.; Xia, L.; Guo, X.; You, J. Facile and ultrasensitive fluorescence sensor platform for tumor invasive biomaker beta-glucuronidase detection and inhibitor evaluation with carbon quantum dots based on inner-filter effect. Biosens. Bioelectron. 2016, 85, 358–362. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, B.; Tan, C.-P.; Lai, O.-M.; Panpipat, W.; Cheong, L.-Z.; Shen, C. Hierarchical macro-microporous ZIF-8 nanostructures as efficient nano-lipase carriers for rapid and direct electrochemical detection of nitrogenous diphenyl ether pesticides. Sens. Actuators B Chem. 2020, 321, 128477. [Google Scholar] [CrossRef]
- Di, L.; Xia, Z.; Li, J.; Geng, Z.; Li, C.; Xing, Y.; Yang, Z. Selective sensing and visualization of pesticides by ABW-type metal-organic framework based luminescent sensors. RSC Adv. 2019, 9, 38469–38476. [Google Scholar] [CrossRef]
- Cheng, Y.; Lai, O.-M.; Tan, C.-P.; Panpipat, W.; Cheong, L.-Z.; Shen, C. Proline-modified UIO-66 as nanocarriers to enhance Candida rugosa lipase catalytic activity and stability for electrochemical detection of nitrofen. ACS Appl. Mater. Interfaces 2021, 13, 4146–4155. [Google Scholar] [CrossRef]
- Yan, M.; Jia, Y.; Qi, P.; Wang, Y.; Ji, Q.; Wang, M.; Wang, Q.; Hao, Y. Determination of three diphenyl ether herbicides in rice by magnetic solid phase extraction using Fe3O4@MOF-808 coupled with high performance liquid chromatography. Chin. J. Chromatogr. 2021, 39, 316–323. [Google Scholar] [CrossRef]
- Turan, N.B.; Bakirdere, S. A miniaturize spray assiste fine droplet formation based liquid phase microextraction method for the simultaneous determination of fenpiclonil, nitrofen and fenoxapropethyl as pesticides in soil samples. Rapid Commun. Mass Spectrom. 2021, 35, e8943. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Zang, X.; Pang, Y.; Chang, Q.; Wang, C.; Wang, Z. Determination of pesticides residues in vegetable and fruit samples by solid-phase microextraction with a covalent organic framework as the fiber coating coupled with gas chromatography and electron capture detection. J. Sep. Sci. 2018, 41, 4038–4046. [Google Scholar] [CrossRef] [PubMed]

| Sample | Spiked Concentration (mg/L) | Detection Concentration (mg/L) | Recovery Rate (%) | RSD |
|---|---|---|---|---|
| Seawater | 1.00 | 1.08 | 108.1 | 1.1% |
| 10.00 | 8.41 | 84.1 | 3.1% | |
| 50.00 | 57.88 | 115.7 | 1.9% | |
| Tap water | 1.00 | 0.86 | 86.6 | 3.1% |
| 10.00 | 10.15 | 101.5 | 4.7% | |
| 50.00 | 51.84 | 103.7 | 2.1% | |
| Purified water | 1.00 | 1.03 | 103.3 | 2.9% |
| 10.00 | 10.18 | 101.9 | 6.7% | |
| 50.00 | 48.25 | 96.5 | 2.6% |
| Biosensor | Analyte | Linear Range (mg/L) | LOD (mg/L) | References |
|---|---|---|---|---|
| nano-BCL@MAC-ZIF-8 | Nitrofen | 0–22.5 | 0.13 | [26] |
| 3D ABW-type Z-MOF | Nitrofen | 0.8~20.00 | 57 | [27] |
| CRL@UIO-66/Pro | Nitrofen | / | 2.93 | [28] |
| Fe3O4@MOF | Nitrofen | 0.002–0.3 | 6 × 10−4 | [29] |
| HPLC | Nitrofen | 0.005~0.1 | 1.56 × 10−5 | [30] |
| CDs@MMIP | Nitrofen | 0.04~0.25 | 5 × 10−5 | [31] |
| CQDs@MIPs | Nitrofen | 0.01~40 | 2.5 × 10−3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, Y.; You, J.; Zhang, Z.; Sun, A.; Liu, H.; Shi, X. Fluorescent Molecular Imprinted Sensor Based on Carbon Quantum Dot for Nitrofen Detection in Water Sample. Polymers 2025, 17, 816. https://doi.org/10.3390/polym17060816
Chen Y, Zhou Y, You J, Zhang Z, Sun A, Liu H, Shi X. Fluorescent Molecular Imprinted Sensor Based on Carbon Quantum Dot for Nitrofen Detection in Water Sample. Polymers. 2025; 17(6):816. https://doi.org/10.3390/polym17060816
Chicago/Turabian StyleChen, Yuge, Yongheng Zhou, Jinjie You, Zeming Zhang, Aili Sun, Hua Liu, and Xizhi Shi. 2025. "Fluorescent Molecular Imprinted Sensor Based on Carbon Quantum Dot for Nitrofen Detection in Water Sample" Polymers 17, no. 6: 816. https://doi.org/10.3390/polym17060816
APA StyleChen, Y., Zhou, Y., You, J., Zhang, Z., Sun, A., Liu, H., & Shi, X. (2025). Fluorescent Molecular Imprinted Sensor Based on Carbon Quantum Dot for Nitrofen Detection in Water Sample. Polymers, 17(6), 816. https://doi.org/10.3390/polym17060816






