Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of TiO2 Nanoparticles
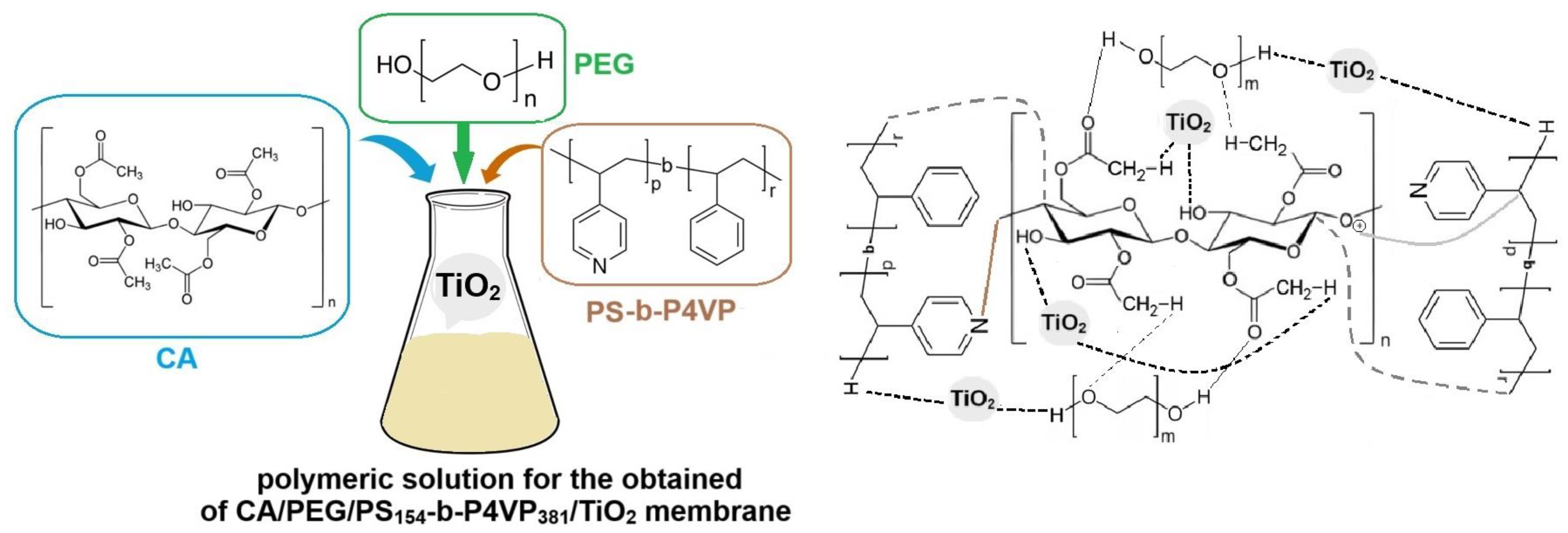
2.3. Synthesis of TiO2 Nanoparticles Polymeric Membrane
2.4. Characterization
2.4.1. Morpho-Structural Characterization of the TiO2 Nanoparticles
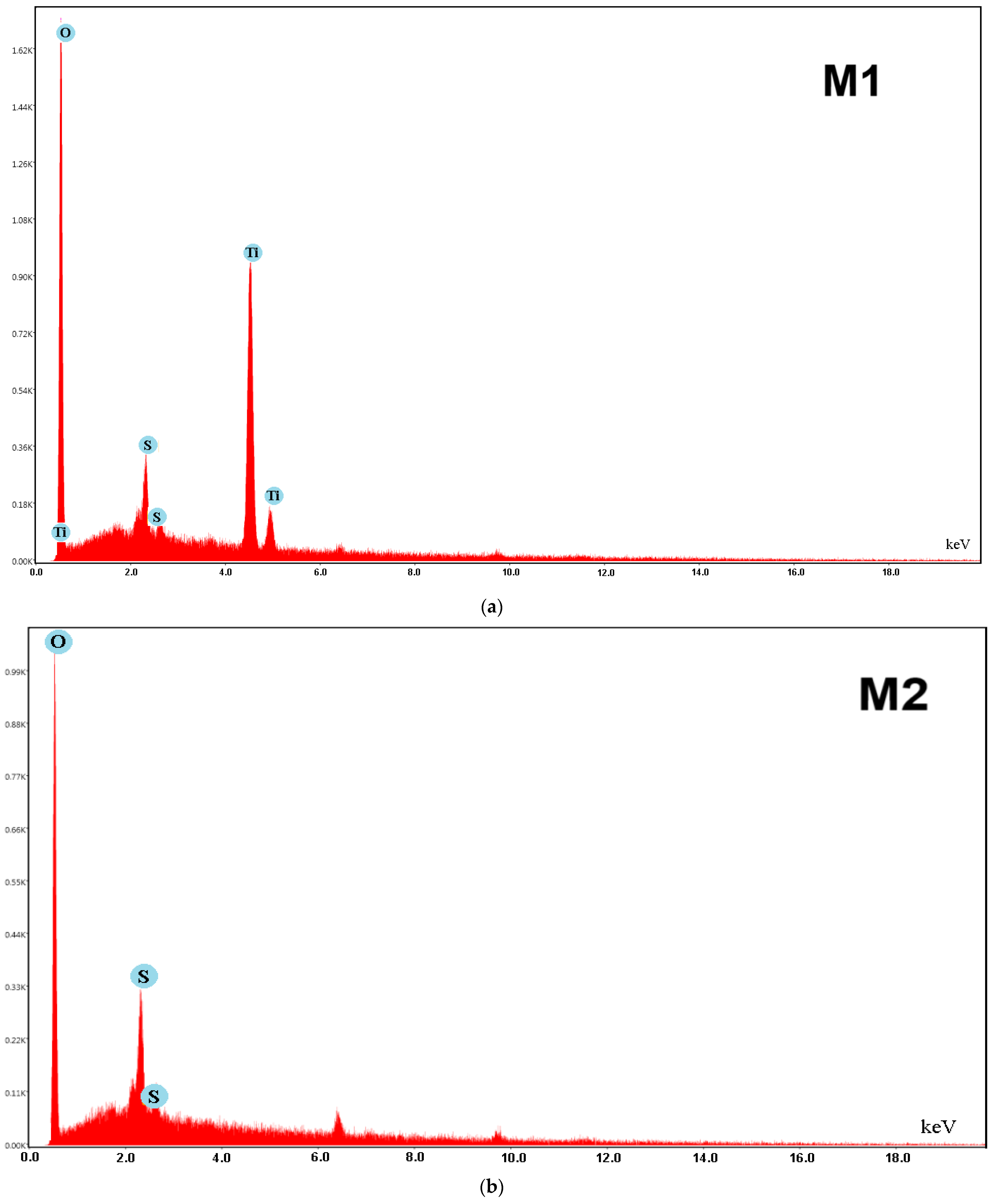
2.4.2. Morpho-Structural Characterization of the Polymeric Membranes
2.4.3. Thermogravimetric Analysis (TGA) of the Polymeric Membranes
2.4.4. Water Retention and Porosity of the Polymeric Membranes
3. Results and Discussion
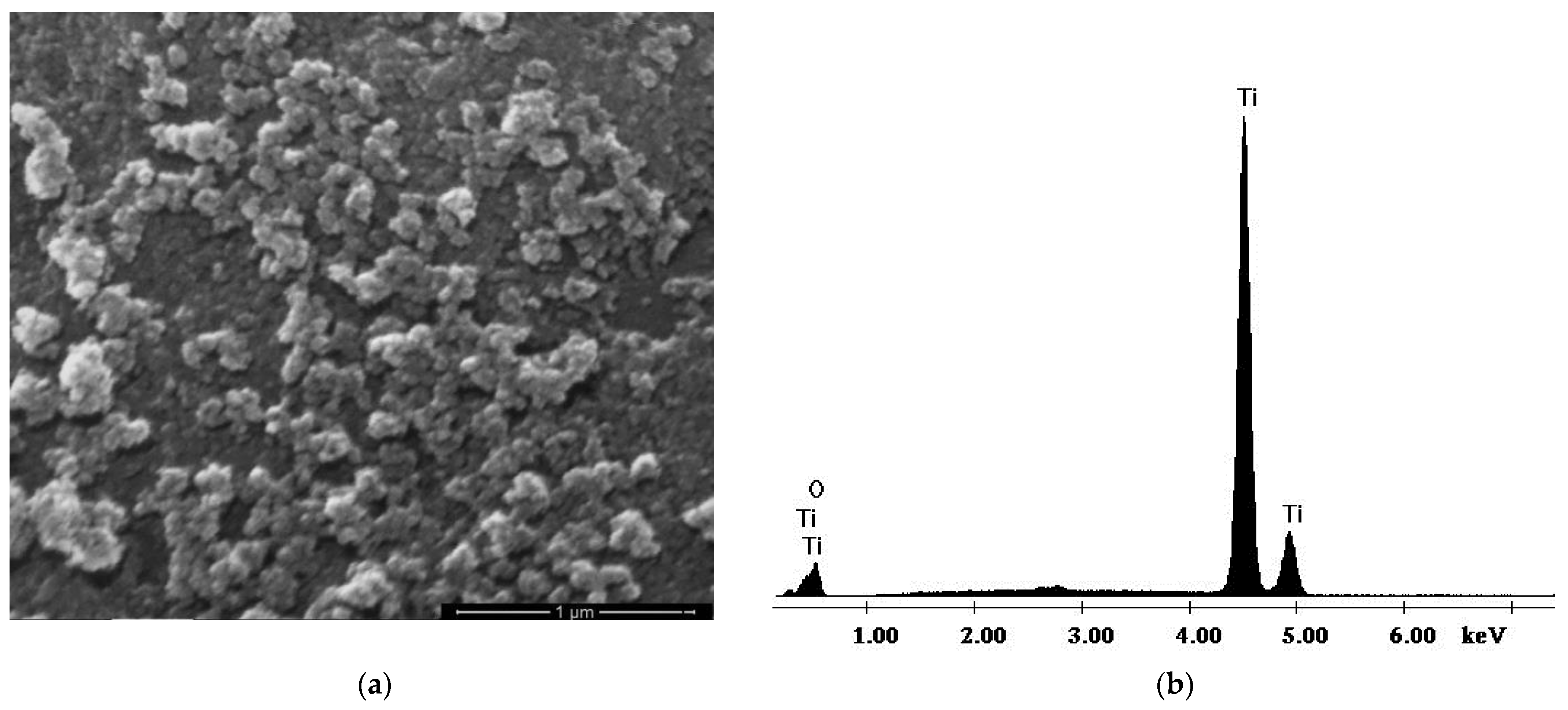
3.1. Characterization of the TiO2 Nanoparticles
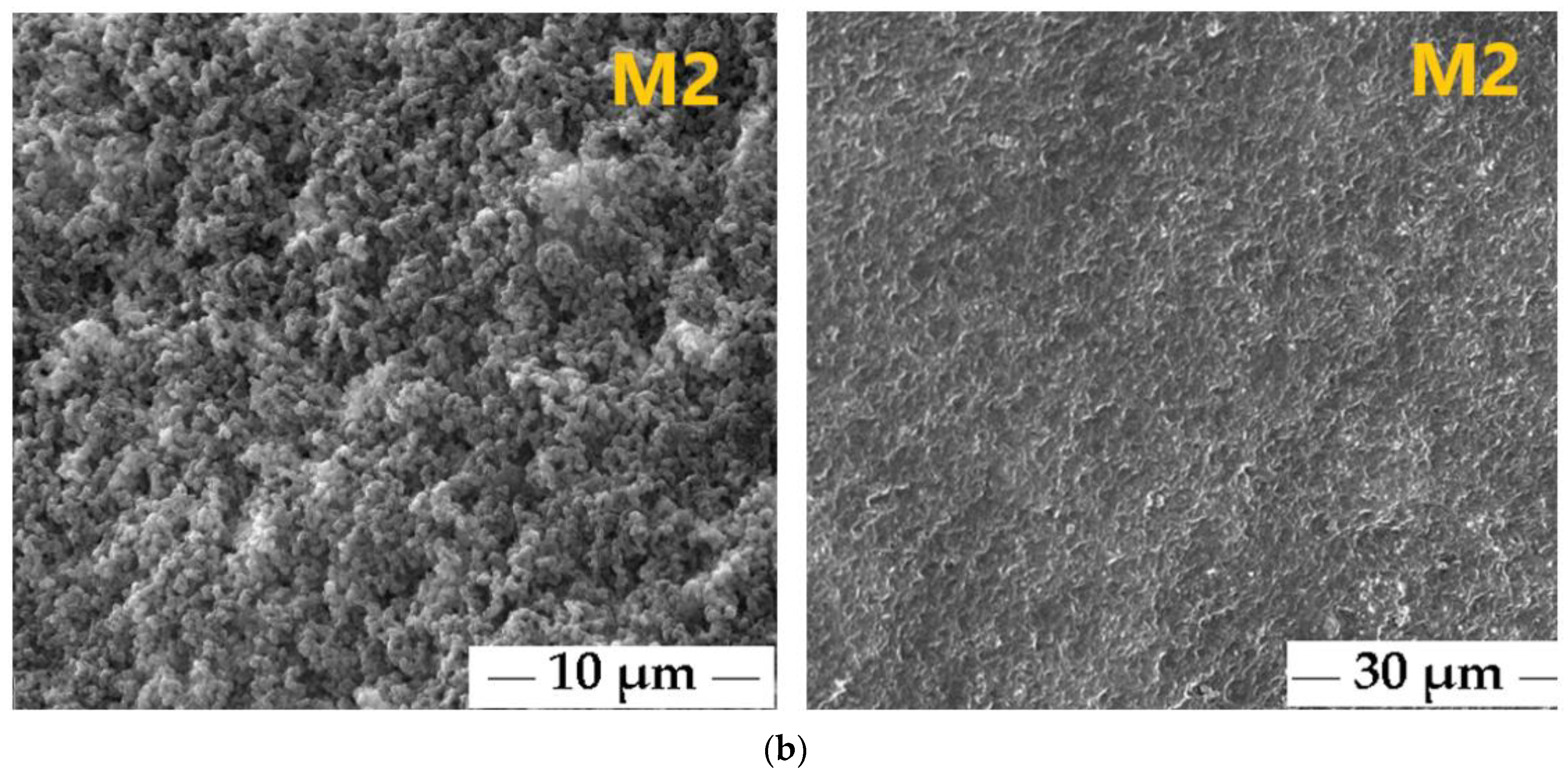
3.2. Characterization of the Polymeric Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourjafar, S.; Rahimpour, A.; Jahanshahi, M. Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 2012, 18, 1398–1405. [Google Scholar] [CrossRef]
- Nain, A.; Sangili, A.; Hu, S.-R.; Chen, C.-H.; Chen, Y.-L.; Chang, H.-T. Recent progress in nanomaterial-functionalized membranes for removal of pollutants. iScience 2022, 25, 104616. [Google Scholar] [CrossRef] [PubMed]
- Dehghankar, M.; Hmtshirazi, R.; Mohammadi, T.; Tofighy, M.A. Synthesis and modification methods of metal-organic frameworks and their application in modification of polymeric ultrafiltration membranes: A review. J. Environ. Chem. Eng. 2023, 11, 109954. [Google Scholar] [CrossRef]
- Farjami, M.; Vatanpour, V.; Moghadassi, A. Fabrication of a new emulsion polyvinyl chloride (EPVC) nanocomposite ultrafiltration membrane modified by para-hydroxybenzoate alumoxane (PHBA) additive to improve permeability and antifouling performance. Chem. Eng. Res. Des. 2020, 153, 8–20. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Yuan, Y.; Wang, Y.; Xu, C.; Fang, Y.; Gu, Y.; Liu, P.; Wan, Y.; Wang, L.; et al. TiO2 nanoparticle embedded PEEKWC/PEI cross-linked ultrafiltration membrane: Improvement in flux and antifouling properties. Chem. Eng. Res. Des. 2024, 202, 480–488. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.-N.; Wang, R.; Fane, A.G. Synthesis and characterization of thin film nanocomposite forward osmosis membranes supported by silica nanoparticle incorporated nanofibrous substrate. Desalination 2017, 401, 142–150. [Google Scholar] [CrossRef]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dăncilă, A.M.; Orbulet, O.D. Study of Polyvinyl Alcohol-SiO2 Nanoparticles Polymeric Membrane in Waste-water Treatment Containing Zinc Ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Mustapa, F.; Selviantori, S.; Idris, M.; Mahmud, A.; Maulidiyah, M.; Muzakkar, M.Z.; Umar, A.A.; Nurdin, M. CA/PEG/chitosan membrane incorporated with TiO2 nanoparticles for strengthening and permselectivity membrane for reverse osmosis desalination. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100848. [Google Scholar] [CrossRef]
- Peyki, A.; Rahimpour, A.; Jahanshahi, M. Preparation and characterization of thin film composite reverse osmosis membranes incorporated with hydrophilic SiO2 nanoparticles. Desalination 2015, 368, 152–158. [Google Scholar] [CrossRef]
- Ahmad, A.; Sabir, A.; Iqbal, S.S.; Felemban, B.F.; Riaz, T.; Bahadar, A.; Hossain, N.; Khan, R.U.; Inam, F. Novel antibacterial polyurethane and cellulose acetate mixed matrix membrane modified with functionalized TiO2 nanoparticles for water treatment applications. Chemosphere 2022, 301, 134711. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Taha, Y.R.; Zrelli, A.; Hajji, N.; Alsalhy, Q.; Shehab, M.A.; Nemeth, Z.; Hernadi, K. Optimum content of incorporated nanomaterials: Characterizations and performance of mixed matrix membranes a review. Desalin. Water Treat. 2024, 317, 100088. [Google Scholar] [CrossRef]
- Yan, H.-F.; Luo, L.-H.; Xu, Z.-L.; Fang, Y.-X.; Pandaya, D.; Dai, J.-Y.; Liang, J.; Xu, S.-J. Incorporating etched ZnO nanoparticles to fabricate positively charged composite membrane for heavy metal removal. Desalination 2024, 574, 117275. [Google Scholar] [CrossRef]
- Heng, Z.W.; Tan, Y.Y.; Chong, W.C.; Mahmoudi, E.; Mohammad, A.W.; Teoh, H.C.; Sim, L.C.; Koo, C.H. Preparation of a novel polysulfone membrane by incorporated with carbon dots grafted silica from rice husk for dye removal. J. Water Proc. Eng. 2021, 40, 101805. [Google Scholar] [CrossRef]
- Amiri, M.K.; Zahmatkesh, S.; Emami, M.R.S.; Bokhari, A. Curve fitting model of Polycarbonate Al2O3-nanoparticle membranes for removing emerging contaminants from waste-water: Effect of temperature and nanoparticles. Chemosphere 2023, 322, 138184. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultratiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Preparation and characterization of novel porous PVDF-ZrO2 composite membranes. Desalination 2002, 146, 35–40. [Google Scholar] [CrossRef]
- Tavangar, T.; Karimi, M.; Rezakazemi, M.; Reddy, K.R.; Aminabhavi, T.M. Textile waste, dyes/inorganic salts separation of cerium oxide loaded loose nanofiltration polyethersulfone membranes. Chem. Eng. J. 2020, 385, 123787. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Zhang, R.; Elzatahry, A.A.; Al-Deyab, S.S.; Zhao, D. Mesoporous titania: From synthesis to application. Nano Today. 2012, 7, 344–366. [Google Scholar] [CrossRef]
- Aparicio, G.M.; Vargas, R.A.; Bueno, P.R. Protonic conductivity and thermal properties of cross-linked PVA/TiO2 nanocomposite polymer membranes. J. Non-Cryst. Solids 2019, 522, 119520. [Google Scholar] [CrossRef]
- Nascimben Santos, E.; Fazekas, Á.; Hodúr, C.; László, Z.; Beszédes, S.; Scheres Firak, D.; Gyulavári, T.; Hernádi, K.; Arthanareeswaran, G.; Veréb, G. Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers 2022, 14, 113. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Gull, N.; Hussain, S.N.; Butt, M.T.Z. Cellulaose acetate based thin film nanocomposite reverse osmosis membrane incorporated with TiO2 nanoparticles for improved performanc. Carbohydr. Polym. 2018, 186, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Chi, M.; Zheng, P.; Wei, M.; Zhu, A.; Zhong, L.; Zhang, Q.; Liu, Q. Polyamide composite nanofiltration membrane modified by nanoporous TiO2 interlayer for enhanced water permeability. J. Ind. Eng. Chem. 2022, 115, 230–240. [Google Scholar] [CrossRef]
- Calderara, F.; Riess, G. Characterization of polystyrene-block-poly(4-vinylpyridine) block copolymer micelles in toluene solution. Macromol. Chem. Phys. 1996, 197, 2115–2132. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Bhat, U.K.; Ismail, A.F.; Obaidd, A.; Fun, H.-K. Preparation and performance studies of polysulfone-sulfated nano-titania (S-TiO2) nanofiltration membranes for dye removal. RSC Adv. 2015, 5, 53874. [Google Scholar] [CrossRef]
- Liu, N.-J.; Yu, J.-Y.; Chen, X.-Y.; Liu, L.-F. A novel organic solvent ultrafiltration membrane of polyimide/polyethyleneimine@TiO2 with high solvent permeability. J. Membr. Sci. 2024, 702, 122796. [Google Scholar] [CrossRef]
- Goyat, R.; Singh, J.; Umar, A.; Saharan, Y.; Ibrahim, A.A.; Akbar, S.; Baskoutas, S. Synthesis and characterization of nanocomposite based polymeric membrane (PES/PVP/GO-TiO2) and performance evaluation for the removal of various antibiotics (amoxicillin, azithromycin & ciprofloxacin) from aqueous solution. Chemosphere 2024, 353, 141542. [Google Scholar]
- Okte, A.N.; Sayinsoz, E. Characterization and photocatalytic activity of TiO2 supported sepiolite catalysts. Sep. Purif. Technol. 2008, 62, 535–543. [Google Scholar] [CrossRef]
- Qi, B.; Wu, L.; Zhang, Y.; Zeng, Q.; Zhi, J. Low-temperature and one-step synthesis of rutile TiO2 aqueous sol by heterogeneous nucleation method. J. Colloid Interface Sci. 2010, 345, 181–186. [Google Scholar] [CrossRef]
- Nicolaescu, M.; Bandas, C.; Orha, C.; Şerban, V.; Lazău, C.; Căprărescu, S. Fabrication of a UV Photodetector Based on n-TiO2/p-CuMnO2 Heterostructures. Coatings 2021, 11, 1380. [Google Scholar] [CrossRef]
- Waseem, S.; Zeeshan, T.; Tariq, H.; Majid, F.; Ali, M.D.; Zohra Nazir, K.; Mongi, A. The influence of transition metals (Fe, Co) on the structural, magnetic and optical properties of TiO2 nanoparticles synthesized by the hydrothermal method. Appl. Phys. A 2022, 128, 690. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Zhang, J. Preparation of 4-vinylpyridine (4VP) resin and its adsorption performance for heavy metal ions. RSC Adv. 2017, 7, 4226–4236. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhao, Y.; Lu, Q. Control over the hydrophobic behavior of polystyrene surface by annealing temperature based on capillary template wetting method. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 136–140. [Google Scholar] [CrossRef]
- Kennemur, J.G. Poly(vinylpyridine) Segments in Block Copolymers: Synthesis, SelfAssembly, and Versatility. Macromolecules 2019, 52, 1354–1370. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, Y.; Su, Z.; Huo, M.; Yang, X.; Huo, H.; Wang, C.; Geng, Z. High performance photodegradation resistant PVA@TiO2/carboxyl-PES self-healing reactive ultrafiltration membrane. Chin. J. Chem. Eng. 2024, 66, 31–39. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Totu, E.E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Song, L.; Lam, Y.M.; Boothroyd, C.; Teo, P.W. One-step synthesis of titania nanoparticles from PS-P4VP diblock copolymer solution. Nanotechnology 2007, 18, 135605. [Google Scholar] [CrossRef] [PubMed]
- Damavandi, F.; Aroujalian, A.; Salimi, P. TiO2 nanoparticle stability via polyacrylic acid-binding on the surface of polyethersulfone membrane: Long-term evaluation. J. Ind. Eng. Chem. 2023, 117, 307–318. [Google Scholar] [CrossRef]

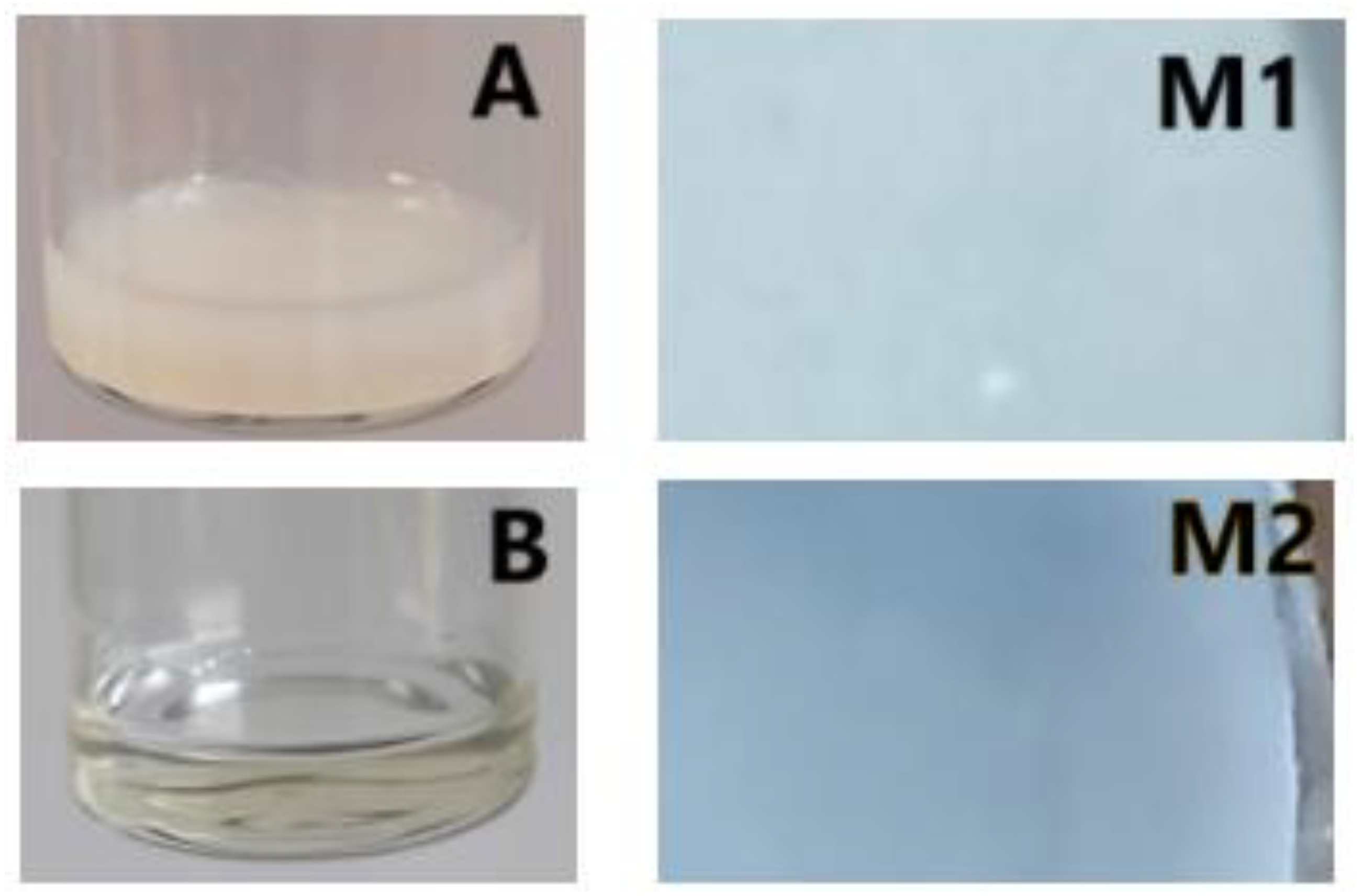





| Liquid | Tyle of Polymeric Membrane | |
|---|---|---|
| CA/PEG/PS154-b-P4VP381/TiO2 (M1) | CA/PEG/PS154-b-P4VP381 (M2) | |
| Water | 15.57 ± 0.45° | 75.62 ± 1.13° |
| DMSO | 18.86 ± 0.24° | 42.73 ± 0.40° |
| Glycerol | 89.13 ± 0.35° | 75.94 ± 0.62° |
| Tyle of Polymeric Membrane | mN m−1 | mN m−1 | mN m−1 |
|---|---|---|---|
| CA/PEG/PS154-b-P4VP381/TiO2 (M1) | 73.4998 | 1.2372 | 74.7370 |
| CA/PEG/PS154-b-P4VP381 (M2) | 11.4258 | 18.6365 | 30.0623 |
| Tyle of Polymeric Membrane | WRC (%) | Ɛ (%) |
|---|---|---|
| CA/PEG/PS154-b-P4VP381/TiO2 (M1) | 81.60 | 87.35 |
| CA/PEG/PS154-b-P4VP381 (M2) | 63.98 | 64.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căprărescu, S.; Tihan, G.T.; Zgârian, R.G.; Grumezescu, A.M.; Lazau, C.; Bandas, C.; Atanase, L.I.; Nicolae, C.-A. Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes. Polymers 2025, 17, 446. https://doi.org/10.3390/polym17040446
Căprărescu S, Tihan GT, Zgârian RG, Grumezescu AM, Lazau C, Bandas C, Atanase LI, Nicolae C-A. Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes. Polymers. 2025; 17(4):446. https://doi.org/10.3390/polym17040446
Chicago/Turabian StyleCăprărescu, Simona, Grațiela Teodora Tihan, Roxana Gabriela Zgârian, Alexandru Mihai Grumezescu, Carmen Lazau, Cornelia Bandas, Leonard Ionuț Atanase, and Cristian-Andi Nicolae. 2025. "Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes" Polymers 17, no. 4: 446. https://doi.org/10.3390/polym17040446
APA StyleCăprărescu, S., Tihan, G. T., Zgârian, R. G., Grumezescu, A. M., Lazau, C., Bandas, C., Atanase, L. I., & Nicolae, C.-A. (2025). Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes. Polymers, 17(4), 446. https://doi.org/10.3390/polym17040446













